Abstract
Melatonin is synthesized and released into the circulation by the pineal gland in a circadian rhythm. Melatonin has been demonstrated to differentially alter blood flow to assorted vascular beds by the activation of different melatonin receptors in animal models. The purpose of the present study was to determine the effect of melatonin on blood flow to various vascular beds in humans. Renal (Doppler ultrasound), forearm (venous occlusion plethysmography), and cerebral blood flow (transcranial Doppler), arterial blood pressure, and heart rate were measured in 10 healthy subjects (29 ± 1 yr; 5 men and 5 women) in the supine position for 3 min. The protocol began 45 min after the ingestion of either melatonin (3 mg) or placebo (sucrose). Subjects returned at least 2 days later at the same time of day to repeat the trial after ingesting the other substance. Melatonin did not alter heart rate and mean arterial pressure. Renal blood flow velocity (RBFV) and renal vascular conductance (RVC) were lower during the melatonin trial compared with placebo (RBFV, 40.5 ± 2.9 vs. 45.4 ± 1.5 cm/s; and RVC, 0.47 ± 0.02 vs. 0.54 ± 0.01 cm·s−1·mmHg−1, respectively). In contrast, forearm blood flow (FBF) and forearm vascular conductance (FVC) were greater with melatonin compared with placebo (FBF, 2.4 ± 0.2 vs. 1.9 ± 0.1 ml·100 ml−1·min−1; and FVC, 0.029 ± 0.003 vs. 0.023 ± 0.002 arbitrary units, respectively). Melatonin did not alter cerebral blood flow measurements compared with placebo. Additionally, phentolamine (5-mg bolus) after melatonin reversed the decrease in RVC, suggesting that melatonin increases sympathetic outflow to the kidney to mediate renal vasoconstriction. In summary, exogenous melatonin differentially alters vascular blood flow in humans. These data suggest the complex nature of melatonin on the vasculature in humans.
Keywords: conductance, renal, limb, cerebral, supplement
melatonin is synthesized and released into the circulation by the pineal gland in a circadian rhythm. The rise in melatonin at night has been demonstrated to promote sleep and decrease body temperature (5). As an over-the-counter supplement, melatonin is commonly ingested as a sleep aid and to overcome jet lag in the general population (5). Melatonin predominantly acts through two membrane-bound receptors (MT1 and MT2). In the vasculature, opposite effects have been demonstrated for the two receptors; the activation of MT1 and MT2 receptors elicits vasoconstriction and vasodilation, respectively (8). In the rat, melatonin has been demonstrated to constrict the coronary artery (24) and decrease in vivo cerebral blood flow (7) while dilating the pulmonary artery (24). When ingested as a supplement in humans, melatonin enhances the cutaneous vasodilating response during heating (1) and blunts the cutaneous vasoconstrictor response during cooling (2). The different vascular effects observed with melatonin are attributed to the relative distribution of MT1 and MT2 receptors. The relative expression levels of MT1 and MT2 receptors in human tissues are unknown. To date, very little information exists regarding the impact that melatonin has on blood flow in different vascular beds in humans. This information is important because over 15.5 million Americans have been reported to use melatonin as a supplement (4). Therefore, the purpose of the present study was to determine the effect of melatonin on renal, forearm, and cerebral blood flow in humans. Because melatonin has been demonstrated to elicit differential vascular responses in animals, we hypothesized that increases in melatonin would elicit differential effects on the vasculature.
METHODS
Subjects
For the main study (study 1), 10 healthy subjects (5 men and 5 women; age, 29 ± 1 yr; height, 169 ± 3 cm; and weight, 62 ± 4 kg) were tested in the experimental group. An additional group of seven healthy subjects (2 men and 5 women; age, 29 ± 1 yr; height, 165 ± 4 cm; and weight, 59 ± 4 kg) were tested as time controls to determine the reproducibility of the measurements. Five healthy subjects (3 men and 2 women; age, 28 ± 2 yr; height, 177 ± 5 cm; and weight, 76 ± 8 kg) were tested separately in a follow-up study (study 2).
All subjects were normotensive, nonsmokers, nonobese, and not taking any medications that would interfere with the measurements. All subjects received a physical examination before participation. Written informed consent was obtained from all subjects after a verbal explanation of the experimental protocol. The Institutional Review Board of The Pennsylvania State University College of Medicine approved this study.
Experimental Protocol
Study 1.
Each subject randomly ingested a tablet in a double-blind manner containing either melatonin (3 mg) or sucrose (placebo). The subjects waited 45 min after ingestion before the experimental protocol began. The subjects returned no sooner than 2 days later at the same time of day to repeat the other trial. Renal, forearm, and cerebral blood flow; heart rate; and mean arterial blood pressure (MAP) were measured continuously while supine for 3 min. Time-control subjects performed the same protocol but ingested sucrose for both trials.
Study 2.
To elucidate the mechanism of altered renal blood flow observed in study 1, five subjects were administered an α-adrenergic receptor antagonist (phentolamine, 5 mg) 45 min after ingestion of melatonin. Measurements of renal blood flow velocity (RBFV), heart rate, and MAP were taken in the supine position before and after melatonin (3 mg) ingestion and then following an intravenous bolus of phentolamine. These measurements were repeated at 10, 15, and 20 min post-phentolamine.
Measurements
Heart rate and arterial blood pressure were continuously recorded during all trials using a Finometer (FMS, Amsterdam, The Netherlands). Before each trial, brachial artery blood pressure was measured by an automated sphygmomanometer (Dinamap, General Electric, Waukesha, WI).
Venous occlusion plethysmography was used to measure forearm blood flow. A Hokanson EC-4 system (Bellevue, WA) with mercury-in-silastic strain gauges was used. A strain gauge was positioned around the maximal circumference of the left forearm. A wrist cuff was inflated to 220 mmHg to arrest circulation to the hand. Forearm blood flow was recorded every 15 s during the trials. During blood flow measurements, a venous collecting cuff was inflated proximal to the elbow to a pressure of 50 mmHg. Forearm vascular conductance (FVC) was calculated by dividing forearm blood flow by MAP.
Doppler ultrasound (HDI 5000, ATL Ultrasound, Bothell, WA) was used to measure RBFV. The renal artery was scanned by the anterior abdominal approach with a curved-array transducer (2–5 MHz) with a 2.5-MHz pulsed Doppler frequency. The probe insonation angle to the artery was ≤60°. The focal zone was set at the depth of the artery. The transducer was held in the same place to record velocity tracings during each trial, and the data were obtained in the same phase of the respiratory cycle. Continuous measurements of RBFV were taken during each trial. Renal vascular conductance (RVC) was calculated by dividing RBFV by MAP.
Transcranial Doppler was used to measure cerebral blood flow. A 500 M Transcranial Doppler System (Multigon Industries, Yonkers, NY) with a 2-MHz pulsed-wave probe was used. The probe was centered over the middle cerebral artery using the transtemporal position. Cerebral blood flow was expressed as mean blood velocity (in cm/s). Cerebrovascular conductance was calculated by dividing mean blood velocity by MAP.
Data Analysis
Statistical analyses of the data for study 1 were performed by paired t-test. Data from study 2 were analyzed using a one-way ANOVA (baseline, melatonin, and phentolamine). Simple effects tests were performed to compare time points if the one-way ANOVA was significant. Significance was set at P < 0.05. All data are presented as means ± SE.
RESULTS
Study 1
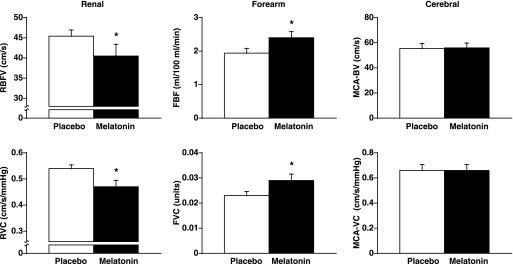
MAP and heart rate were not different between the two trials (Table 1). Vascular blood flow responses after placebo and melatonin ingestion are presented in Fig. 1. RBFV and RVC were significantly decreased by melatonin compared with placebo (Δ −4.9 ± 2.2 cm/s and Δ −0.06 ± 0.02 cm·s−1·mmHg−1, respectively). Forearm blood flow and FVC were significantly increased by melatonin compared with placebo (Δ 0.4 ± 0.2 ml·100 ml−1·min−1 and Δ 0.006 ± 0.002 arbitrary units, respectively). Cerebral blood flow and cerebrovascular conductance were not significantly different between placebo and melatonin trials (Δ 0 ± 4 cm/s and Δ 0 ± 0.04 cm·s−1·mmHg−1, respectively).
Table 1.
Hemodynamic values for study 1
| Placebo | Melatonin | |
|---|---|---|
| Mean arterial pressure, mmHg | 84 ± 1 | 85 ± 2 |
| Heart rate, beats/min | 63 ± 3 | 60 ± 3 |
Values are means ± SE. Hemodynamic values for placebo and melatonin trials are shown.
Fig. 1.
Vascular blood flow responses after melatonin and placebo ingestion. Melatonin (3 mg) significantly decreased renal blood flow velocity (RBFV) and renal vascular conductance (RVC), significantly increased forearm blood flow (FBF) and forearm vascular conductance (FVC), and did not alter cerebral blood flow velocity (MCA-BV) and cerebrovascular conductance (MCA-VC). Values are means ± SE. *P < 0.05, significantly different from placebo.
Time control.
Heart rate and MAP were not different between time control trials (heart rate, 70 ± 4 vs. 68 ± 3 beats/min; and MAP, 87 ± 2 vs. 86 ± 2 mmHg). Renal, forearm, and cerebral blood flows and vascular conductances were not different between time control trials. The differences between trials for renal, forearm, and cerebral conductance were as follows: RVC, Δ −0.03 ± 0.06 cm·s−1·mmHg−1; FVC, Δ −0.002 ± 0.005 arbitrary units; and cerebrovascular conductance, Δ0.04 ± 0.05 cm·s−1·mmHg−1.
Study 2
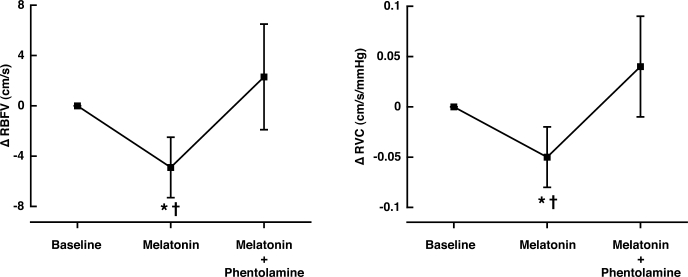
Heart rate and MAP were not different from baseline after melatonin ingestion (Table 2). Heart rate increased (Δ11 ± 7 beats/min) and MAP decreased (Δ −3 ± 1 mmHg) significantly from baseline during the first minute of phentolamine infusion (P < 0.001) but returned to baseline levels ∼3 min later. RBFV and RVC decreased significantly after melatonin ingestion as observed in study 1 (48.5 ± 3.2 to 43.5 ± 3.3 cm/s and 0.57 ± 0.04 to 0.52 ± 0.04 cm·s−1·mmHg−1, respectively; P < 0.002; Fig. 2). Phentolamine significantly increased RBFV and RVC to values observed at baseline before melatonin ingestion (P < 0.002; Fig. 2). Ten minutes after phentolamine, RBFV and RVC returned to values observed after melatonin ingestion and were maintained throughout the remainder of the study.
Table 2.
Hemodynamic values for study 2
| Baseline | Melatonin | |
|---|---|---|
| Mean arterial pressure, mmHg | 85 ± 1 | 84 ± 1 |
| Heart rate, beats/min | 58 ± 6 | 57 ± 6 |
Values are means ± SE. Hemodynamic values for baseline and melatonin time points are shown.
Fig. 2.
Renal vascular responses after melatonin ingestion (3 mg) and phentolamine (5-mg bolus). Melatonin significantly decreased RBFV and RVC. Phentolamine reversed these changes. Values are means ± SE. *Significantly different from baseline. †P < 0.002, significantly different from phentolamine infusion.
DISCUSSION
The novel finding of the present study was that melatonin differentially alters blood flow in vascular beds in humans. Renal blood flow was significantly decreased and forearm blood flow was significantly increased after melatonin ingestion, whereas cerebral blood flow was not altered after melatonin ingestion. The reduction in renal blood flow was reversed by an α-adrenergic antagonist, indicating that melatonin augments renal sympathetic outflow to the kidney.
Previous research in animal models indicates that melatonin may have a differential effect on the vasculature. In the rat, melatonin has been demonstrated to vasoconstrict the tail and cerebral arteries (7–9, 12, 22). Additionally, vasoconstrictive responses have been observed with melatonin in porcine coronary arteries (24). In contrast, melatonin-mediated vasodilator responses have been reported in rat and rabbit aorta, iliac, and renal arteries (16–19). The current study is the first to demonstrate that melantonin differentially affects blood flow in multiple vascular beds in humans.
Mechanisms for melatonin's action on the vasculature have been demonstrated to include direct melatonin receptor activation (12, 24) and through intercellular pathways (17, 20, 23). Melatonin binding to MT1 receptors on the vascular smooth muscle cells has been demonstrated to cause vasoconstriction by potentiating norepinephrine signaling (8, 11, 12, 22–24). Through a series of experiments using 4-phenyl-2-acetamidotetraline, a MT2 selective antagonist, on rat caudal arteries, Doolen et al. (8) demonstrated that MT2 receptors mediate vasodilation. Masana et al. (12) confirmed the vasodilation function of MT2 receptors. In addition to receptor-mediated vasodilation, previous research has demonstrated a vasodilation effect without specific melatonin binding. Satake et al. (17) observed a dilation response in rat aorta with melatonin administration, although Viswanathan et al. (23) demonstrated no specific [125I]-labeled melatonin binding in rat aorta. Similar nonreceptor-mediated vasodilation responses to melatonin were observed in porcine vascular smooth muscle (20). Together, these data suggest that melatonin alters vascular blood flow through a combination of mechanisms.
[125I]-labeled melatonin binding is commonly used to determine the presence of melatonin receptors in various vascular beds (13, 23). In the rat, specific [125I]-labeled melatonin binding was not observed in the renal artery, suggesting the melatonin receptors are not located in the renal artery (23). Therefore, the decrease in RVC observed in the present study could be from two possible sources. First, there may be species-related differences in melatonin receptor distribution with MT1 receptors located in human renal arteries but not in rat renal arteries. Second, exogenous melatonin could stimulate neural centers of the brain that increase renal sympathetic nerve activity. Exogenous melatonin has been demonstrated to increase adrenal nerve activity via the suprachiasmatic nucleus in the rat (14). Therefore, melatonin may alter renal sympathetic nerve activity via a similar mechanism in humans. To address this possibility that melatonin-induced reductions in renal blood flow are related to increased renal sympathetic outflow, we measured RBFV after phentolamine, an α-adrenergic receptor antagonist. We observed that following phentolamine, the observed increase in renal vasoconstriction induced by melatonin was eliminated. This finding indicates that melatonin augmented sympathetic outflow to the kidney and therefore is the likely mechanism for this observed response.
Forearm blood flow increased with melatonin in the present study. Previous research by Aoki et al. (1, 2) demonstrated melatonin's cutaneous vasodilator response in the forearm. Additionally, an increase in peripheral blood flow by intravenous melatonin was observed with a distal to proximal skin temperature gradient and finger pulse volume (21). Because MT1 receptor activation causes vasoconstriction and MT2 receptor activation causes vasodilation (8), these data suggest that there are more MT2 receptors expressed in the forearm than MT1 receptors. Unfortunately, specific receptor blockers in humans are not currently available, which would specifically delineate MT1 versus MT2 changes in vascular blood flow.
Previous research demonstrated that melatonin decreases regional cerebral blood flow in the rat (7). However, we did not observe a change in middle cerebral artery blood flow with melatonin ingestion in the present study as measured by transcranial Doppler. Additionally, van der Helm-van Mil et al. (21) did not observe a change in basilar artery blood flow as measured by magnetic resonance imaging with a single bolus of intravenous melatonin in humans. If melatonin alters regional cerebral blood flow in humans, our data in combination with previous results suggest that melatonin alters blood flow in other cerebral arteries involved in brain perfusion in humans.
In contrast to previous research (3, 6), blood pressure did not change in the present study after ingesting melatonin. Arangino and colleagues (3, 6) used 1 mg of melatonin to decrease MAP in men and women. However, in the current study, 3 mg of melatonin were ingested. Previous research from our laboratory suggests that 3 mg of melatonin do not alter blood pressure (15). Therefore, the effect of exogenous melatonin on blood pressure appears to be dose related. Research in animals confirms a dose response to melatonin concentration in relation to vascular changes (8, 20), adrenal nerve activity (14), and hormonal secretion responses (10). These data suggest that melatonin functions differently within the body depending on the ingested dose and might explain the different observed responses in blood pressure with acute melatonin supplementation (3, 6, 15).
Plasma melatonin levels were not measured in the present study. Previous research from our laboratory demonstrates unequivocally that ingesting 3 mg of melatonin increases plasma melatonin >100-fold than endogenous daytime plasma melatonin levels and that the time course to reach near maximal plasma melatonin levels in young, healthy subjects is ∼45 min (15). Because young, healthy subjects were also used for the present study, a deviation from our laboratory's previous observations of plasma melatonin levels is not expected.
In summary, melatonin differentially alters vascular blood flow in humans. The different vascular effects observed with melatonin are attributed to the relative distribution of MT1 and MT2 receptors. Additionally, the reduction in renal blood flow observed after melatonin was reversed by phentolamine, indicating that melatonin increases renal sympathetic outflow. These findings add to the knowledge of melatonin's effect on the vasculature in humans and are important for understanding the effects of melatonin on blood pressure regulation in humans.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
This study was supported by National Heart, Lung, and Blood Institute Grant P01-HL-077670 and a National Aeronautics and Space Administration space grant fellowship.
REFERENCES
- 1. Aoki K, Stephens DP, Zhao K, Kosiba WA, Johnson JM. Modification of cutaneous vasodilator response to heat stress by daytime exogenous melatonin administration. Am J Physiol Regul Integr Comp Physiol 291: R619–R624, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Aoki K, Zhao K, Yamazaki F, Sone R, Alvarez GE, Kosiba WA, Johnson JM. Exogenous melatonin administration modifies cutaneous vasoconstrictor response to whole body skin cooling in humans. J Pineal Res 44: 141–148, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Arangino S, Cagnacci A, Angiolucci M, Vacca AM, Longu G, Volpe A, Melis GB. Effects of melatonin on vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am J Cardiol 83: 1417–1419, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Bliwise DL, Ansari FP. Insomnia associated with valerian and melatonin usage in the 2002 National Health Interview Survey. Sleep 30: 881–884, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brzezinski A. Melatonin in humans. N Engl J Med 336: 186–195, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Cagnacci A, Arangino S, Angiolucci M, Maschio E, Melis GB. Influences of melatonin administration on the circulation of women. Am J Physiol Regul Integr Comp Physiol 274: R335–R338, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Capsoni S, Stankov BM, Fraschini F. Reduction of regional cerebral blood flow by melatonin in young rats. Neuroreport 6: 1346–1348, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Doolen S, Krause DN, Dubocovich ML, Duckles SP. Melatonin mediates two distinct responses in vascular smooth muscle. Eur J Pharmacol 345: 67–69, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Ersahin C, Masana MI, Dubocovich ML. Constitutively active melatonin MT1 receptors in male rat caudal arteries. Eur J Pharmacol 439: 171–172, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Forsling ML, Wheeler MJ, Williams AJ. The effect of melatonin administration on pituitary hormone secretion in man. Clin Endocrinol (Oxf) 51: 637–642, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Geary GG, Krause DN, Duckles SP. Melatonin directly constricts rat cerebral arteries through modulation of potassium channels. Am J Physiol Heart Circ Physiol 273: H1530–H1536, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Masana MI, Doolen S, Ersahin C, Al-Ghoul WM, Duckles SP, Dubocovich ML, Krause DN. MT2 melatonin receptors are present and functional in rat caudal artery. J Pharmacol Exp Ther 302: 1295–1302, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Morgan PJ, Barrett P, Howell HE, Helliwell R. Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem Int 24: 101–146, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Niijima A, Chun SJ, Shima T, Bizot-Espiard JG, Guardiola-Lemaitre B, Nagai K. Effect of intravenous administration of melatonin on the efferent activity of the adrenal nerve. J Auton Nerv Syst 71: 134–138, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Ray CA. Melatonin attenuates the sympathetic nerve responses to orthostatic stress in humans. J Physiol 551: 1043–1048, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Satake N, Oe H, Sawada T, Shibata S. The mode of vasorelaxing action of melatonin in rabbit aorta. Gen Pharmacol 22: 219–221, 1991 [DOI] [PubMed] [Google Scholar]
- 17. Satake N, Oe H, Shibata S. Vasorelaxing action of melatonin in rat isolated aorta: possible endothelium dependent relaxation. Gen Pharmacol 22: 1127–1133, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Satake N, Shibata S, Takagi T. The inhibitory action of melatonin on the contractile response to 5-hydroxytryptamine in various isolated vascular smooth muscles. Gen Pharmacol 17: 553–558, 1986 [DOI] [PubMed] [Google Scholar]
- 19. Shibata S, Satake N, Takagi T, Usui H. Vasorelaxing action of melatonin in rabbit basilar artery. Gen Pharmacol 20: 677–680, 1989 [DOI] [PubMed] [Google Scholar]
- 20. Ting N, Thambyraja A, Sugden D, Scalbert E, Delagrange P, Wilson VG. Pharmacological studies on the inhibitory action of melatonin and putative melatonin analogues on porcine vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol 361: 327–333, 2000 [DOI] [PubMed] [Google Scholar]
- 21. van der Helm-van Mil AH, van Someren EJ, van den Boom R, van Buchem MA, de Craen AJ, Blauw GJ. No influence of melatonin on cerebral blood flow in humans. J Clin Endocrinol Metab 88: 5989–5994, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Vandeputte C, Giummelly P, Atkinson J, Delagrange P, Scalbert E, Capdeville-Atkinson C. Melatonin potentiates NE-induced vasoconstriction without augmenting cytosolic calcium concentration. Am J Physiol Heart Circ Physiol 280: H420–H425, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Viswanathan M, Laitinen JT, Saavedra JM. Expression of melatonin receptors in arteries involved in thermoregulation. Proc Natl Acad Sci USA 87: 6200–6203, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weekley LB. Effects of melatonin on pulmonary and coronary vessels are exerted through perivascular nerves. Clin Auton Res 3: 45–47, 1993 [DOI] [PubMed] [Google Scholar]