Abstract
σ-Receptors are integral membrane proteins that have been implicated in a number of biological functions, many of which involve the modulation of ion channels. A wide range of synthetic ligands activate σ-receptors, but endogenous σ-receptor ligands have proven elusive. One endogenous ligand, dimethyltryptamine (DMT), has been shown to act as a σ-receptor agonist. Progesterone and other steroids bind σ-receptors, but the functional consequences of these interactions are unclear. Here we investigated progesterone binding to σ1- and σ2-receptors and evaluated its effect on σ-receptor-mediated modulation of voltage-gated Na+ channels. Progesterone binds both σ-receptor subtypes in liver membranes with comparable affinities and blocks photolabeling of both subtypes in human embryonic kidney 293 cells that stably express the human cardiac Na+ channel Nav1.5. Patch-clamp recording in this cell line tested Na+ current modulation by the σ-receptor ligands ditolylguanidine, PB28, (+)SKF10047, and DMT. Progesterone inhibited the action of these ligands to varying degrees, and some of these actions were reduced by σ1-receptor knockdown with small interfering RNA. Progesterone inhibition of channel modulation by drugs was consistent with stronger antagonism of σ2-receptors. By contrast, progesterone inhibition of channel modulation by DMT was consistent with stronger antagonism of σ1-receptors. Progesterone binding to σ-receptors blocks σ-receptor-mediated modulation of a voltage-gated ion channel, and this novel membrane action of progesterone may be relevant to changes in brain and cardiovascular function during endocrine transitions.
Keywords: neurosteroids, heart muscle, heart rhythmicity
σ-receptors bind to a wide variety of drugs and have profound effects on membrane excitability. σ-Receptors comprise at least two subtypes designated σ1 and σ2 (21, 44). The σ1-receptor has been better characterized: its gene encodes a 25.3-kDa protein (17, 27, 48) thought to harbor two transmembrane segments with an extracellular loop and cytoplasmic COOH and NH2 termini (1). The σ2-receptor has a distinct pharmacological signature and a lower molecular mass of ∼18–21 kDa (22, 23), but its molecular structure is unknown. σ-Receptors modulate the activity of a wide variety of ion channels, including N-, L-, P/Q- and R-type Ca2+ channels in rat sympathetic and parasympathetic neurons (61), several types of K+ channels in hippocampus, intracardiac neurons, neurohypophysis, and tumor cells (28, 36, 57, 58, 60), and Na+ channels in rodent cardiac myocytes (14, 25). σ-Receptors have also been shown to modulate Ca2+ influx induced by the acid-sensing ion channel ASIC1a (24), and ionotropic glutamate receptors in cortex (18). Ion channel modulation by σ-receptors does not depend on G proteins or phosphorylation (30, 40, 60) but is likely to result from protein-protein interactions within a complex that contains both the receptor and channel (1, 7). In addition to its role in ion channel modulation, the σ1-receptor has recently been proposed to serve as a Ca2+-sensitive, ligand-regulated molecular chaperone in the endoplasmic reticulum (20). Studies of the cellular functions of σ2-receptors are very limited, primarily focusing on the connections with cancer. Activation of σ2-receptors has been shown to lead to changes in intracellular calcium, changes in cell morphology, and apoptosis (5, 6, 53–55).
σ-Receptors were originally identified in binding studies with opioid receptor ligands (32), but the subsequent demonstration that they do not bind opioid peptides (49) immediately posed the problem of identifying endogenous σ-receptor ligands. Recent work with N,N-dimethyltryptamine (DMT) showed that this natural hallucinogen binds σ-receptors, acts as an agonist in σ-receptor-mediated Na+ channel modulation, and induces hyperactivity in mice in a σ-receptor-dependent fashion (14). Thus, a strong case has been made that DMT serves as an endogenous ligand in the activation of σ-receptors. σ-Receptors also bind steroids such as progesterone and testosterone and neuroactive steroids such as dehydroepiandrosterone (DHEA) (9, 15, 21, 51, 59), as well as cholesterol (41). Furthermore, the σ1-receptor sequence shows homology with a steroid-metabolizing enzyme, yeast sterol isomerase (17). However, physiological tests of several steroids failed to demonstrate activity in σ-receptor-mediated modulation of K+ channels in rodent neurohypophysis (57). Thus, the functional significance of steroid binding to σ-receptors remains unclear. Many steroid effects on behavior have been linked to the modulation of neuronal excitability through actions at cell surface receptors (31, 42, 46), and σ-receptors could contribute to these responses (34, 50). However, at the cellular level the physiological consequences of steroid-σ-receptor interactions are poorly understood. A number of studies have indicated that steroids can facilitate pre- and postsynaptic function in the brain (2, 10–12, 33, 43). Although the mechanisms of these actions remain unclear, the involvement of σ-receptors has been proposed (4, 37, 62). In light of its many effects on membrane excitability, we tested the action of progesterone, the steroid with the highest affinity for σ-receptors, on σ-receptor-mediated modulation of voltage-gated Na+ (Nav) channels (25). We confirmed that progesterone binds σ-receptors (51) and determined nanomolar affinities for both σ1- and σ2-subtypes. Furthermore, we found that progesterone acts as an antagonist in σ-receptor-mediated modulation of cardiac Na+ channels expressed in human embryonic kidney (HEK)293 cells. Progesterone binds to both the σ1- and σ2-receptors and blocks their activation to varying degrees depending on choice of ligand. Thus, progesterone can act as an endogenous ligand at σ-receptors. σ-Receptors represent novel membrane targets of progesterone.
METHODS
Ethical approval.
All procedures in this study were approved by the Animal Care and Use Committee of the University of Wisconsin School of Medicine and Public Health (Madison, WI), in compliance with the National Institutes of Health (Bethesda, MD).
Cell culture and transfection.
HEK293 cells stably expressing Nav1.5 were provided by Dr. J. C. Makielski [University of Wisconsin-Madison (52)]. Cells were cultured on glass coverslips at 37°C in 5% CO2-air atmosphere, in minimum essential Eagle's medium (Fisher Scientific, Pittsburg, PA) with 10% fetal bovine serum, 1% penicillin-streptomycin, 1% l-glutamine, 1% sodium pyruvate, and 400 μg/ml gentamicin to maintain the selection for human Nav1.5 expression.
A σ1-receptor small interfering RNA (siRNA) construct was designed using the pRNAT-U6.1/Neo plasmid (GenScript, Piscataway, NJ). An siRNA sequence corresponding to nucleotides 500–519 of the human σ1-receptor open reading frame (PubMed Nucleotide ID: NM005866) was inserted into the GenScript plasmid for transfection into mammalian cells. HEK293 cells stably expressing Nav1.5 were transiently transfected with this construct by electroporation (ECM 830, BTX Harvard Apparatus, Holliston, MA).
Electrophysiology.
Na+ current was recorded using whole cell patch-clamp techniques, as described previously (25, 52). Cultured cells were superfused with external recording solutions (compositions stated below) by gravity feed at ∼1–2 ml/min. All experiments were conducted at room temperature (22–24°C). Individual cells were located with an upright microscope equipped with a Zeiss ×40 water immersion objective (Carl Zeiss MicroImaging, Thornwood, NY). Patch pipettes were fabricated from borosilicate or aluminosilicate glass (Garner Glass, Claremont, CA), and pipette shanks were coated with Sylgard to reduce electrode capacitance (16). Before contact with the cell membrane, resistances ranged from 1 to 3 MΩ. Immediately after breaking in, cell capacitance and series resistance were determined by transient cancellation. Series resistance was compensated 85–95% to reduce the effective series resistance to ∼1 MΩ. When the product of peak Na+ current times the series resistance indicated a series resistance voltage error of >5 mV, the data from that recording were discarded. Recordings were made using an Axopatch-200B patch-clamp amplifier (Axon Instruments/Molecular Devices, Foster City, CA) interfaced to a PC. Data acquisition, voltage control, and analysis were carried out with pCLAMP7 software (Axon Instruments/Molecular Devices).
The external solution for recordings consisted of (in mM) 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 glucose, and 10 HEPES (pH 7.4 adjusted with NaOH). The pipette solution consisted of (in mM) 139 KCl, 1 NaCl, 2 MgCl2, 1 CaCl2, 5 EGTA, 10 glucose, and 10 HEPES (pH 7.2 KOH) (52). Note that ATP and GTP were not included. Na+ current was typically elicited with pulses from −80 mV to −10 mV for 25 ms, but for current-voltage plots the test pulses ranged from −70 to +70 mV in 10-mV increments.
Drug application.
Progesterone, (+)SKF10047, 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetra-hydronaphthalen-1-yl)propyl]piperazine (PB28), haloperidol, and ditolylguanidine (DTG) were obtained from Sigma-Aldrich (St. Louis, MO). DMT was synthesized as previously reported (14). DTG was first dissolved in DMSO and then diluted in external solution to obtain the desired drug concentration. Final DMSO never exceeded 0.1% (by volume), and control experiments verified that this level of DMSO had no effect on Na+ currents. Progesterone was first dissolved in ethanol and diluted in external solution to the desired concentrations. At higher concentrations, ethanol weakly inhibited Na+ current. Drugs were applied by changing the perfusing bathing solution. In general, currents were recorded at 15-s intervals for ∼5 min to obtain a stable baseline, after which the drug was applied. Drug effects typically appeared within 1–2 min of solution change and were recorded until a stable inhibition level was achieved. Reversal of response following drug removal was checked routinely.
σ-Receptor binding assays.
Competitive binding assays were performed in rat liver membranes using (+)[3H]pentazocine and [3H]ditolylguanidine (New England Nuclear/Perkin-Elmer, Waltham, MA) as previously described (39).
Membrane preparation and photoaffinity labeling.
Photoaffinity labeling was performed in HEK293 cells using the σ1/σ2-receptor photolabel iodoazidofenpropimorph [1-N-(2′,6′-dimethyl-morpholino)-3-(4-azido-3-[125I]iodo-phenyl)propane; [125I]IAF], as previously described (14, 39).
Data analysis.
Current recordings were analyzed with pCLAMP7. Simple statistical analyses were performed on exported data using Microsoft Excel or Origin (Microcal Software, Northampton, MA). Concentration dependence of Na+ current inhibition was analyzed using Origin, by fitting to a single-site saturation equation of the form I = Icontrol/(IC50 + [X]), where I is peak current for a voltage step to −10 mV, [X] is drug concentration, and IC50 is the concentration producing 50% block. Arithmetic means were computed and are presented with the standard error of the mean. Statistical significance was calculated using one-way analysis of variance (ANOVA) followed by the post hoc Tukey test.
RESULTS
Binding of progesterone to σ-receptors.
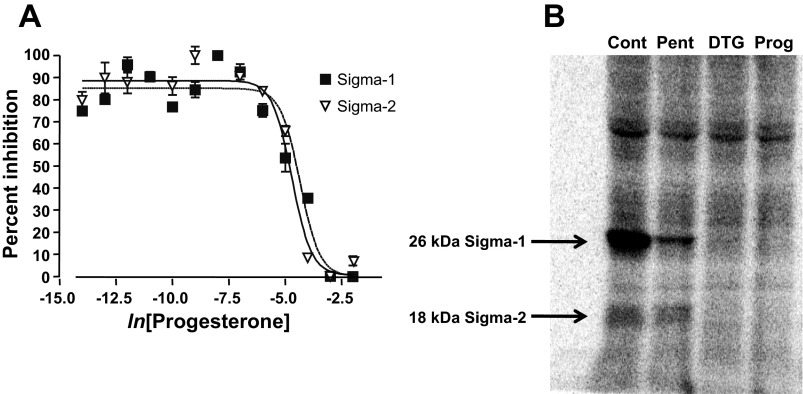
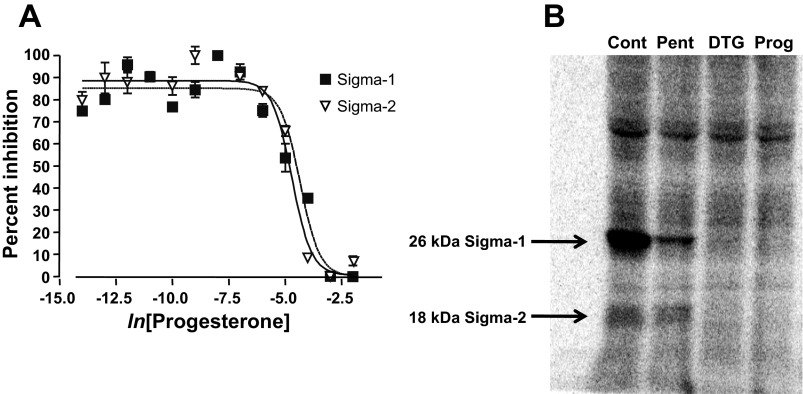
Progesterone binds to σ-receptors (51), but the subtype specificity of this interaction has not been determined. To address this question, we performed a ligand-binding study using radiolabeled ligands that are either σ1-receptor specific [(+)[3H]pentazocine] or that bind to both receptor subtypes ([3H]ditolylguanidine) (39). Competitive binding assays in rat liver membranes showed that progesterone displaces both of these ligands. [3H]pentazocine (10 nM) binding resolved σ1-receptors, and [3H]ditolylguanidine (30 nM) in the presence of 100 nM pentazocine resolved σ2-receptors (Fig. 1A). Binding in the presence of haloperidol (5 μM) was used to determine nonspecific binding. These experiments showed that progesterone binds to both σ1- and σ2-receptors, and yielded similar dissociation constants for progesterone of 239 nM and 441 nM, respectively. These values are similar to those reported previously in guinea pig brain and spleen (51).
Fig. 1.
Progesterone binding to σ-receptors. A: radioligand binding curves demonstrating the competitive displacement by progesterone of ligand binding to σ1- and σ2-receptors. (+)[3H]-pentazocine was used as a ligand for σ1-receptors. [3H]ditolylguanidine ([3H]DTG) with cold (+)pentazocine (μM) was used to resolve σ2-receptors. The binding affinity of progesterone was determined to be 239 nM for σ1-receptors and 441 nM for σ2-receptors. B: σ-Receptor photolabeling with the σ1/σ2-receptor photolabel 1-N-(2′,6′-dimethyl-morpholino)-3-(4-azido-3-[125I]iodo-phenyl)propane ([125I]IAF) indicated that σ1- and σ2-receptors are expressed in human embryonic kidney (HEK)293 cells. The first lane represents HEK293 cell homogenates alone [control (Cont)], the second in 10 μM (+)pentazocine (Pent), the third in 20 μM DTG, and the fourth in 50 μM progesterone (Prog).
Our electrophysiology experiments were performed in HEK293 cells, in which both σ1- and σ2-receptor ligands have proven efficacious in ion channel modulation (25). These results indicate that HEK293 cells express σ-receptors. Here we used photolabeling as a biochemical method to determine that both σ-receptor subtypes are present in this cell line. A photoprobe based on the fungicide fenpropimorph, [125I]IAF, labeled both σ1- and σ2-receptors, revealing the σ1-receptor as a 26-kDa protein and the σ2-receptor as an 18-kDa protein (39) (Fig. 1B). The photolabeling of both σ1- and σ2-receptors was blocked by DTG (20 μM) and progesterone (50 μM), while only σ1-receptor photolabeling was blocked by pentazocine (10 μM). These results confirm previous physiological experiments (25) that HEK293 cells express both σ-receptor subtypes, and are consistent with the results in liver membranes (Fig. 1A) showing progesterone binding to both molecules.
Na+ channel modulation by synthetic ligands.
In HEK293 cells expressing the human cardiac voltage-gated Na+ channel Nav1.5, voltage steps from −80 mV to −10 mV evoked large Na+ currents. Addition of progesterone failed to alter this current, leaving it within 3 ± 2% of controls (data not shown). Thus, progesterone is not a σ-receptor agonist in the modulation of cardiac Na+ channels. This lack of action mirrors results obtained with progesterone on voltage-activated K+ channels in rodent neurohypophysis (57).
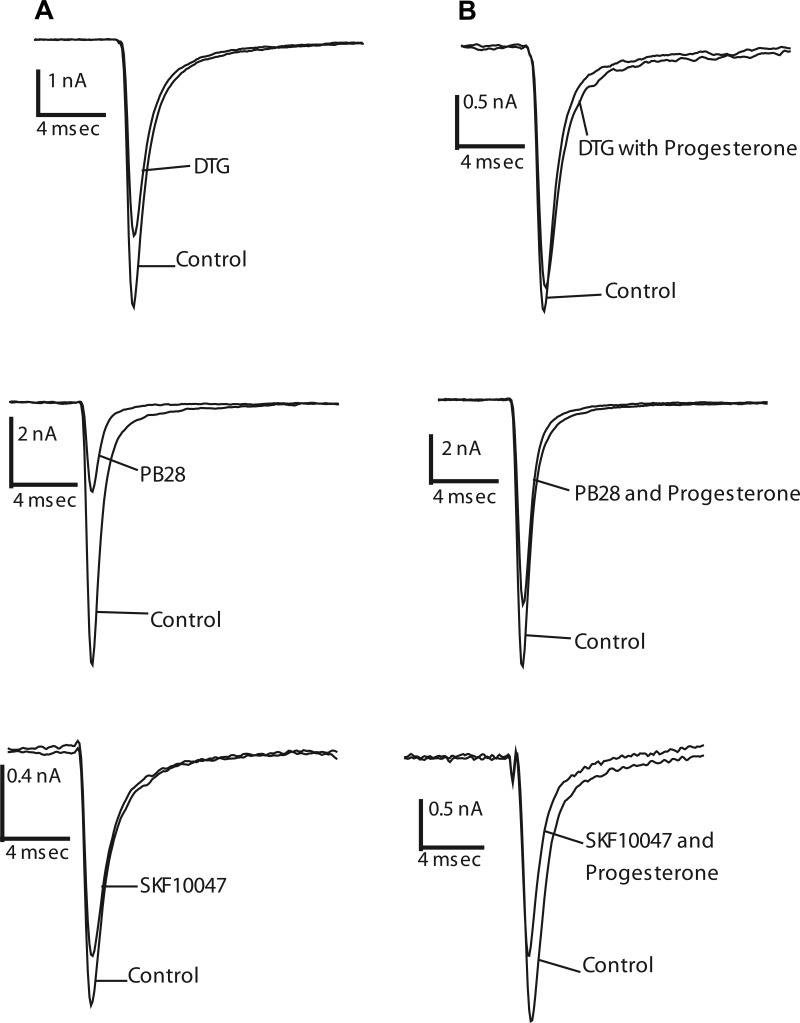
As reported previously (25), the σ-receptor ligands DTG and (+)SKF10047 activate σ-receptors to inhibit voltage-activated Na+ channels in HEK293 cells. The present study confirmed these results, showing similar Na+ current inhibition of 42 ± 3% and 51 ± 9%, respectively (Fig. 2A; n = 4–7). Furthermore, the σ2-receptor selective ligand PB28 (3, 24) inhibited Na+ current by 61 ± 4%, (n = 5) (Fig. 2A). Inhibition of Na+ current by all ligands tested was reversible (25); currents recovered to >75% of control levels within 10–15 min of a return to drug-free solution (data not shown).
Fig. 2.
Progesterone actions on Na+ channel inhibition by σ-receptor ligands in HEK293 cells. Na+ currents evoked by voltage steps from −80 mV to −10 mV before and after addition of 10 μM DTG, 10 μM PB28, and 100 μM (+)SKF10047. A and B: current inhibition by σ-receptor ligands in the absence of progesterone (A) and in the presence of 10 μM progesterone (B).
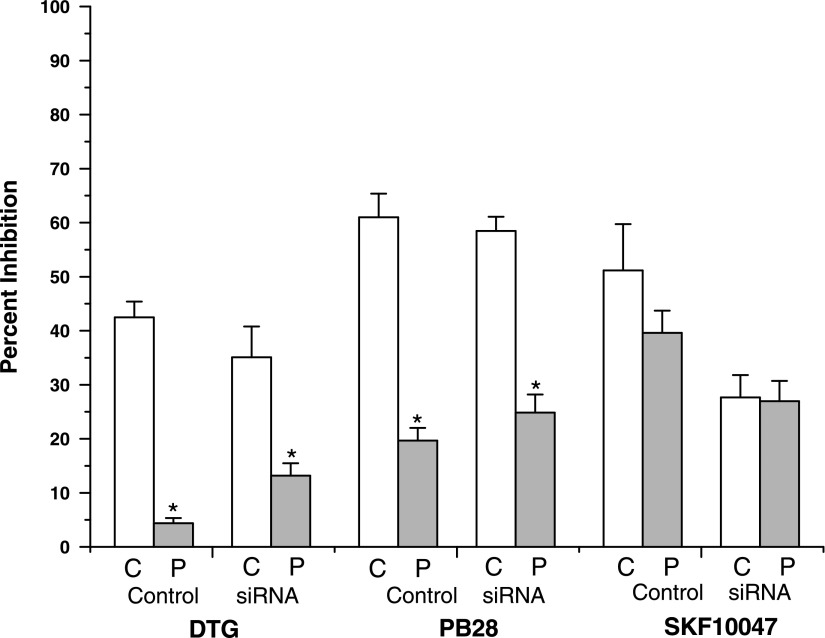
Progesterone blocked σ-receptor activation by these ligands; 10 μM progesterone reduced the inhibition of Na+ current to 4 ± 1, 20 ± 2, and 39 ± 4%, for DTG, PB28, and (+)SKF10049, respectively (Fig. 2B; n = 3–5). Thus, progesterone blocked the activation of σ-receptors by different agonists to varying degrees. The antagonism by progesterone was statistically significant for the σ2-receptor selective ligand PB28, and the σ1/σ2-receptor ligand DTG (Fig. 4, P < 0.005), but not for the σ1-selective ligand (+)SKF10047. These results, therefore, provide a preliminary indication that progesterone acts as an antagonist at σ2-receptors but not at σ1-receptors. The finding that progesterone antagonized a σ-receptor-mediated response contrasts with findings in rat neurohypophysis, where progesterone failed to antagonize the inhibition of voltage-activated K+ current (57).
Fig. 4.
Comparison of Na+ current inhibition with and without progesterone in control HEK293 cells and HEK293 cells with σ1-receptor siRNA knockdown. Average inhibition of each ligand was determined from current traces such as shown in Figs. 2 and 3. C, control; P, 10 μM progesterone. Bars represent means ± SE for n = 4–7. The values for DTG and PB28 differ significantly in both control HEK293 cells and with the addition of sig1RsiRNA, between recordings with and without 10 μM progesterone (*P < 0.005).
To explore the receptor specificity further, HEK293 cells were transfected with a siRNA construct based on the σ1-receptor gene sequence (sig1RsiRNA, see methods). This construct reduces the expression of σ1-receptors to 33 ± 9% of control levels (25). In transfected HEK293 cells, DTG, PB28, and (+)SKF10047 still inhibited Na+ current by 35 ± 6, 58 ± 3, and 28 ± 4%, respectively (Fig. 3A). In the presence of 10 μM progesterone, Na+ current inhibition by these ligands was reduced to 13 ± 2, 25 ± 3, and 27 ± 4%, respectively (n = 4–7) (Fig. 3B). Antagonism of Na+ current inhibition by progesterone was again significant for DTG and PB28 (P < 0.005), but not for (+)SKF10047. These results are summarized in Fig. 4.
Fig. 3.
Progesterone actions on Na+ channel inhibition by σ-receptor ligands in HEK293 cells with σ1-receptor small interfering RNA (siRNA) knockdown. A and B: Na+ currents evoked by voltage steps from −80 mV to −10 mV recorded from HEK293 cells transfected with sig1RsiRNA in the absence and presence of 10 μM DTG, 10 μM PB28, and 100 μM (+)SKF10047 before (A) and after (B) the addition of 10 μM progesterone.
The reduction of σ1-receptor protein expression by sig1RsiRNA produced a significant reduction of Na+ current inhibition by the σ1-receptor ligand (+)SKF10047, but the residual response showed no significant progesterone sensitivity. The residual responses in sig1RsiRNA-transfected cells to ligands that bind to σ2-receptors were still inhibited by progesterone. Thus, these results support the hypothesis that progesterone antagonizes responses to σ2-receptors but not σ1-receptors. However, a more detailed examination of the σ1-receptor ligand (+)SKF10047 presented below revealed a small but significant antagonism by progesterone.
Na+ channel modulation by DMT.
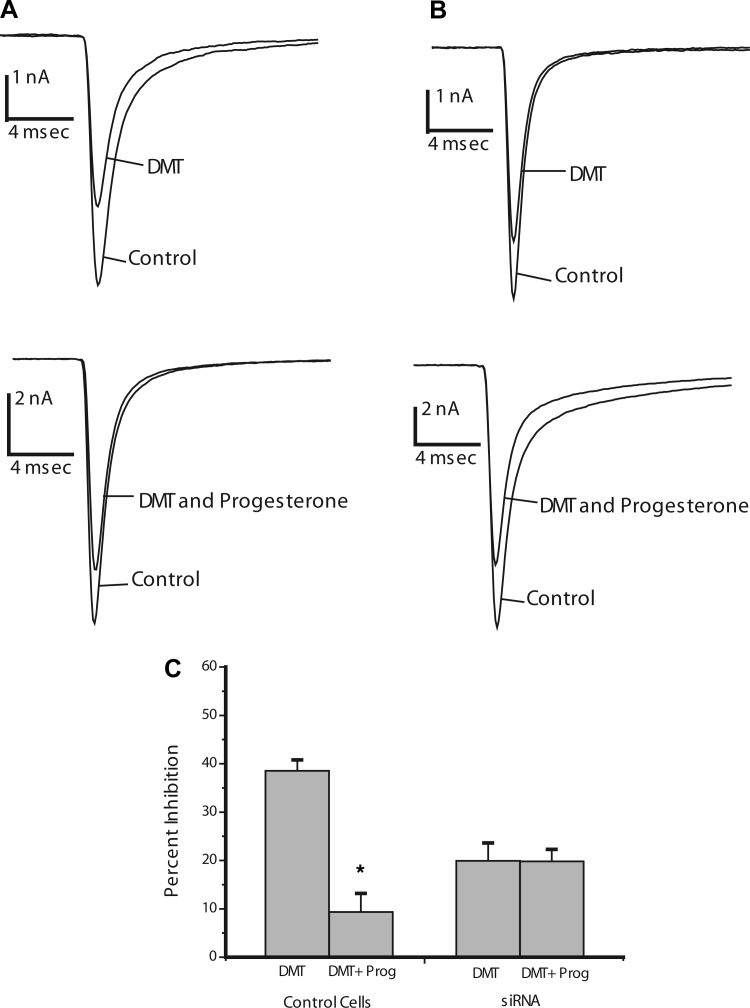
DMT has been recently identified as a candidate endogenous ligand for σ-receptors (14). DMT binds with similar affinities to σ1- and σ2-receptors and modulates Nav1.5 (14), so we tested progesterone as an antagonist of Na+ current inhibition by DMT. In HEK293 cells, DMT inhibited Na+ current by 38 ± 2% (n = 6), and 10 μM progesterone reduced this inhibition to 9 ± 4% (n = 5) (Fig. 5, A and C). This reduction was statistically significant (P < 0.005) and indicates that progesterone can antagonize the action of DMT.
Fig. 5.
Progesterone actions on Na+ current inhibition by N,N-dimethyltryptamine (DMT) in HEK293 cells. A and B: Na+ currents evoked by voltage steps from −80 mV to −10 mV recorded from control HEK293 cells (A) and cells with reduced σ1-receptor expression (B) by transfection with sig1RsiRNA in the absence and presence of 100 μM DMT before and after the addition of 10 μM progesterone. C: average Na+ current inhibition by DMT in each condition. Bars represent means ± SE for n = 5–7. The percent inhibition differs significantly in control HEK293 cells between recordings with and without 10 μM progesterone (*P < 0.005).
In HEK293 cells transfected with sig1RsiRNA, DMT inhibited Na+ current by 20 ± 4% (Fig. 5, B and C, n = 5) without progesterone. In contrast to control (untransfected) HEK293 cells, 10 μM progesterone failed to block the Na+ current inhibition by DMT in sig1RsiRNA-transfected HEK293 cells; DMT still inhibited Na+ current by 20 ± 3% (Fig. 5, B and C, n = 5). The loss of the progesterone-sensitive component of Na+ current inhibition following σ1-receptor knockdown stands in contrast to our results with synthetic ligands described above. Progesterone antagonism of DMT responses appears to depend on σ1-receptors rather than σ2-receptors.
Concentration dependence of progesterone action.
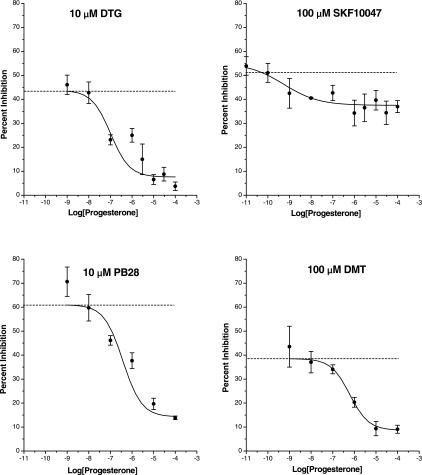
We tested a range of progesterone concentrations in the block of channel modulation by DTG, DMT, PB28, and (+)SKF10047 (Fig. 6). For all four of these σ-receptor agonists, the plots of peak Na+ current versus progesterone concentration were well fitted by a single-site saturation equation (see methods), yielding IC50 values of 105, 380, 620, and 0.3 nM, respectively. However, in the DTG plot the point at 1 μM may indicate the presence of a second binding site. Although the tests described above of progesterone against (+)SKF10047 appeared to show no effect (Figs. 2 and 3), these experiments were all done with one concentration of progesterone. The plot of Na+ current inhibition by (+)SKF10047 versus progesterone concentration in Fig. 6 appears to show a weak inhibition. With this larger data set, ANOVA indicated that this action is statistically significant (P < 0.05). The fit to the (+)SKF10047 plot yielded an IC50 of only 0.3 nM, but the small effect made the determination of this value inaccurate. Saturating concentrations of progesterone reduced the maximal inhibition of Na+ current by DTG, DMT, and PB28 by >75%. By contrast, a saturating concentration of progesterone reduced the maximal inhibition of Na+ current by (+)SKF10047 by only ∼30%. This analysis showed that progesterone weakly inhibited the action of (+)SKF10047. This could indicate either a weak action of progesterone at σ1-receptors or a weak action of (+)SKF10047 at σ2-receptors that is blocked by progesterone.
Fig. 6.
Concentration dependence of σ-receptor antagonism by progesterone in HEK293 cells. Na+ current evoked by voltage steps from −80 mV to −10 mV, before and after addition of the indicated concentration of agonist, in the presence of various concentrations of progesterone. Peak current was normalized to control and plotted versus drug concentration. The horizontal lines indicate Na+ current inhibition by agonist in the absence of progesterone. Data points represent means ± SE for n = 5–7 cells. Curves represent best fits to the data using the single-site saturation equation given in methods. Half-maximal inhibition (IC50) values were 105, 380, 620, and 0.3 nM for DTG, PB28, DMT, and (+)SKF10047, respectively.
DISCUSSION
Previous studies have shown that progesterone and other steroids bind to σ-receptors (9, 15, 21, 41, 51, 59). An earlier effort failed to demonstrate a physiological action of steroids in the σ-receptor-mediated modulation of K+ channels (57), but the present study found that progesterone can antagonize the inhibition of Na+ current in HEK293 cells by DTG, PB28, DMT, and (+)SKF10047. The discrepancy may reflect differences in σ-receptor pharmacology between the rodent neurohypophysis and human HEK293 cells. In comparing results on the modulation of different channels, it is important to bear in mind that σ-receptors are likely to be part of a complex with the ion channels they modulate (1, 7). Association with a channel could alter the binding properties of the receptor and lead to different pharmacological properties in different preparations.
This work showed that HEK293 cells express σ1- and σ2-receptors, and that progesterone binds with similar affinity to both (Fig. 1). We tested four σ-receptor agonists with a wide range of preferences between the two σ-receptor subtypes and found that the actions of all were sensitive to progesterone. Results with the synthetic ligands suggested that σ2-receptor-mediated responses can be blocked by progesterone much more readily than σ1-receptor-mediated responses. However, the action of the σ1-receptor-specific ligand (+)SKF10047 was weakly blocked by progesterone, and the progesterone sensitivity to DTG underwent a modest reduction after σ1-receptor knockdown (Fig. 4). The results with the endogenous σ-receptor ligand DMT indicated that σ1-receptors can account for all of the inhibition induced by this compound. Thus, with DMT we have the reverse situation that progesterone can block responses mediated by σ1-receptors more readily than responses mediated by σ2-receptors.
The pharmacological data in control cells and cells treated with sig1RsiRNA indicate that progesterone can antagonize both σ1- and σ2-receptors. The apparent dependence of antagonist action on the choice of agonist is difficult to explain. Evidence suggests that structurally distinct σ-receptor ligands bind to different domains and that the binding sites may be allosterically coupled (56). σ1-Receptor ligands bind to residues in two steroid binding-like domains (38, 39, 47), as well as a third region (13). These three parts of the protein are thought to come together to form a single ligand binding site which includes parts of the protein implicated in steroid binding along with other parts not implicated in steroid binding. Steroids and other ligands compete in their binding to σ-receptors (15). Differences in the regions of overlap of the agonist binding sites with progesterone's binding site may explain why progesterone varies in its effectiveness as an antagonist of various agonists. The interpretation in terms of overlapping binding sites was developed using structural insights into the σ1-receptor, but comparable knowledge of the molecular structure of the σ2-receptor is lacking. This gap in our knowledge also prevented us from using siRNA to knock down σ2-receptors. Indeed, one potential complication in the interpretation of our results is that sig1RsiRNA could also alter σ2-receptor levels. However, this is unlikely because although the σ2-receptor gene sequence is unknown, it is so different from the σ1-receptor sequence that searches of gene databases have failed to identify σ1-receptor homologues. Thus, cloning the σ2-receptor is an important task, which, once achieved, will enable investigators to explore these and other important questions about σ-receptor pharmacology and physiology.
DMT is an endogenous ligand that activates σ-receptors (14). It is significant that progesterone blocked Na+ channel modulation by DMT. Thus, one endogenous compound activates σ-receptors and another blocks them. The action of progesterone suggests a bidirectional control over σ-receptors in their modulation of membrane excitability. The activation of σ-receptors by DMT can thus be countered by progesterone to make σ-receptor modulation varied and conditional.
Plasma progesterone concentrations in women range from 30 to 450 nM depending on reproductive state (26), and the actions reported here were observed with progesterone in this range. σ-Receptor ligands have profound effects on function in the brain as well as the heart (19, 21, 35). Progesterone block of σ-receptors in the brain could play a role in the differences in mood and behavior associated with reproductive state (31, 42, 46). The modulation of the human cardiac Na+ channel Nav1.5 has implications for cardiovascular function. Sex hormone regulation of cardiac ion channels plays an important role in sex-related differences in susceptibility to arrhythmias (29). Sex-related differences have been noted in the incidence of drug-induced QT interval prolongation (8, 45). Although most drug-induced dysrhythmias have been linked to blockade of cardiac delayed rectifier K+ channels, many of these drugs are also σ-receptor ligands. This raises the possibility that progesterone blockade of σ-receptor-mediated ion channel modulation in the heart plays a role in female cardiovascular function, and testing this hypothesis may lead to a better understanding of how reproductive transitions influence heart rhythmicity. Given the wide range of ion channels modulated by σ-receptors, it will be important to test other channels for σ-receptor ligand/progesterone interactions. σ-Receptors bind other steroids such as testosterone, DHEA, and cholesterol (15, 21, 41, 51, 59), and the evaluation of additional steroids in σ-receptor-mediated ion channel modulation represents another important area for future research to identify other endocrine systems that can target σ-receptors.
GRANTS
This work was supported by National Institutes of Health Grants NS30016 (to M. B. Jackson) and MH065503 and DA027181 (to A. E. Ruoho). M. Johannessen was supported by a predoctoral fellowship from the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. J. Michael Edwardson for comments on this manuscript and Dr. Nicholas V. Cozzi for providing DMT.
Present address of M. Johannessen: Ross University Medical School, Dominica.
REFERENCES
- 1. Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron 34: 399–410, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav 64: 53–57, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Berardi F, Ferorelli S, Abate C, Colabufo NA, Contino M, Perrone R, Tortorella V. 4-(Tetralin-1-yl)- and 4-(naphthalen-1-yl)alkyl derivatives of 1-cyclohexylpiperazine as sigma receptor ligands with agonist sigma2 activity. J Med Chem 47: 2308–2317, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. J Neurosci 16: 1193–1202, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowen WD. Sigma receptors: recent advances and new clinical potentials. Pharm Acta Helv 74: 211–218, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Bowen WD, Walker JM, de Costa BR, Wu R, Tolentino PJ, Finn D, Rothman RB, Rice KC. Characterization of the enantiomers of cis-N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1- pyrrolidinyl)cyclohexylamine (BD737 and BD738): novel compounds with high affinity, selectivity and biological efficacy at sigma receptors. J Pharmacol Exp Ther 262: 32–40, 1992 [PubMed] [Google Scholar]
- 7. Carnally SM, Johannessen M, Henderson RM, Jackson MB, Edwardson JM. Demonstration of a direct interaction between sigma-1 receptors and acid-sensing ion channels. Biophys J 98: 1182–1191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cavero I, Mestre M, Guillon JM, Crumb W. Drugs that prolong QT interval as an unwanted effect: assessing their likelihood of inducing hazardous cardiac dysrhythmias. Expert Opin Pharmacother 1: 947–973, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Chen Y. Characterization of the Sigma receptor - a cocaine binding protein (PhD thesis). Madison, WI: University of Wisconsin, 2001 [Google Scholar]
- 10. Dluzen DE, Ramirez VD. Progesterone effects upon dopamine release from the corpus striatum of female rats. I. Evidence for interneuronal control. Brain Res 476: 332–337, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Farmer CJ, Isakson TR, Coy DJ, Renner KJ. In vivo evidence for progesterone dependent decreases in serotonin release in the hypothalamus and midbrain central grey: relation to the induction of lordosis. Brain Res 711: 84–92, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Feng XQ, Dong Y, Fu YM, Zhu YH, Sun JL, Wang Z, Sun FY, Zheng P. Progesterone inhibition of dopamine-induced increase in frequency of spontaneous excitatory postsynaptic currents in rat prelimbic cortical neurons. Neuropharmacology 46: 211–222, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Fontanilla D, Hajipour AR, Pal A, Chu UB, Arbabian M, Ruoho AE. Probing the steroid binding domain-like I (SBDLI) of the sigma-1 receptor binding site using N-substituted photoaffinity labels. Biochemistry 47: 7205–7217, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 323: 934–937, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ganapathy ME, Prasad PD, Huang W, Seth P, Leibach FH, Ganapathy V. Molecular and ligand-binding characterization of the sigma-receptor in the Jurkat human T lymphocyte cell line. J Pharmacol Exp Ther 289: 251–260, 1999 [PubMed] [Google Scholar]
- 16. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 17. Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci USA 93: 8072–8077, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayashi T, Kagaya A, Takebayashi M, Shimizu M, Uchitomi Y, Motohashi N, Yamawaki S. Modulation by sigma ligands of intracellular free Ca++ mobilization by N-methyl-d-aspartate in primary culture of rat frontal cortical neurons. J Pharmacol Exp Ther 275: 207–214, 1995 [PubMed] [Google Scholar]
- 19. Hayashi T, Su T. The sigma receptor: evolution of the concept in neuropsychopharmacology. Curr Neuropharmacol 3: 267–280, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131: 596–610, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Hayashi T, Su TP. Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs 18: 269–284, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Hellewell SB, Bowen WD. A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res 527: 244–253, 1990 [DOI] [PubMed] [Google Scholar]
- 23. Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol 268: 9–18, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Herrera Y, Katnik C, Rodriguez JD, Hall AA, Willing A, Pennypacker KR, Cuevas J. sigma-1 receptor modulation of acid-sensing ion channel a (ASIC1a) and ASIC1a-induced Ca2+ influx in rat cortical neurons. J Pharmacol Exp Ther 327: 491–502, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Johannessen M, Ramachandran S, Riemer L, Ramos-Serrano A, Ruoho AE, Jackson MB. Voltage-gated sodium channel modulation by σ-receptors in cardiac myocytes and heterologous systems. Am J Physiol Cell Physiol 296: C1049–C1057, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johansson ED. Plasma levels of progesterone in pregnancy measured by a rapid competitive protein binding technique. Acta Endocrinol (Copenh) 61: 607–617, 1969 [DOI] [PubMed] [Google Scholar]
- 27. Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1). Biochem Biophys Res Commun 229: 553–558, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Kennedy C, Henderson G. Inhibition of potassium currents by the sigma receptor ligand (+)-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine in sympathetic neurons of the mouse isolated hypogastric ganglion. Neuroscience 35: 725–733, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Kurokawa J, Suzuki T, Furukawa T. New aspects for the treatment of cardiac diseases based on the diversity of functional controls on cardiac muscles: acute effects of female hormones on cardiac ion channels and cardiac repolarization. J Pharm Sci 109: 334–340, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Lupardus PJ, Wilke RA, Aydar E, Palmer CP, Chen Y, Ruoho AE, Jackson MB. Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J Physiol 526: 527–539, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol 38: 379–395, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther 197: 517–532, 1976 [PubMed] [Google Scholar]
- 33. Matuszewich L, Lorrain DS, Hull EM. Dopamine release in the medial preoptic area of female rats in response to hormonal manipulation and sexual activity. Behav Neurosci 114: 772–782, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Maurice T, Gregoire C, Espallergues J. Neuro(active)steroids actions at the neuromodulatory sigma1 (sigma1) receptor: biochemical and physiological evidences, consequences in neuroprotection. Pharmacol Biochem Behav 84: 581–597, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Monassier L, Bousquet P. Sigma receptors: from discovery to highlights of their implications in the cardiovascular system. Fundam Clin Pharmacol 16: 1–8, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Monassier L, Manoury B, Bellocq C, Weissenburger J, Greney H, Zimmermann D, Ehrhardt JD, Jaillon P, Baro I, Bousquet P. sigma(2)-receptor ligand-mediated inhibition of inwardly rectifying K(+) channels in the heart. J Pharmacol Exp Ther 322: 341–350, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Monnet FP, Mahe V, Robel P, Baulieu EE. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-d-aspartate in the rat hippocampus. Proc Natl Acad Sci USA 92: 3774–3778, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pal A, Chu UB, Ramachandran S, Grawoig D, Guo LW, Hajipour AR, Ruoho AE. Juxtaposition of the steroid binding domain-like I and II regions constitutes a ligand binding site in the sigma-1 receptor. J Biol Chem 283: 19646–19656, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pal A, Hajipour AR, Fontanilla D, Ramachandran S, Chu UB, Mavlyutov T, Ruoho AE. Identification of regions of the sigma-1 receptor ligand binding site using a novel photoprobe. Mol Pharmacol 72: 921–933, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Palmer CP, Aydar E, Jackson MB. σ receptor modulation of ion channels. In: Sigma Receptors: Chemistry, Cell Biology, and Clinical Implications. New York: Springer Science, 2007, p. 127–149 [Google Scholar]
- 41. Palmer CP, Mahen R, Schnell E, Djamgoz MB, Aydar E. Sigma-1 receptors bind cholesterol and remodel lipid rafts in breast cancer cell lines. Cancer Res 67: 11166–11175, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Paul SM, Purdy RH. Neuroactive steroids. FASEB J 6: 2311–2322, 1992 [PubMed] [Google Scholar]
- 43. Petitclerc M, Bedard PJ, Di Paolo T. Progesterone releases dopamine in male and female rat striatum: a behavioral and microdialysis study. Prog Neuropsychopharmacol Biol Psychiatry 19: 491–497, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci 13: 85–86, 1992 [DOI] [PubMed] [Google Scholar]
- 45. Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PK, Strang I, Sullivan AT, Wallis R, Camm AJ, Hammond TG. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res 58: 32–45, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci 22: 410–416, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Seth P, Ganapathy ME, Conway SJ, Bridges CD, Smith SB, Casellas P, Ganapathy V. Expression pattern of the type 1 sigma receptor in the brain and identity of critical anionic amino acid residues in the ligand-binding domain of the receptor. Biochim Biophys Acta 1540: 59–67, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Seth P, Leibach FH, Ganapathy V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem Biophys Res Commun 241: 535–540, 1997 [DOI] [PubMed] [Google Scholar]
- 49. Su TP. Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J Pharmacol Exp Ther 223: 284–290, 1982 [PubMed] [Google Scholar]
- 50. Su TP, Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem 10: 2073–2080, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science 240: 219–221, 1988 [DOI] [PubMed] [Google Scholar]
- 52. Valdivia CR, Nagatomo T, Makielski JC. Late Na currents affected by alpha subunit isoform and beta1 subunit co-expression in HEK293 cells. J Mol Cell Cardiol 34: 1029–1039, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Vilner BJ, Bowen WD. Modulation of cellular calcium by sigma-2 receptors: release from intracellular stores in human SK-N-SH neuroblastoma cells. J Pharmacol Exp Ther 292: 900–911, 2000 [PubMed] [Google Scholar]
- 54. Vilner BJ, Bowen WD. Sigma receptor-active neuroleptics are cytotoxic to C6 glioma cells in culture. Eur J Pharmacol 244: 199–201, 1993 [DOI] [PubMed] [Google Scholar]
- 55. Vilner BJ, de Costa BR, Bowen WD. Cytotoxic effects of sigma ligands: sigma receptor-mediated alterations in cellular morphology and viability. J Neurosci 15: 117–134, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa B, Rice KC. Sigma receptors: biology and function. Pharmacol Rev 42: 355–402, 1990 [PubMed] [Google Scholar]
- 57. Wilke RA, Lupardus PJ, Grandy DK, Rubinstein M, Low MJ, Jackson MB. K+ channel modulation in rodent neurohypophysial nerve terminals by sigma receptors and not by dopamine receptors. J Physiol 517: 391–406, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wilke RA, Mehta RP, Lupardus PJ, Chen Y, Ruoho AE, Jackson MB. Sigma receptor photolabeling and sigma receptor-mediated modulation of potassium channels in tumor cells. J Biol Chem 274: 18387–18392, 1999 [DOI] [PubMed] [Google Scholar]
- 59. Yamada M, Nishigami T, Nakasho K, Nishimoto Y, Miyaji H. Relationship between sigma-like site and progesterone-binding site of adult male rat liver microsomes. Hepatology 20: 1271–1280, 1994 [PubMed] [Google Scholar]
- 60. Zhang H, Cuevas J. Sigma receptor activation blocks potassium channels and depresses neuroexcitability in rat intracardiac neurons. J Pharmacol Exp Ther 313: 1387–1396, 2005 [DOI] [PubMed] [Google Scholar]
- 61. Zhang H, Cuevas J. Sigma receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons. J Neurophysiol 87: 2867–2879, 2002 [DOI] [PubMed] [Google Scholar]
- 62. Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol 89: 134–152, 2009 [DOI] [PubMed] [Google Scholar]