Abstract
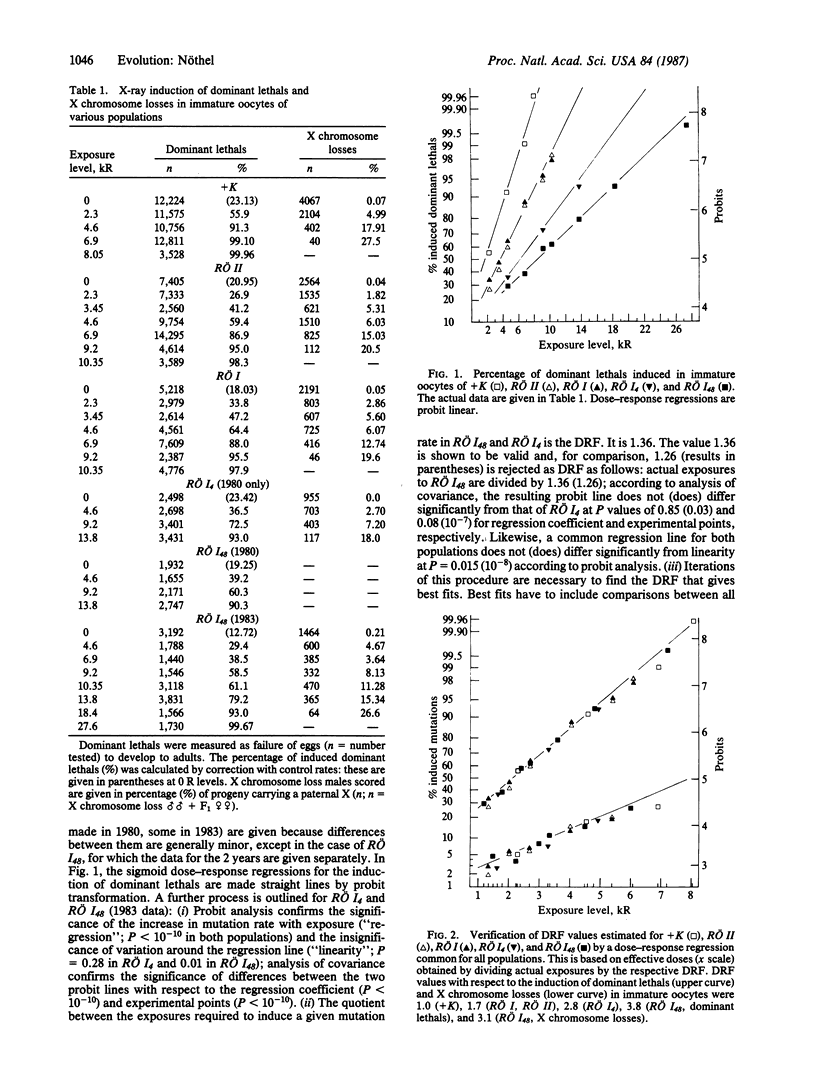
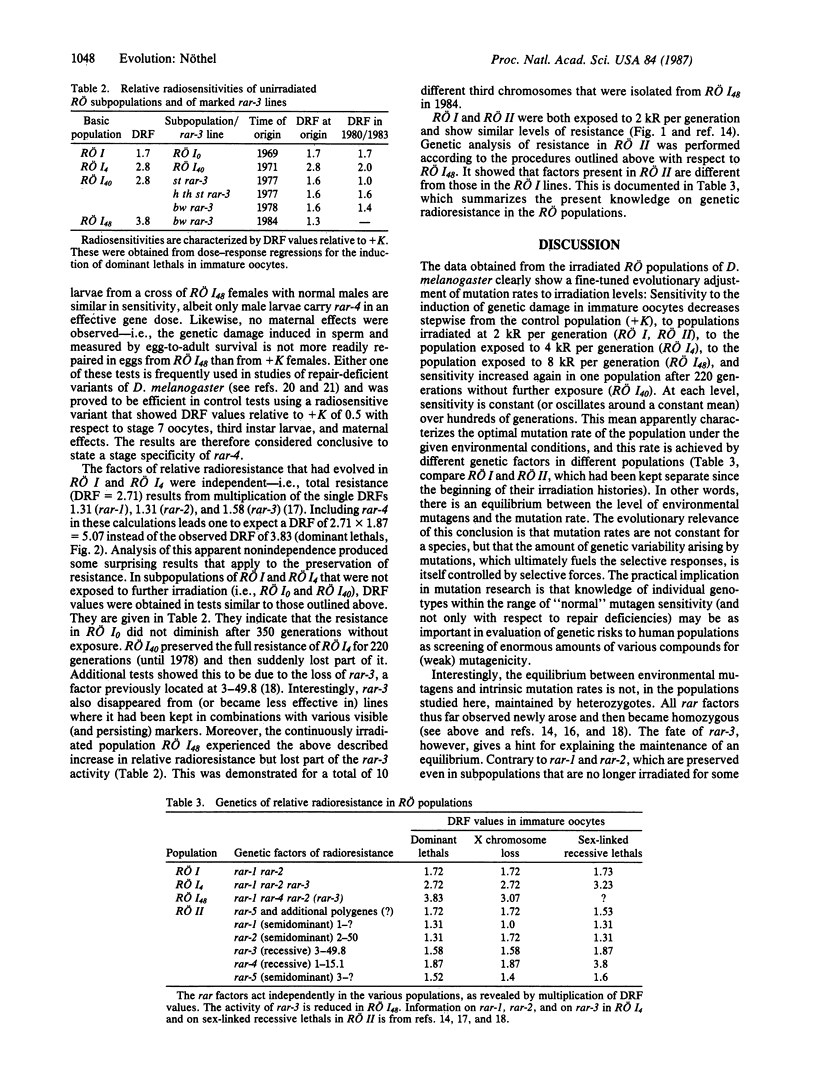
Evolutionary aspects of high mutation pressure were studied in laboratory populations of Drosophila melanogaster that have irradiation histories up to 600 generations. Dose-response regressions for the x-ray induction of various types of mutation were obtained from six of these populations. The sensitivity of these irradiated populations relative to an unirradiated control population was characterized by dose reduction factors. Sensitivity decreased stepwise with the stepwise increase in irradiation levels to which the populations had been exposed every generation (0 R, 2 kR, 4 kR, 8 kR; 1 R = 0.258 mC/kg) but remained the same over hundreds of generations when the irradiation levels were constant. Resistance is controlled by single genetic factors. Additional factors evolved in subpopulations exposed to increased irradiation levels, and different factors evolved in populations that were kept separate from the beginning of their irradiation histories. Two of three factors persisted in subpopulations no longer irradiated, but one factor disappeared; this last one behaved like a transposon. Factors of relative radio-resistance are stage specific (immature oocytes) and some of them are assumed to modify or control mutation-rate genes. The resistance factors enable populations to achieve an equilibrium between the amounts of environmental mutagens and intrinsic mutation rates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd J. B., Golino M. D., Shaw K. E., Osgood C. J., Green M. M. Third-chromosome mutagen-sensitive mutants of Drosophila melanogaster. Genetics. 1981 Mar-Apr;97(3-4):607–623. doi: 10.1093/genetics/97.3-4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E. C., Gibson T. C. Selection for high mutation rates in chemostats. Genetics. 1974 Jun;77(2):169–184. doi: 10.1093/genetics/77.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Ganesan A. K., Smith C. A. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- Holsinger K. E., Feldman M. W. Modifiers of mutation rate: Evolutionary optimum with complete selfing. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6732–6734. doi: 10.1073/pnas.80.21.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Nöthel H. Investigation on radiosensitive and radioresistant populations of Drosophila melanogaster. VI. Selection for further increase in relative radioresistance of stage-7 oocytes of the irradiated population RO I. Mutat Res. 1974 Oct;25(1):135–139. doi: 10.1016/0027-5107(74)90226-7. [DOI] [PubMed] [Google Scholar]
- Nöthel H. Investigations on radiosensitive and radioresistant populations of Drosophila melanogaster. IV. The genetics of the relative radioresistance in stage-7 oocytes of the irradiated population RO I. Mutat Res. 1974 May;23(2):163–177. doi: 10.1016/0027-5107(74)90137-7. [DOI] [PubMed] [Google Scholar]
- Nöthel H. Investigations on radiosensitive and radioresistant populations of Drosophila melanogaster. X. The resistance factor rar-3: genetics. Mutat Res. 1981 Dec;84(2):291–304. doi: 10.1016/0027-5107(81)90199-8. [DOI] [PubMed] [Google Scholar]
- Nöthel H. Investigations on radiosensitive and radioresistant populations of Drosophila melanogaster. XII. The resistance factor rar-3: stage specificity. Mutat Res. 1981 Dec;84(2):315–329. doi: 10.1016/0027-5107(81)90201-3. [DOI] [PubMed] [Google Scholar]
- Nöthel H. Investigations on radiosensitive and radioresistant populations of Drosophila melanogaster. XVI. Adaptation to the mutagenic effects of X-rays in several experimental populations with irradiation histories. Mutat Res. 1983 Nov;111(3):325–340. doi: 10.1016/0027-5107(83)90030-1. [DOI] [PubMed] [Google Scholar]
- Nöthel H., Weber M. Investigations on radio-sensitive and radio-resistant populations of Drosophila melanogaster. VII. High relative radio resistance to the induction of sex-linked recessive lethals in stage-7 oocytes of RO I4. Mutat Res. 1976 Aug;36(2):245–248. doi: 10.1016/0027-5107(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Zimmering S., Deitemeyer N. A further note on the utility of the excision repair-deficient mei-9a females of Drosophila melanogaster in detecting chromosome breakage induced by procarbazine in male germ cells. Environ Mutagen. 1981;3(3):293–295. doi: 10.1002/em.2860030312. [DOI] [PubMed] [Google Scholar]


