Abstract
Obstructive sleep apnea (OSA) increases cardiovascular morbidity and mortality, which have been attributed to intermittent hypoxia (IH). The effects of IH on lung structure and function are unknown. We used a mouse model of chronic IH, which mimics the O2 profile in patients with OSA. We exposed adult C57BL/6J mice to 3 mo of IH with a fraction of inspired oxygen (FiO2) nadir of 5% 60 times/h during the 12-h light phase. Control mice were exposed to room air. Lung volumes were measured by quasistatic pressure-volume (PV) curves under anesthesia and by water displacement postmortem. Lungs were processed for morphometry, and the mean airspace chord length (Lm) and alveolar surface area were determined. Lung tissue was stained for markers of proliferation (proliferating cell nuclear antigen), apoptosis (terminal deoxynucleotidyl transferase dUTP nick-end labeling), and type II alveolar epithelial cells (surfactant protein C). Gene microarrays were performed, and results were validated by real-time PCR. IH increased lung volumes by both PV curves (air vs. IH, 1.16 vs. 1.44 ml, P < 0.0001) and water displacement (P < 0.01) without changes in Lm, suggesting that IH increased the alveolar surface area. IH induced a 60% increase in cellular proliferation, but the number of proliferating type II alveolocytes tripled. There was no increase in apoptosis. IH upregulated pathways of cellular movement and cellular growth and development, including key developmental genes vascular endothelial growth factor A and platelet-derived growth factor B. We conclude that IH increases alveolar surface area by stimulating lung growth in adult mice.
Keywords: murine model, lung function, lung morphometry, microarray
obstructive sleep apnea (OSA) affects ∼4% of men and 2% of women in the US, but prevalence may exceed 50% in the obese population (4, 38, 61). OSA is independently associated with increased cardiovascular morbidity and mortality (37, 60). OSA causes chronic intermittent hypoxia (IH) during sleep. IH has been implicated in the pathogenesis of complications of OSA by inducing dysfunction of central nervous, cardiovascular, endocrine, gastrointestinal, immune, and reproductive systems (7, 15, 17, 23, 24, 29, 33, 35, 38, 41, 42, 47, 54). Compared with other organs, lungs are exposed to extreme fluctuations in oxygen concentrations during IH. Nevertheless, although effects of OSA and IH on pulmonary vasculature have been studied (11, 40), the effects of IH on lung structure and function are unknown.
There is a wide body of literature on sustained hypoxia, particularly in the context of high-altitude research, but only a few studies have analyzed the direct effect of sustained hypoxia on lung tissues (1, 55). Alveolar epithelial cells and microvascular endothelial cells are highly sensitive to hypoxia (45). They exhibit a rapid and sustained adaptive response, including activation of key transcription factors and signaling molecules. However, IH results in different physiological and molecular changes compared with sustained hypoxia (36, 39, 59).
In this study, we assessed the effects of IH on lung structure and function. We used adult mice to better model conditions resembling OSA, which is more prevalent in older adults (61). We exposed animals to IH for 3 mo, quantified changes in lung structure, and analyzed the gene expression profile in the lung parenchyma.
MATERIALS AND METHODS
Animals.
In total, sixty-five 5- to 6-mo-old male lean C57BL/6J mice purchased from The Jackson Laboratory (Bar Harbor, ME) were used in the study. Five animals died during IH because of a technical malfunction of the IH system and were excluded from the analysis. Thirty-eight animals were used for physiological, biochemical, and morphometric measurements. Ten animals were used solely for obtaining microarray data. Twelve mice were used for assessing the effects of weight loss through IH, which were observed in the initial experiment. The study was approved by the Johns Hopkins University Animal Care and Use Committee and complied with National Institutes of Health guidelines (Guide for the Care and Use of Laboratory Animals).
Experimental design.
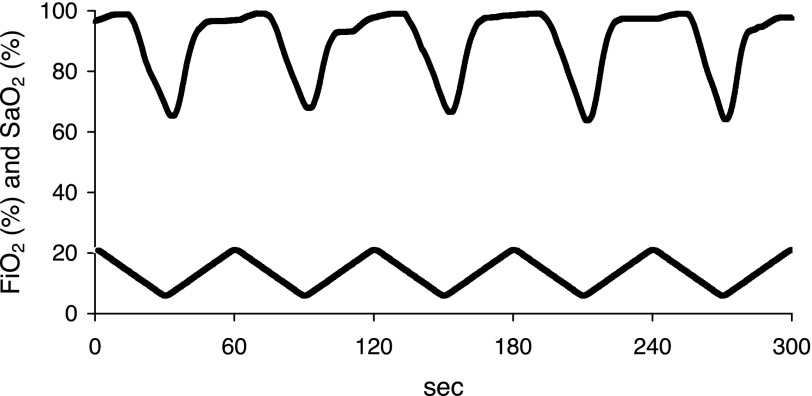
An automatic gas control delivery system (HyCon 0520) was designed to regulate the flow of room air, nitrogen, and oxygen into customized cages housing the mice, similar to our previously described system (34). A maximum of five mice were housed continuously in a single customized cage (dimensions 27 × 17 × 17 cm) with constant access to food and water. During each period of IH, the fractional inhaled O2 was reduced from 20.9 to 5% over a 30-s period and then reoxygenated to room air levels in the subsequent 30-s period, resulting in 60 hypoxic events per hour (Fig. 1). We have determined previously that an average arterial partial pressure of O2 over a hypoxic cycle was 51.7 ± 4.2 mmHg (41). Thus, our murine model mimicked IH of severe OSA. During the 3-mo experiment, the IH mice were exposed only during the 12-h light phase (lights on at 0900), alternating with 12 h of constant room air during the dark phase. Control mice had continuous exposure to room air.
Fig. 1.
The real-time intermittent hypoxia (IH) profile. During each period of IH, the fraction of inspired oxygen (FiO2) was reduced from room air levels to 5% within 30 s, followed by a reoxygenation to room air levels within the subsequent 30 s. This protocol was used for 3 mo during the 12-h light phase. A corresponding representative SaO2 tracing from 1 mouse is shown at the top.
Pulmonary function testing.
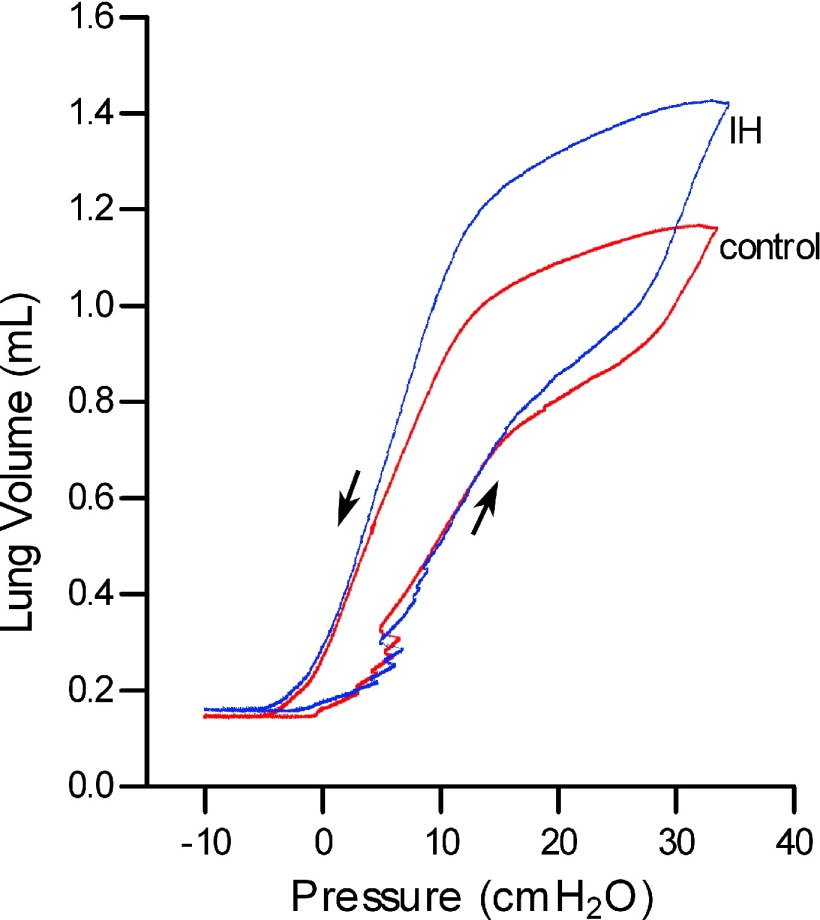
Pulmonary function testing was performed in 20 mice (10 mice/group), as described previously (3, 48). Briefly, each mouse was anesthetized with ketamine-xylazine intraperitoneally at a dose of 75/15 mg/kg body wt. After anesthesia, a tracheostomy was performed, and the lungs were connected to a ventilator. To measure dynamic resistance and elastance, animals were ventilated in the supine position with a tidal volume of 0.2 ml of 100% oxygen at a rate of 150 breaths/min, with a positive end-expiratory pressure of 3 cmH2O. After 5–10 min, a sinusoidal oscillation at 2.5 Hz was applied to determine dynamic resistance and elastance (3). Following this procedure the lungs were sealed off with a stopcock for 4 min. This led to complete degassing of the lungs by absorption atelectasis and cessation of cardiac activity. Quasistatic pressure-volume curves were performed as reported previously (Fig. 2) (48), and lung volume at full inflation (defined as the volume at 35 cmH2O) was recorded. Residual volume was the volume at −10 cmH2O. Functional residual capacity (FRC) was the volume at 0 cmH2O. The specific compliance (Csp) was calculated from the static compliance (Cst; slope of deflation limb from 3–8 cmH2O) and FRC (Csp = Cst/FRC). In one mouse from the control group, there was a leak in the ventilator circuit, resulting in erroneous values. This mouse was excluded from the experiment.
Fig. 2.
Representative pressure-volume curves from control mice (red) and IH mice (blue). Quasistatic pressure-volume curves were performed by inflating the lungs to a maximum pressure of 35 cmH2O, which was defined as the lung volume at full inflation (total lung capacity). Residual volume was the volume at −10 cmH2O. Functional residual capacity was the volume at 0 cmH2O.
Sample collection.
After pulmonary function testing, retroorbital blood was drawn with a capillary tube to assess the hematocrit. Animals were exsanguinated. The epididymal fat was surgically removed and weighed. The right bronchus was clamped and the left lung inflated with warmed (50°C) 1% low-melt agarose at a pressure of 25 cmH2O. Pressure was maintained until the agarose began to gel substantially (12). The lungs were then sealed with a stopcock. The agarose in the excised left lung was allowed to harden on ice for 15 min. After the inflated total left lung volume (V; airspace plus tissue) was measured by water displacement, the lung was fixed for histological examination by immersion in buffered 10% formalin for ≥24 h. Right lung tissue was frozen in liquid nitrogen and kept at −80°C for further analysis. The heart was surgically removed. The right ventricle was separated from the left ventricle, and both were weighed.
Histology.
The left lung was dehydrated in ethanol and embedded in paraffin. For morphometry, 5-μm-thick sections were cut from transverse blocks and stained with hematoxylin and eosin. Images were acquired using a Nikon Eclipse 80i microscope at ×40 magnification. The entire sections were photographed, and a systematic random sampling was done to yield ∼25 fields/section. Mean airspace chord length (Lm) was measured with sampling grid lines using Nikon NIS-Elements AR 3.00 software. Overall, this technique resulted in the measurement of ∼14,000 chords/mouse, which were averaged to obtain the Lm of each animal. Alveolar surface area (SA) was estimated as described previously (56) from the Lm and lung volume (SA = 4 V/Lm). The alveolar surface area calculations are based on robust stereological measurements (6, 57). The volumes used for this calculation were those of the agarose-filled left lung.
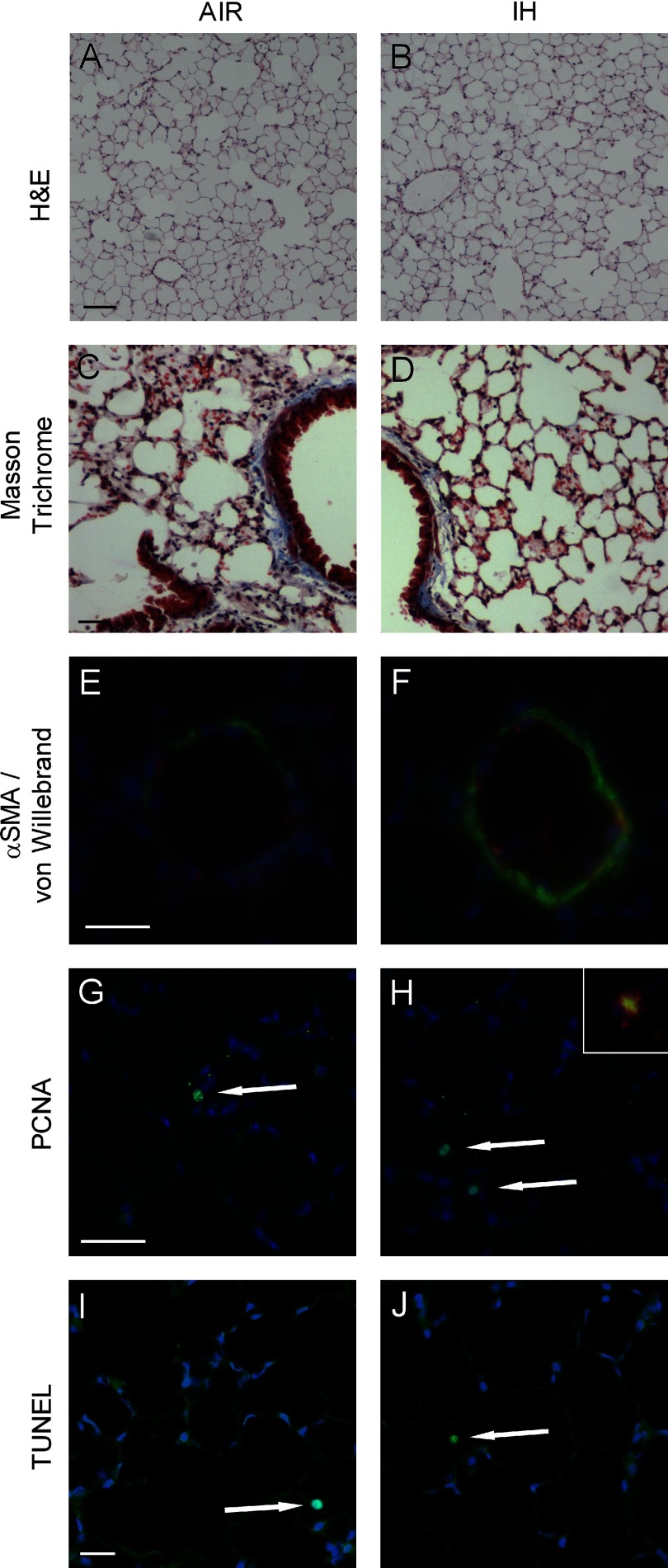
Proliferating cell nuclear antigen (PCNA) staining was performed in lung sections to detect proliferation using PCNA (PC10) Mouse mAb (catalog no. 2586) from Cell Signaling Technology (Danvers, MA). 4,6′-Diamidino-2-phenylindole (DAPI) staining was used for labeling cell nuclei. To determine the percentage of proliferating cells, all cells on one section (excluding cells from arteries, veins, and bronchioles) were counted under fluorescence microscopy at an excitation wavelength of 330–380 nm. Results are expressed as percentage of PCNA-positive cells/total cells. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining was performed in lung sections using FragEL DNA Fragmentation Kit (product no. QIA39) from EMD Chemicals (Gibbstown, NJ). Positive and negative controls were generated according to the manufacturer's protocol. For identification of alveolar epithelial type II cells (AEC), surfactant protein C (SPC) staining was performed using Anti-Prosurfactant Protein C (catalog no. AB3786) from Millipore (Billerica, MA). The percentage of type II AEC among all cells was determined in a randomly chosen view field (200×). Proliferating type II AEC were identified by PCNA(+)SPC(+) staining in the whole lung section. Masson trichrome staining was performed to assess collagen.
The degree of muscularization of small pulmonary arteries was assessed by double staining of lung sections for α-smooth muscle actin (F3777; Sigma, St. Louis, MO) and von Willebrand factor (A0082; Dako). Sections were counterstained with DAPI for labeling cell nuclei. At ×400 magnification, a total of 30–40 pulmonary arteries/mouse (external diameter 20–90 μm) were analyzed in a blinded fashion. Percent media wall thickness was determined by the formula (2 × media thickness)/external diameter × 100, and the shortest external diameter was measured to allow for vessels not being perfectly symmetrical circles (32).
Gene microarrays.
Total RNA was isolated from the lungs and analyzed by gene microarrays using Affymetrix 430A_2.0 gene chips (Santa Clara, CA) in each animal individually. Microarray studies have been performed using standard protocols. Total RNA was isolated from lung tissue using the TRIzol Reagent method (cat. no. 15596-026; Invitrogen, Carlsbad, CA) with subsequent RNEasy clean up (cat. no. 74104; Qiagen, Valencia, CA) (19). Purified RNA from each sample (5 μg) was converted into double-stranded cDNA using SuperScript Choice system (Invitrogen). Each double-stranded cDNA was subsequently used as a template to make biotin-labeled cRNA using the BioArray HighYield RNA Transcript Kit (Enzo Life Science, Farmingdale, NY), and 15 μg of fragmented, biotin-labeled murine lung cRNA from each sample was hybridized to the Affymetrix GeneChip Mouse Genome 430A 2.0 Array (MG_430A_2.0; Affymetrix, Santa Clara, CA) at 45°C for 16 h. The arrays were washed and stained in the Affymetrix GeneChip Fluidics Station 450 using the supplier's reagents. Produced fluorescent images were read using the Affymetrix GeneChip Scanner 3000 and converted into GeneChip Cell files (CEL) using the Affymetrix GeneChip Operating Software (version 1.0; Affymetrix), with global normalization of target intensity set to 150. Quality control for generated expression profiles was conducted according to Affymetrix Guideline for Assessing Sample and Array Quality specifications. Gene expression values for 22,626 transcripts on the MG 430A 2.0 Array were calculated using Robust Microarray Analysis (RMA; Bioconductor affy package) (19). Briefly, the RMA module of this package (18) was used for background correction across array normalization and identification of the probe set fluorescence intensity values. Then gene expression profiles from normoxic and hypoxic lungs were grouped (5 samples in each group) for direct comparison. The normoxic/hypoxic fold change ratio and false discovery rate (q value) were computed with Significance Analysis of Microarrays (SAM 2.0) software (53), using the RMA generated expression matrix as an input.
The microarray sample size determination for class comparisons was calculated as described previously (16). Identified standard deviations for control lung (σ = 0.274) tissues were submitted to the microarray sample size identifying formula with power (1 − β) = 90% and fold change log2 (Δ = 0.585, numerical 1.5) for n = 5. The power.t.test function of the R2.3.1 program (www.r-project.org) identified false discovery rate for this setting; q = 3.8% (significance level α = 0.038).
The functional analysis that identifies the biological functions that were significantly associated with identified candidate genes was conducted using the Ingenuity Pathways Knowledge Base tool (http://www.ingenuity.com), as described previously (16). Fischer's exact test was used to calculate a P value determining the probability that each biological function assigned to our candidate genes was due to chance alone. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (10) and are accessible through GEO series accession no. GSE21409 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE21409).
Real-time PCR.
Total RNA was extracted from lung using TRIzol (Invitrogen), and complementary DNA was synthesized using Advantage RT for PCR kit from Clontech (Palo Alto, CA). Real-time reverse transcriptase PCR was performed with primers and probe specific for platelet-derived growth factor B (PDGF-B; forward 5′-TCCCGTCCCCGTTCCT-3′, reverse 5′-GAAAGCCCCCAAAAAATATAATCA-3′, probe 5′-CTGCTTCCTTCAGTTTGT-3′), transforming growth factor-β2 (TGF-β2; forward 5′-CACGGTCAAAAGTCTGACAATACAT-3′, reverse 5′-ATGGTTCTTTGCCAGTTTAAGCA-3′, probe 5′-TCAAAACAGGTCAACATTT-3′), vascular endothelial growth factor A (VEGF-A; Mm00437304_m1), and 18S (forward 5′-CTCTTTCGAGGCCCTGTAATTG-3′, reverse 5′-AACTGCAGCAACTTTAATATACGCTATT-3′, probe 5′-AGTCCACTTTAAATCCTT-3′) from Applied Biosystems (Foster City, CA) and Invitrogen. The mRNA expression levels were normalized to 18S ribosomal RNA concentrations using the standard ΔΔCT approach (http://wwww.ambion.com/techlib/basics/rtpcr/index.html), as we have done previously (26, 27), and then expressed as a ratio of IH to control.
Collagen assay.
Collagen levels in lung tissues were determined by SIRCOL collagen assay (Biocolor) according to the manufacturer's instructions. Briefly, right lung lobes were homogenized, and collagen was solubilized in 0.5 M acetic acid. Extracts were incubated with Sirius red dye, and absorbance was determined at 550 nm with a spectrophotometer (Model 680; Bio-Rad, Hercules, CA). The amount of collagen was expressed in micrograms per milligram protein in lung tissue.
Separate series of experiments.
In the separate series of experiments, 12 mice were exposed to control conditions only. Half of the mice were fed ad libitum, whereas the other six were food restricted to match the weight profile of the IH group from the initial experiment. Measurement of lung volumes by water displacement and lung morphometry were performed as described above.
Statistical analyses.
All values are reported as means ± SE. Comparisons of the results between the IH and control groups of mice were performed using unpaired t-test (between groups). A P value of <0.05 was considered significant. See Gene microarrays above for the statistical analyses of microarrays.
RESULTS
Table 1 shows the basic characteristics of mice from the control and IH groups. Mice exposed to IH weighed significantly less than control animals despite being the same age. There was no corresponding decrease in the epididymal fat. There was evidence of mild left and right ventricular hypertrophy when normalized to the body weight. IH also resulted in a moderate but significant increase in the hematocrit.
Table 1.
Basic characteristics of C57BL/6J mice
| Control (n = 22) | IH (n =16) | |
|---|---|---|
| Age, wk | 36 | 36 |
| Body weight, g | 35.3 ± 1.2 | 27.6 ± 1.2*** |
| Epididymal fat, g | 0.536 ± 0.052 | 0.449 ± 0.023 |
| Epididymal fat/body weight, % | 1.73 ± 0.16 | 1.70 ± 0.08 |
| Heart weight, g | 0.134 ± 0.003 | 0.122 ± 0.003** |
| Heart weight/body weight, % | 0.390 ± 0.014 | 0.440 ± 0.006** |
| LV + septum weight, g | 0.105 ± 0.002 | 0.094 ± 0.003** |
| LV + septum/heart weight, % | 78.3 ± 0.7 | 77.0 ± 1.0 |
| LV + septum/body weight, % | 0.30 ± 0.01 | 0.34 ± 0.01* |
| RV weight, g | 0.029 ± 0.001 | 0.028 ± 0.001 |
| RV/heart weight, % | 21.7 ± 0.7 | 23.0 ± 1.0 |
| RV/body weight, % | 0.09 ± 0.01 | 0.10 ± 0.005* |
| Hematocrit, % | 44.4 ± 1.2 | 50.8 ± 0.5*** |
Values are means ± SE.
IH, intermittent hypoxia; LV, left ventricle; RV, right ventricle.
P < 0.05
P < 0.01, and
P < 0.0001.
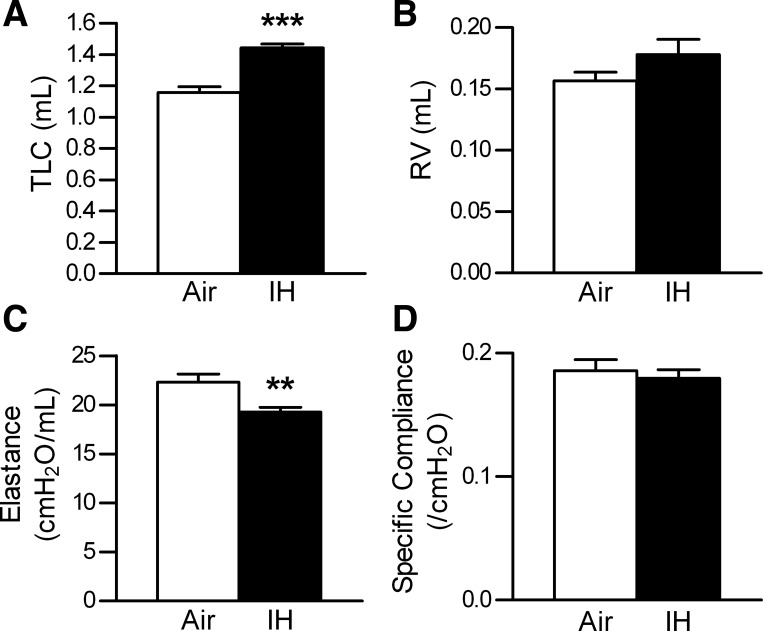
Pulmonary function testing revealed a significant increase in the total lung capacity (TLC; Figs. 2 and 3A). The residual volume remained unchanged (Figs. 2 and 3B). Lung elastance was significantly decreased, but because the lung volumes were increased with IH, the specific compliance was unchanged (Fig. 3, C and D).
Fig. 3.
Results of the pulmonary function testing. Values are means ± SE. A: total lung capacity (TLC). B: residual volume (RV). C: dynamic elastance. D: specific compliance; n = 9 for air, n = 10 for IH. **P < 0.01 and ***P < 0.0001.
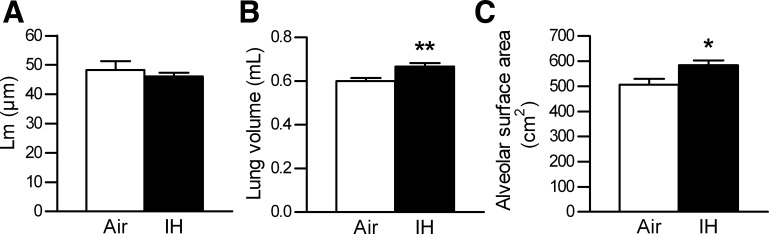
Morphological examination of the lungs showed no difference in Lm between the IH and control groups (Figs. 4A and 5, A and B). IH increased the left lung volume (Fig. 4B), consistent with the measured increase in the TLC. Given that Lm was unchanged, the increase in the lung volume resulted in a significant increase in the calculated alveolar surface area (Fig. 4C). Total collagen assay (μg/mg lung protein) showed no difference between the IH and control groups (control, 299 ± 13 μg/mg; IH, 330 ± 22 μg/mg; P = 0.23), which was confirmed by Masson Trichrome staining (Fig. 5, C and D). PCR for collagen 1α1 (P = 0.11) and collagen 3α1 (P = 0.73) further verified these results.
Fig. 4.
Morphometry results of the left lung. Values are means ± SE. A: mean airspace chord length (Lm). B: left lung volume, using water displacement. C: alveolar surface area, calculated as 4 V/Lm; n = 9 for air, n = 9 for IH. *P < 0.05 and **P < 0.01.
Fig. 5.
Representative bright-field (A–D) and immunofluorescence (E–J) images of the lungs. Original magnification: ×40 (A and B), ×100 (C and D), and ×400 (E–J). A and B: hematoxylin and eosin (H & E). C and D: Masson Trichrome. E and F: muscularization of small pulmonary arteries as assessed by double staining for α-smooth muscle actin (αSMA; green) and von Willebrand factor (red). Nuclei are counterstained with 4,6′-diamidino-2-phenylindole (DAPI) in blue. G and H: proliferating cell nuclear antigen (PCNA) stained in green; nuclei are counterstained with DAPI in blue. H, inset, shows a magnified PCNA positive cell, the cytoplasma of which is positive for SPC stained in red. I and J: terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining. White arrows point to PCNA- (G and H) and TUNEL-positive cells (I and J). Scale bars for A and B are 100 μm, for C and D 40 μm, and for E–J 20 μm each.
Quantitative analysis of small pulmonary artery remodeling showed a marked increase in percent media wall thickness in the IH group (control, 6.4 ± 0.2%; IH, 12.5 ± 0.9%; P < 0.01; Fig. 5, E and F), suggesting the development of pulmonary hypertension.
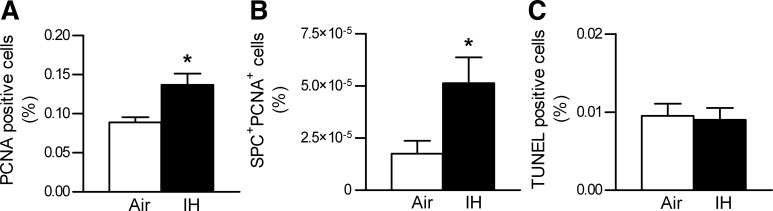
The increased lung volume and alveolar surface area with IH suggested the possibility of lung growth, prompting us to examine a cell proliferation marker, PCNA. PCNA staining revealed a significant increase in cellular proliferation during IH (Figs. 5, G and H, and 6A). Moreover, hypoxic mice showed a disproportionally higher percentage of proliferating type II AEC (Fig. 6B). Of note, type II AEC accounted for a higher percentage among all cells in the IH group (control, 16.3 ± 1.2%; IH, 20.9 ± 1.1%; P < 0.05). TUNEL staining showed no increase in apoptosis (Figs. 5, I and J, and 6C).
Fig. 6.
Indices of proliferation and apoptosis in lung tissue. A: results of the PCNA staining of lung tissue, shown as %PCNA positive cells/total cells. B: results of the PCNA + surfactant protein C (SPC) double staining, shown as %SPC+ PCNA+ cells/total cells. C: results of the TUNEL staining of lung tissue, shown as %TUNEL positive cells/total cells. Values are means ± SE; n = 10 for air, n = 10 for IH. *P < 0.05.
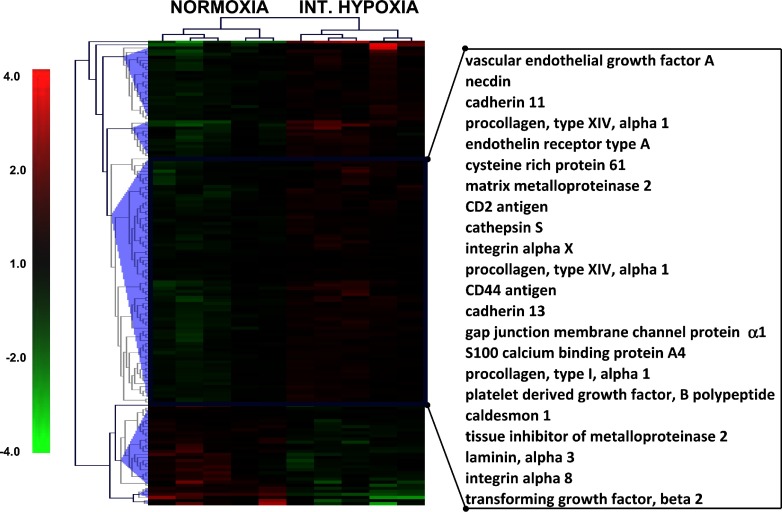
Gene microarrays of lung tissue were performed in a separate subset of mice, as described in materials and methods (IH, n = 5; control, n = 5). Microarrays showed a significant (1.5 < fold change < −1.5; q value <3.88) upregulation of 291 genes and downregulation of 87 genes in the IH mice (Supplemental Tables S1 and S2; Supplemental Material for this article can be found on the AJP-Lung Cellular and Molecular Physiology web site). Ingenuity analysis demonstrated a significant association of cellular movement and cellular growth and development pathways with IH (Fig. 7 and Supplemental Fig. S1). Among the upregulated genes there were multiple markers of lung development as well as fibrosis, including VEGF-A, TGF-β2, PDGF-B, and several isoforms of collagen (9, 31, 50).
Fig. 7.
Hierarchical clustering of genes representing cell movement pathway. The 151 genes that represent cell movement pathway as identified by Ingenuity analysis (Supplemental Fig. S1) were clustered using default settings of MeV software. Expression difference values (log2) for each gene were calculated by subtracting the average of expression values of a given gene in all tested conditions from individual gene expression value of each biological replicate. Each column represents an experimental condition of lung sample, and each row represents expression pattern of a gene throughout given experimental conditions. Hierarchical clustering conducted using Euclidian correlation (average linkage) identified 5 major clusters (blue triangles), of which 1 cluster demonstrated clear upregulation after IH. Genes from these clusters are highlighted with a blue rectangle, and the most representative are listed on the right. Red color indicates upregulation, and green color indicates downregulation of gene expression relative to combined average, with color intensity corresponding to the expression difference (scale is shown on the left).
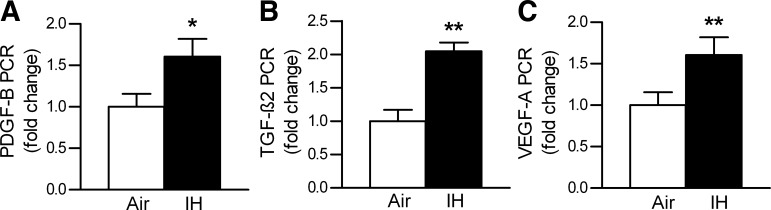
Gene microarray data were validated by real-time PCR in the main subset of mice, in which lung function and morphology were also examined. PCR confirmed upregulation of VEGF-A, TGF-β2, and PDGF-B by IH (Fig. 8).
Fig. 8.
Results of the real-time PCR in lung tissue. A: platelet-derived growth factor B (PDGF-B). B: transforming growth factor-β2 (TGF-β2). C: vascular endothelial growth factor A (VEGF-A), shown as mRNA expression levels normalized to 18s ribosomal RNA concentrations and then expressed as a ratio of IH to control. Values are means ± SE; n = 10 for air, n = 10 for IH. *P < 0.05 and **P < 0.01.
In the separate set of mice, as expected, liberally fed mice consumed more food and weighed more than food-restricted mice (average food intake 4.23 ± 0.04 vs. 3.10 ± 0.06 g/day, respectively, P < 0.0001; body weight 33.5 ± 0.9 vs. 28.0 ± 0.6 g, respectively, P < 0.001). Mice fed ad libitum had a similar body weight as the control mice in the initial experiment (P = 0.23; compare with Table 1), and food-restricted mice had a similar body weight as the IH mice (P = 0.12; compare with Table 1). Lung volumes by water displacement were not different between either group (fed ad libitum mice, 0.65 ± 0.02 ml vs. food-restricted mice, 0.68 ± 0.01 ml, P = 0.16). The Lm (48.7 ± 1.0 vs. 48.6 ± 1.3 μm, respectively, P = 0.94) and the calculated SA (562 ± 11 vs. 536 ± 15 cm2, respectively, P = 0.20) showed no differences between either group.
DISCUSSION
The purpose of this study was to examine the effects of IH on lung structure and function. The main novel finding of our work was that IH increased alveolar surface area and proliferation of lung parenchymal cells, especially type II AEC, in adult mice. Our genomic analysis confirmed that pathways for lung development were upregulated. In the following discussion we explore effects of IH on lung structural remodeling and the potential implications of our work.
We have shown that IH increases both the total lung air volume and lung volume fixed for histology. The increased lung volume was not associated with a change in Lm, which resulted in a significant increase in calculated alveolar surface area. There may have been stretching of the existing alveolar walls, leading to more surface at any given pressure. Simple stretching of alveolar walls does not account for all of our observations, since we also found a 60% increase in PCNA staining, a marker of cell proliferation. TUNEL staining showed no difference between both groups, indicating that there is increased proliferation rather than increased turnover of cells in lung parenchyma. Thus, we have shown that IH induces lung growth by activating proliferation of parenchymal cells, especially type II AEC. Type II AEC have many functions in addition to the production of surfactant phospholipids. After exposure to acute hyperoxia, adult rat type II AEC were significantly more migratory compared with those from control rats, suggesting that these cells have an important function for alveolar repair (5). Our data indicate that IH might activate AEC in a similar fashion.
Effects of sustained hypoxia on lung parenchyma were previously reported. However, the majority of animal studies looking at changes of lung structure due to hypoxia, either intermittent or sustained, have focused primarily on pulmonary arteries and pulmonary hypertension (62). Although we did not measure pulmonary artery pressure, we found evidence of mild right ventricular hypertrophy, which had previously been reported in the mouse model of IH by Fagan (11). We have also found that IH increased muscularization of small pulmonary arteries, which also suggests that IH caused pulmonary hypertension in mice.
To the best of our knowledge, our study is the first to report that prolonged exposure to IH induces lung growth in adult animals. Although the chronic hypoxic stimulus arrested lung development in neonatal rats (1, 55), adult rats were not affected (55). The impact of IH of OSA on lung volumes and function in humans is often difficult to determine because of the confounding effects of obesity.
The effects of sustained hypoxia on lung function were elucidated in denizens of high altitudes. Exposure to high altitude from birth leads to a significantly higher vital capacity compared with newcomers. The extent of the increase depends on the age when acclimatization took place. Lowland natives acclimatized as adults had lower vital capacity than highland natives (8, 14, 58). It is conceivable that compensatory lung growth in response to hypoxia observed in adult mice is turned off with age in humans. Nevertheless, gene expression analysis in our study suggests putative pathways activated by IH, which might be relevant in humans.
Our gene microarray data identified pathways of lung growth and development as a target of IH, which may account for lung growth during hypoxic exposure. Genes involved in lung development during maturation include VEGF, PDGF, and TGF-β, which were affected by IH in our study. VEGF is upregulated during the development of capillaries in fetal and newborn rat lungs (28). Neutralization of VEGF through fetal lung development disrupts normal lung septation and development, leading to bronchopulmonary dysplasia, whereas VEGF receptor blockade results in endothelial cell apoptosis and emphysema (52). VEGF gene therapy increased lung capillary growth and improved alveolization in experimental bronchopulmonary dysplasia (51). Both VEGF and VEGF receptor genes are positively regulated by hypoxia through hypoxia-inducible factor-1 (HIF-1) (13, 43). Thus, an increase in VEGF-A during IH may contribute to lung growth.
Lung growth in our model may have also been due to upregulation of PDGF-B. PDGF-B is vital for lung development. PDGF-B knockout is embryonically lethal with retarded lung growth (22, 25). PDGF-B antisense oligonucleotides inhibit cell proliferation during embryonic rat lung development (49). PDGF-B in lung parenchyma is also known to be upregulated by hypoxia (2) via HIF-1 (13). An increase in PDGF-B may work in concert with VEGF-A during IH to promote lung growth. In our study, both PDGF-B and VEGF-A were increased in the mature lung by IH.
Although both described targets are HIF-1 responsive, the increase in expression of HIF-1 itself was not vigorous enough to be called significant using our filtering criteria. The expression of HIF-1α subunit demonstrated an upregulating trend with a fold change of 1.3 and significant q value of 0.86. However, HIF-1α is regulated posttranscriptionally by hydroxylase-dependent degradation in the presence of O2 (44). Therefore, an increase in expression of HIF-1-dependent genes may indicate that the HIF-1 pathway is activated even in the absence of an increase in HIF-1α RNA. The observed increase in hematocrit during IH is also consistent with HIF-1α upregulation (44).
In contrast to VEGF and PDGF, TGF-β signaling can inhibit alveolar development, whereas upregulation of the TGF-β pathway causes interstitial fibrosis (30, 46, 63). In the present study, TGF-β2 mRNA was upregulated by IH, but there was no concomitant increase in collagen deposition or evidence of lung fibrosis. It is conceivable that IH does not augment TGF-β signaling despite an increase in gene expression. Thus, IH induces lung growth despite upregulation of TGF-β2 mRNA.
Our study had several limitations. First, IH induced weight loss. We have described previously that the weight loss occurs during the 1st wk of IH (20, 21, 26). Our previous publications and present data suggest that this weight loss is attributable to anorectic effects of IH. In the complementary experiment, we have shown that food restriction did not affect lung volume and Lm. In addition, a carefully controlled study by Bishai and Mitzner (3) showed that even severe calorie restriction does not lead to increased lung volume or surface area. Second, it is conceivable that IH attenuated the lung tissue processing shrinkage, resulting in elevated lung volumes, and since we did not quantify the tissue shrinkage with dehydration and paraffin embedding in each group, we cannot rule this out. However, differences in shrinkage could not explain accelerated cellular proliferation and upregulation of developmental pathways in hypoxic animals. Third, type II AEC proliferation was assessed with a single marker, PCNA. Fourth, our study analyzed a single time point. It is conceivable that IH may turn different genes on and off throughout the 3-mo time course. Future studies would be important to elucidate temporal relationships between IH-induced lung growth and the gene expression profile.
Conclusions.
In summary, we have shown that IH increases lung volume and alveolar surface area and upregulates genes of lung growth in adult mice. Our work provides a basis for future studies to discover mechanisms by which IH turns on pathways of lung development.
GRANTS
This work was supported by Research Fellowship Grant RE 2842/1-1 of the German Research Foundation to C. Reinke, Conselho Nacional de Desenvolvimento Científico e Tecnológico Grant 200032/2009-7 and Fundação Zerbini, Brazil, to L. F. Drager, National Institutes of Health (NIH) Specialized Center of Clinically Oriented Research Grant 5P50-HL-084945 to R. A. Wise, NIH Grant HL-80105 and American Heart Association Grant 10GRNT3360001 to V. Y. Polotsky, and the National Institute on Aging (through the Johns Hopkins Older Americans Independence Center; P30-AG-021334).
DISCLOSURES
No conflicts of interest, financial or otherwise, that are relevant to this article are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Stanislav Savransky for the design of the automatic gas control delivery system (HyCon 0520) and to Enid R. Neptune for helpful comments.
REFERENCES
- 1. Ambalavanan N, Nicola T, Hagood J, Bulger A, Serra R, Murphy-Ullrich J, Oparil S, Chen YF. Transforming growth factor-β signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 295: L86–L95, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berg JT, Breen EC, Fu Z, Mathieu-Costello O, West JB. Alveolar hypoxia increases gene expression of extracellular matrix proteins and platelet-derived growth factor-B in lung parenchyma. Am J Respir Crit Care Med 158: 1920–1928, 1998. [DOI] [PubMed] [Google Scholar]
- 3. Bishai JM, Mitzner W. Effect of severe calorie restriction on the lung in two strains of mice. Am J Physiol Lung Cell Mol Physiol 295: L356–L362, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bixler EO, Vgontzas AN, Lin HM, Ten HT, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 163: 608–613, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Buckley S, Driscoll B, Shi W, Anderson K, Warburton D. Migration and gelatinases in cultured fetal, adult, and hyperoxic alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 281: L427–L434, 2001. [DOI] [PubMed] [Google Scholar]
- 6. Campbell H, Tomkeieff SI. Calculation of the internal surface of a lung. Nature 170: 116–117, 1952. [PubMed] [Google Scholar]
- 7. Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med 172: 915–920, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Chen QH, Ge RL, Wang XZ, Chen HX, Wu TY, Kobayashi T, Yoshimura K. Exercise performance of Tibetan and Han adolescents at altitudes of 3,417 and 4,300 m. J Appl Physiol 83: 661–667, 1997. [DOI] [PubMed] [Google Scholar]
- 9. Chinoy MR. Lung growth and development. Front Biosci 8: d392–d415, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fagan KA. Selected Contribution: Pulmonary hypertension in mice following intermittent hypoxia. J Appl Physiol 90: 2502–2507, 2001. [DOI] [PubMed] [Google Scholar]
- 12. Fields MJ, Bishai JM, Mitzner W, Wagner EM. Effects of pulmonary ischemia on lung morphology. Am J Physiol Lung Cell Mol Physiol 293: L254–L258, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Fong GH. Regulation of angiogenesis by oxygen sensing mechanisms. J Mol Med 87: 549–560, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Frisancho AR, Martinez C, Velasquez T, Sanchez J, Montoye H. Influence of developmental adaptation on aerobic capacity at high altitude. J Appl Physiol 34: 176–180, 1973. [DOI] [PubMed] [Google Scholar]
- 15. Gozal D, Row BW, Kheirandish L, Liu R, Guo SZ, Qiang F, Brittian KR. Increased susceptibility to intermittent hypoxia in aging rats: changes in proteasomal activity, neuronal apoptosis and spatial function. J Neurochem 86: 1545–1552, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol 19: 547–558, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O'Doherty RM, Polotsky VY, O'Donnell CP. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med 175: 851–857, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Irizarry RA, Gautier L, Cope LM. An R package for analyses of affymetrix oligonucleotide arrays. In: The Analysis of Gene Expression Data: Methods and Software, edited by Parmigiani G, Garrett ES, Irizarry RA, Zeger SL. New York: Springer, 2003, p. 102–119. [Google Scholar]
- 20. Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 209: 381–386, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jun J, Savransky V, Nanayakkara A, Bevans S, Li J, Smith PL, Polotsky VY. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol 295: R1274–R1281, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar VH, Lakshminrusimha S, El Abiad MT, Chess PR, Ryan RM. Growth factors in lung development. Adv Clin Chem 40: 261–316, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Lavie L, Polotsky V. Cardiovascular Aspects in Obstructive Sleep Apnea Syndrome—Molecular Issues, Hypoxia and Cytokine Profiles. Respiration 78: 361–370, 2009. [DOI] [PubMed] [Google Scholar]
- 24. Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 27: 123–128, 2004. [PubMed] [Google Scholar]
- 25. Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 8: 1875–1887, 1994. [DOI] [PubMed] [Google Scholar]
- 26. Li J, Savransky V, Nanayakkara A, Smith PL, O'Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol 102: 557–563, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O'Donnell CP, Polotsky VY. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res 97: 698–706, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Marszalek A, Daa T, Kashima K, Nakayama I, Yokoyama S. Expression of vascular endothelial growth factor and its receptors in the developing rat lung. Jpn J Physiol 51: 313–318, 2001. [DOI] [PubMed] [Google Scholar]
- 29. McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched controlled study. Am J Respir Crit Care Med 175: 190–195, 2007. [DOI] [PubMed] [Google Scholar]
- 30. McDevitt TM, Gonzales LW, Savani RC, Ballard PL. Role of endogenous TGF-β in glucocorticoid-induced lung type II cell differentiation. Am J Physiol Lung Cell Mol Physiol 292: L249–L257, 2007. [DOI] [PubMed] [Google Scholar]
- 31. Merry CL, Wilson VA. Role of heparan sulfate-2-O-sulfotransferase in the mouse. Biochim Biophys Acta 1573: 319–327, 2002. [DOI] [PubMed] [Google Scholar]
- 32. Nakagawa Y, Kishida K, Kihara S, Funahashi T, Shimomura I. Adiponectin ameliorates hypoxia-induced pulmonary arterial remodeling. Biochem Biophys Res Commun 382: 183–188, 2009. [DOI] [PubMed] [Google Scholar]
- 33. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342: 1378–1384, 2000. [DOI] [PubMed] [Google Scholar]
- 34. Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 552: 253–264, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Polotsky VY, Patil SP, Savransky V, Laffan A, Fonti S, Frame LA, Steele KE, Schweizter MA, Clark JM, Torbenson MS, Schwartz AR. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med 179: 228–234, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prabhakar NR, Kline DD. Ventilatory changes during intermittent hypoxia: importance of pattern and duration. High Alt Med Biol 3: 195–204, 2002. [DOI] [PubMed] [Google Scholar]
- 37. Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 6: e1000132, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 165: 677–682, 2002. [DOI] [PubMed] [Google Scholar]
- 39. Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112: 2660–2667, 2005. [DOI] [PubMed] [Google Scholar]
- 40. Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 51: 363–370, 2009. [DOI] [PubMed] [Google Scholar]
- 41. Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol 293: G871–G877, 2007. [DOI] [PubMed] [Google Scholar]
- 42. Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med 175: 1290–1297, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Semenza GL. Regulation of hypoxia-induced angiogenesis: a chaperone escorts VEGF to the dance. J Clin Invest 108: 39–40, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 24: 97–106, 2009. [DOI] [PubMed] [Google Scholar]
- 45. Signorelli S, Jennings P, Leonard MO, Pfaller W. Differential effects of hypoxic stress in alveolar epithelial cells and microvascular endothelial cells. Cell Physiol Biochem 25: 135–144, 2010. [DOI] [PubMed] [Google Scholar]
- 46. Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100: 768–776, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Soukhova-O'Hare GK, Shah ZA, Lei Z, Nozdrachev AD, Rao CV, Gozal D. Erectile dysfunction in a murine model of sleep apnea. Am J Respir Crit Care Med 178: 644–650, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soutiere SE, Mitzner W. On defining total lung capacity in the mouse. J Appl Physiol 96: 1658–1664, 2004. [DOI] [PubMed] [Google Scholar]
- 49. Souza P, Sedlackova L, Kuliszewski M, Wang J, Liu J, Tseu I, Liu M, Tanswell AK, Post M. Antisense oligodeoxynucleotides targeting PDGF-B mRNA inhibit cell proliferation during embryonic rat lung development. Development 120: 2163–2173, 1994. [DOI] [PubMed] [Google Scholar]
- 50. Sozo F, Wallace MJ, Zahra VA, Filby CE, Hooper SB. Gene expression profiling during increased fetal lung expansion identifies genes likely to regulate development of the distal airways. Physiol Genomics 24: 105–113, 2006. [DOI] [PubMed] [Google Scholar]
- 51. Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 112: 2477–2486, 2005. [DOI] [PubMed] [Google Scholar]
- 52. Tuder RM, Yun JH. Vascular endothelial growth factor of the lung: friend or foe. Curr Opin Pharmacol 8: 255–260, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 27: 194–201, 2004. [DOI] [PubMed] [Google Scholar]
- 55. Vicencio AG, Eickelberg O, Stankewich MC, Kashgarian M, Haddad GG. Regulation of TGF-β ligand and receptor expression in neonatal rat lungs exposed to chronic hypoxia. J Appl Physiol 93: 1123–1130, 2002. [DOI] [PubMed] [Google Scholar]
- 56. Weibel ER. Morphometry of the Human Lung. New York: Academic, 1963. [Google Scholar]
- 57. Weibel ER. Principles and methods for the morphometric study of the lung and other organs. Lab Invest 12: 131–155, 1963. [PubMed] [Google Scholar]
- 58. Weitz CA, Garruto RM, Chin CT, Liu JC, Liu RL, He X. Lung function of Han Chinese born and raised near sea level and at high altitude in Western China. Am J Hum Biol 14: 494–510, 2002. [DOI] [PubMed] [Google Scholar]
- 59. Wu W, Dave NB, Yu G, Strollo PJ, Kovkarova-Naumovski E, Ryter SW, Reeves SR, Dayyat E, Wang Y, Choi AM, Gozal D, Kaminski N. Network analysis of temporal effects of intermittent and sustained hypoxia on rat lungs. Physiol Genomics 36: 24–34, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 31: 1071–1078, 2008. [PMC free article] [PubMed] [Google Scholar]
- 61. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993. [DOI] [PubMed] [Google Scholar]
- 62. Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 103: 691–696, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zeng X, Gray M, Stahlman MT, Whitsett JA. TGF-beta1 perturbs vascular development and inhibits epithelial differentiation in fetal lung in vivo. Dev Dyn 221: 289–301, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.