Abstract
The protein kinase encoded by the Tpl2 proto-oncogene regulates ERK activation and cytokine gene expression in macrophages in response to LPS and TNF-. In this study we show that OVA-immunized Tpl2−/− mice express high levels of IgE and develop more severe bronchoalveolar eosinophilic inflammation than Tpl2+/+ controls, when challenged with OVA intranasally. Bronchoalveolar exudates and supernatants of OVA-stimulated splenocytes from immunized Tpl2−/− mice express elevated levels of IL-4 and IL-5, suggesting that Tpl2 ablation promotes the Th2 polarization of the T cell response. Anti-CD3 stimulation of CD4+ T cells of wild-type and Tpl2 knockout mice revealed that Tpl2 ablation gives rise to a cell autonomous T cell defect that is primarily responsible for the Th2 polarization of the T cell response to Ag. This observation was further supported by experiments addressing the expression of Th1 and Th2 cytokines in OVA-stimulated mixed cultures of CD4+ T cells from Tpl2+/+/OT2 or Tpl2−/−/OT2 mice and dendritic cells from Tpl2+/+ or Tpl2−/− mice. Further studies revealed that Th1 cells express significantly higher levels of Tpl2 than Th2 cells. As a result, Tpl2−/− Th1 cells exhibit a stronger defect in ERK activation by anti-CD3 than Th2 cells and express low levels of T-bet. Given that the development of Th1 and Th2 cells depends on positive feedback signals from the T cells, themselves, the functional defect of the Tpl2−/− Th1 cells provides a mechanistic explanation for the T cell autonomous Th2 polarization in Tpl2−/− mice.
Introduction
T helper cells differentiate in response to external signals toward Th1, Th2, and Th17 cells. The differentiation depends on epigenetically controlled global changes in gene expression and it is regulated by cytokines and chemokines produced by T cells or other hematopoietic cells and the signaling pathways they activate (1). Th1 cells produce IFN-, are involved in cell-mediated immunity, and contribute to the defense against intracellular bacteria, viruses, and tumor cells. Th2 cells produce IL-4, IL-5, and IL-13, are involved in humoral immunity, and contribute to the defense against extracellular parasites. Th17 cells produce IL-17, activate neutrophils, and contribute to the defense against bacteria at mucosal sites (2–4). Our understanding of the mechanisms responsible for the polarization of the Th cell response toward Th1, Th2, and Th17 depends, to a significant degree, on the successful exploitation of animal genetic models (5).
The work presented in this paper uses Tpl2−/− mice to determine the role of the Tpl2 kinase in the polarization of the Th cell response in animals exposed to OVA. Animals immunized by i.p. injection of OVA in an alum formulation, make Abs that belong to different isotype subclasses, including IgG1 and IgE (6). When challenged by OVA administered intranasally, immunized animals develop bronchoalveolar inflammation (7–10). The severity of inflammation depends on the level of IgE in the serum and the main cell type in the inflammatory exudates is the eosinophil (8, 11). The role of IgE in allergen-induced bronchoalveolar inflammation has been inferred from numerous epidemiologic studies demonstrating a relationship between asthma and IgE levels (12, 13), and from human and animal studies showing that anti-IgE immunotherapy can improve the clinical course of asthma (14–16). Functional studies provided evidence that IgE binds its high-affinity receptor Fc--RI (17) and contributes to mast cell degranulation and release of potent proinflammatory mediators, such as histamine and leukotrienes (18, 19).
Ig isotype switching toward IgE depends on costimulatory molecules and cytokines expressed by the APCs (20), as well as cytokines produced by T cells during the immune response. T cell cytokines that shift the Ig isotype balance toward IgE and promote bronchial inflammation include the Th2-type cytokines IL-4 and IL-5 (8, 9, 21). These cytokines play very important roles in OVA-induced bronchoalveolar inflammation in both animal models and in humans (8, 9, 21–24). In addition to its role in the polarization of the Th cell response, IL-5 contributes to the recruitment of eosinophils. For this, it operates in concert with eotaxin, an eosinophil chemoattractant that is produced by endothelial and alveolar epithelial cells (25, 26). IL-5 mobilizes eosinophils from the marrow and increases their sensitivity to eotaxin (27), whereas eotaxin targets the accumulation of eosinophils at specific sites (25, 28, 29).
Tpl2 is a serine-threonine protein kinase that, when overexpressed, activates all the MAP kinase pathways (30–32), and the transcription factors NFAT and NF-B in a variety of cell types (33–35). Tpl2 ablation in mice revealed an obligatory role for this kinase in the transduction of TLR, death receptor, and G-protein–coupled receptor signals (36–39). However, of the pathways activated by these signals in macrophages and dendritic cells, only ERK depends on Tpl2 (40). The loss of ERK activation renders Tpl2−/− macrophages defective in the induction of TNF- and other proinflammatory molecules. These findings suggest that Tpl2 is involved in the regulation of innate immunity (36–38, 40). Tpl2 also plays an important role in T cell function and the regulation of adaptive immunity. TCR-stimulated Tpl2−/− T cells exhibit a defect in ERK activation and CTLA4 induction and they hyperproliferate in response to Ag (41).
In this paper we present evidence that OVA-immunized Tpl2−/− mice produce significantly higher levels of both OVA-specific and total IgE than Tpl2+/+ mice. The upregulation of IgE correlates with increased secretion of Th2 cytokines and decreased secretion of IFN-. The OVA-treated T cells of Tpl2−/− mice exhibit a cell autonomous defect that shifts the balance of cytokine production toward the Th2 cytokines. Ample evidence to date indicates that Tpl2 has an important role in the biology of APCs [(36–38, 40) and unpublished observations]. The Th2 polarization of the T cell response to OVA in Tpl2−/− mice is in agreement with and complements our recent data showing that the defense of Tpl2 knockout mice to the obligate intracellular pathogen Toxoplama gondii is impaired because of a T cell autonomous defect of Th1 responses (42).
Materials and Methods
Animals
Tpl2 knockout mice have been described previously (30, 36). These mice carry a partial deletion of exon 3, a complete deletion of intron 3, exon 4, and intron 4, and a partial deletion of exon 5. The deletion removed the sequences encoding the ATP binding site of the Tpl2 kinase, and gave rise to a mutant gene that fails to produce stable RNA or protein (36). Experimental mice were 6- to 8-wk-old male littermates, and they were generated by crossing heterozygous Tpl2−/+ mice. The Tpl2−/− mice used in these experiments had been backcrossed nine times to C57BL/6 mice. OT2 transgenic mice carry a TCR transgene that recognizes the OVA peptide OVA323–339 (43). Tpl2−/−/OT2 transgenic C57BL/6 mice were produced by crossing the OT2 transgene (also on the C57BL/6 background) into the Tpl2−/− genetic background. All mice were housed under pathogen-free conditions. All animal studies were approved by the Institutional Animal Care and Use Committees (IACUC) in the authors’ institutions.
Ag sensitization and challenge
Mice were inoculated i.p. with 10 μg OVA (Sigma-Aldrich, St. Louis, MO) in 100 μl PBS/alum suspension or with an equivalent volume of PBS/alum on days 0 and 5. The OVA inoculum was prepared by mixing OVA dissolved in PBS at a concentration of 200 μg/ml, with an equal volume of a 2 mg/ml Alum suspension (Sigma-Aldrich). The final concentration of OVA in the 100 μl OVA inoculum was 100 μg/ml and the final concentration of alum was 1 mg/ml. Seven days after the 5-d boost, mice received 100 μg OVA dissolved in 30 μl PBS intranasally. Intranasal OVA application was repeated daily for 3 d. Prior to the intranasal application of the Ag, the mice were anesthetized with isoflurane (Abbott Laboratories, Abbott Park, IL).
Bronchoalveolar exudates
Bronchoalveolar exudates were collected by bronchoalveolar lavage (BAL) 1 d after the last intranasal application of OVA. Mice were anesthetized by i.p. injection of 2 mg pentobarbital (Abbott Laboratories). After this, their tracheas were cannulated with a polyethylene catheter (20 gauge; 1.16 inch), and their lungs were lavaged twice with 0.6 ml PBS. The volume recovered from each animal was 0.8 ml. Cells in the recovered BAL were counted in a hemocytometer. Cytospin preparations of BAL were stained with Wright’s stain (Protocol HEMA 3, Biochemical Science, Swedesboro, NJ). BAL supernatants obtained by centrifugation were used to measure eosinophil peroxidase, eotaxin, IgE, and cytokine (IL-4 and IL-5) levels.
Measurement of anti-OVA IgG1, IgE, and total IgE in serum and BAL
Ninety-six-well microtiter plates were coated with a purified anti-mouse IgE mAb (R35-72, BD Pharmingen, San Diego, CA). Undiluted BAL or dilutions of serum samples were then added to the coated plates. The captured IgE was detected either with a biotinylated anti-mouse IgE mAb (R35-92, BD Pharmingen) or with biotinylated-OVA (40 μg/ml). The IgE Ab measured total IgE levels, whereas OVA measured IgE-specific anti-OVA Abs. The total IgE levels were calculated based on standard curves generated from known amounts of purified mouse IgE (isotype standard, [anti-TNP], 03121D, BD Pharmingen). OVA-specific IgG1 Abs were measured in serum samples using standard ELISA protocols. Microtiter plates were coated with OVA (10 μg/ml). Dilutions of serum samples were then added to the plates. Biotinylated mAbs against mouse IgG1 (A85-1, BD Pharmingen) were used for detection. The levels of anti-OVA IgG1 were expressed as relative OD values.
Quantitation and functional analysis of mast cells and basophils
Quantitation of mast cells in vivo was performed by staining sections of skin and stomach with toluidine blue at low pH. The numbers of brightly stained mast cells were counted per field of view for skin and per section for stomach. Basophils are characterized as FcERI+Kit−CD11b+CD49b+ cells and were quantified in blood and spleen as described previously (44).
Bone marrow-derived mast cells (BMMCs) were obtained by flushing bone marrow cells from mouse tibiae and femurs with media and culturing them with recombinant mouse IL-3 (20 ng/ml) and stem cell factor (20 ng/ml) for >1 mo. IgE receptor expression was determined by flow cytometry using FITC-conjugated anti-mouse IgE or an isotype control Ab (BD Pharmingen).
To test for BMMC function, Ag-induced β-hexosaminidase was measured. Briefly, 2 × 106 BMMCs/ml were sensitized in medium without IL-3 for 3 h with 1 μg/ml of DNP-specific IgE and challenged with DNP-HSA at 1–1000 ng/ml in Tyrode’s buffer (10 mM HEPES buffer [pH 7.4], 130 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 0.1% BSA). β-hexosaminidase release was measured as described previously (45). The enzymatic activities of β-hexosaminidase in supernatants and in the cell pellets, after solubilizing with 0.5% Triton X-100 in Tyrode’s buffer, were measured with p-nitrophenyl N-acetyl-β-D-glucosaminide in 0.1 M sodium citrate (pH 4.5) for 60 min at 37°C. The reaction was stopped by addition of 0.2 M glycine (pH 10.7). The release of the product 4-p-nitrophenol was detected by absorbance at 405 nm. The extent of degranulation was calculated as the percentage of 4-p-nitrophenol absorbance in the supernatants over the sum of absorbance in the supernatants and in cell pellets solubilized in detergent. For maximal IgE-independent β-hexosaminidase release, cells were alternatively stimulated with PMA plus ionomycin.
Splenocyte cultures, bone marrow-derived dendritic cells, and CD4+ T cells
Single-cell suspensions of mouse splenocytes (2 × 107cells/ml) derived from Tpl2+/+ and Tpl2−/− mice were cultured in DMEM supplemented with penicillin and streptomycin (Life Technologies, Rockville, MD), nonessential amino acids (Life Technologies), sodium pyruvate (Life Technologies), and FBS (10%, Life Technologies). Splenocyte cultures were treated with OVA (100 μg/ml) for 48 h. Culture supernatants were tested for IL-4, IL-5, and IFN- by ELISA.
Bone marrow-derived dendritic cells (BMDCs) were produced by culture of bone marrow cells from femurs and tibias of six 12-wk-old mice. Cells (1 × 106 cells/ml) were cultured at 37°C and 5% CO2 in complete RPMI (RPMI 1640 containing 10% FCS [BioSource International, Camarillo, CA], 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine [Invitrogen], and 50 μM 2-mercaptoethanol [Sigma-Aldrich]) supplemented with 40 ng/ml GM-CSF (PeproTech, Rocky Hill, NJ). On days 3 and 5, fresh medium equal to half of the initial volume of the culture containing 40 ng/ml GM-CSF was added. On day 7, nonadherent cells were collected, incubated with anti-mouse CD11c labeled microbeads (Miltenyi Biotech, Auburn, CA), and CD11c+ cells were selected using an AutoMACS (Miltenyi Biotec) according to the manufacturer’s instructions. The purity of the cell population was determined to be >90% CD11c+ by flow cytometry.
To determine whether LPS-stimulated cells express dendritic cell markers, on day 6 of culture, cells were either stimulated with LPS, zymosan, or polyinosinic-polycytidylic acid overnight. On day 7, cells were harvested and stained with Abs to CD11c (HL-3, BD Pharmingen) and CD11b (M1/70, BD Pharmingen) in combination with Abs against costimulatory markers, including CD86 (GL1, BD Pharmingen), MHC class II/I-Ab (25-9-17, BD Pharmingen), CD40 (HM40-3, BD Pharmingen), and CD80 (16-10A1,BD Pharmingen).
Total CD4+ T cells were purified from spleen and lymph nodes by negative selection using a CD4+ T cell isolation kit and an AutoMACS instrument (Miltenyi Biotec). Cells isolated in this way were routinely >90% pure CD4+ T cells. Unless indicated otherwise, naive CD4+CD62L+CD44loCD25− T cells were further purified to >99% by cell sorting using a FACSAria II (BD Biosciences) or MoFlo sorter (DakoCytomation, Carpintera, CA).
Anti-CD3 stimulation of total splenocytes and CD4+ T cells
For cytokine secretion, 5 × 105 whole splenocytes were stimulated in a 200-μl volume with 0–3 μg/ml anti-CD3 for 48 h, and IFN- and IL-4 in cell culture supernatants were quantified by Th1/2 mouse cytometric bead array (BD Pharmingen). For mRNA analysis, 1–2 × 106 cells/ml were stimulated with 5 μg/ml immobilized anti-CD3, or 5 μg/ml immobilized anti-CD3 and anti-CD28 for 18 h. Gene expression was measured by real-time PCR. Briefly, RNA was isolated and reverse transcribed using a first-strand cDNA synthesis kit (Roche, Mannheim, Germany). Real-time PCR was performed using the ABI PRISM7700 Sequence Detection System (Applied Biosystems, Foster City, CA). Analysis of IL-4, IFN-, and 18S mRNA levels was performed using commercially available primer/probe sets (Applied Biosystems). Relative levels of all genes were determined by normalization to endogenous 18S levels and are presented as relative to the unstimulated control for each individual experiment, which was arbitrarily designated a value of 1.
Th1 and Th2 differentiation of CD4+ T cells and maintenance of Th1 and Th2 polarized cultures
Naive CD4+CD62L+CD44loCD25− T cells were sorted to >99% purity. Cells were cultured at 1 × 106/ml complete RPMI 1640 in tissue culture plates that had been precoated with 5 μg/ml anti-CD3 and anti-CD28 in the presence of various cytokines and anticytokine Ab mixtures to direct distinct Th lineages as follows: Th0, no cytokines or anticytokines; Th1, 20 ng/ml IL-12 and 10 μg/ml anti-IL-4; and Th2, 10 ng/ml IL-4 and 10 μg/ml anti-IFN-. Cells were cultured for 3 d and subsequently transferred into T75 flasks containing 15 ml medium with 40 U/ml IL-2 alone (Th0) or in combination with IL-12 (Th1) or IL-4 (Th2) and grown an additional 4 d. Fully polarized Th0, Th1, or Th2 cells were harvested after 7 d of culture.
For biochemical analyses of fully polarized wild-type (WT) and Tpl2−/− Th cell lineages, T cells were harvested on day 7 of culture. Cells were washed in complete RPMI 1640 and rested for 8 h in cytokine-free medium. After harvest, cells were adjusted to 2.5 × 106 cells/ml medium and stimulated with 5 μg/ml anti-CD3 for the indicated times. Lysates of cell pellets were prepared in Triton lysis buffer. Proteins were separated by SDS-PAGE and transferred to nylon membranes for Western blotting with phosphor ERK1/2 and total ERK1/2 Abs (Cell Signaling Technology, Beverly, MA) as previously described (36).
For quantitation of transcription factor expression, total CD4 cells were subjected to the same 7-d Th0, Th1, and Th2 polarization regimen as described previously. On day 7, cells pellets were lysed in Triton lysis buffer. Proteins were separated by SDS-PAGE, transferred to nylon membranes, and probed for the Th1 transcription factor, T-bet (Santa Cruz Biotechnology, Santa Cruz, CA) as well as the Th2 transcription factor, Gata-3 (Santa Cruz Biotechnology) as previously described (36).
OVA stimulation of mixed cultures of T cells and dendritic cells
For coculture experiments, 104 WT or Tpl2−/− BMDCs (10,000/well) were incubated with a 10-fold excess of naive OT2 CD4+ cells (100,000/well) and varying amounts of OVA peptide (OVA323–339, Peptides International, Louisville, KY) in 96-well microtiter plates in a volume of 200 μL. On day 3, supernatants were harvested and analyzed by ELISA for the expression of IFN-, IL-4, and IL-5 as described below.
Measurement of cytokines, chemokines, and eosinophil peroxidase
To measure IL-4, IL-5, and IFN-, in BAL and/or culture supernatants, we used ELISA kits from BD Pharmingen (OptEIA Kit). Alternatively, IFN- and IL-4 were measured in cell culture supernatants using a mouse Th1/2 cytometric bead array (BD Pharmingen) where indicated.
Eotaxin levels were also measured by ELISA. The capture Ab, used to coat the microtiter plates, was an anti-mouse eotaxin polyclonal Ab (AF-420-NA, R&D Systems, Minneapolis, MN). The detection Ab was a biotinylated anti-mouse eotaxin Ab (BAF420, R&D Systems). Purified anti-mouse eotaxin Ab (the capture Ab) was diluted in coating buffer (0.4 μg/ml) and was adsorbed onto the wells of a 96-well microtiter plate. Uncoated sites were blocked with blocking buffer. The undiluted BAL samples were then incubated with the plate-bound antieotaxin Ab. After this, 40 ng/ml biotinylated anti-mouse eotaxin Ab (detection Ab) in blocking buffer was added to each well, followed by Avidin conjugated to HRP (BD Pharmingen) (1/1000 dilution), and the substrate 3,3′,5.5′ tetramethylbenzidine (BD Pharmingen). Eotaxin levels were calculated by reference to standard curves of known amounts of recombinant mouse eotaxin (420-ME, R&D Systems).
Eosinophil peroxidase in BAL was measured using o-phenylene-diamine hydrochloride as the substrate. Seventy-five microliters of the substrate solution (16 mM o-phenylene-diamine hydrochloride, 0.1% Triton X-100, 0.01% H2O2 in 100 mM Tris-HCl) was added to 50 μl of the sample in 96-well microtiter plates and the mixture was incubated at room temperature for 30 min. The reaction was stopped by adding 50 μl 4 M sulfuric acid. The OD of the reaction product was read at 450 nm. Eosinophil peroxidase levels were calculated by reference to standard curves of HRP (BD Pharmingen). This method has been shown to detect eosinophil but not neutrophil peroxidase activity (46).
Statistical analysis
Given the variability between individual mice, all the experiments were carried out on multiple animals (3–11 per group per experiment), and they were repeated multiple (3–18) times. Tpl2+/+ and Tpl2−/− mice within a given experiment were compared by t test or the Wilcoxon/Mann-Whitney U test, a nonparametric equivalent that does not assume normality of the measurements (it compares medians instead of means).
Results
Total IgE, Ag-specific IgG1, and IgE levels in the serum of OVA-immunized Tpl2+/+ and Tpl2−/− mice
To determine the role of Tpl2 in adaptive immunity and allergen-induced pulmonary inflammation, Tpl2+/+ and Tpl2−/− mice were inoculated i.p. with OVA (10 μg) in alum. The i.p. inoculation of the OVA/alum formulation elicits a response characterized by the production of IgG1 and IgE Abs. Five days later, the mice were given a boost of 10 μg OVA i.p. Finally, on days 12–14 the mice were challenged with the same Ag intranasally. One day after the intranasal challenge, blood samples were collected, and serum levels of total IgE and OVA-specific IgG1 and IgE were measured by ELISA. The results showed that serum levels of OVA-specific IgG1 Abs were lower in Tpl2−/− mice (Fig. 1A). In contrast, total IgE and OVA-specific IgE Abs were significantly higher in Tpl2−/− mice (Fig. 1B, 1C). These results demonstrate that inactivation of Tpl2 enhances total and Ag-specific IgE responses.
FIGURE 1.
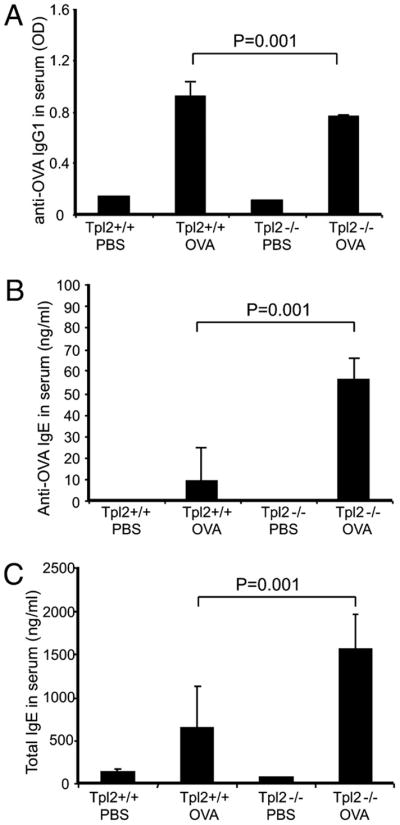
Isotype-specific Abs in the serum of OVA immunized Tpl2+/+ and Tpl2−/− mice. Tpl2 knockout and WT mice were immunized and challenged with OVA. Age- and sex-matched control mice were inoculated with PBS. Serum was collected 1 d after OVA challenge. Ig isotype levels in serum were measured by ELISA: (A) Levels of anti-OVA IgG1, (B) levels of anti-OVA IgE, and (C) levels of total IgE. Data of anti-OVA IgG1 were presented as the mean OD ± SD. Data of anti-OVA IgE and total IgE were presented as the mean concentration (ng/ml) ± SD. Each group consisted of three mice. This was 1 of 18 similar experiments. p Values were calculated based on the results of all 18 experiments by the stratified version of the Wilcoxon test. WT/PBS: WT mice receiving PBS and OVA challenge; WT/OVA: WT mice receiving OVA immunization and OVA challenge; KO/PBS: Tpl2 knockout mice receiving PBS and OVA challenge; KO/OVA: Tpl2 knockout mice receiving OVA immunization and OVA challenge.
Our previous studies had shown that the histology of the bone marrow, thymus, spleen, and lymph nodes of the Tpl2−/− mice was normal and that the numbers of CD4, CD8, Thy1.2, B220, CD11b, Ter-1 (CCR8), CD3, IL-2R, TCR/β, and TCR/positive cells in these organs, were also normal (36). These findings suggest that the shift in the relative levels of Ig isotypes observed in OVA-immunized Tpl2−/− mice were not due to major structural defects of these organs or to major defects in lymphoid cell differentiation and distribution. Our previous studies had also shown that isolated B cells from Tpl2−/− mice produced low levels of IgE when stimulated with IL-4 or CD40 ligand, two molecules that control IgE synthesis in B cells (47). The increased levels of IgE in OVA-immunized mice therefore were unexpected and could not be explained on the basis of a cell autonomous B cell defect. Instead, the combination of these seemingly incompatible observations suggests that IgE levels may be higher in OVA-immunized Tpl2−/− mice because of the effects of Tpl2 ablation on the complex interactions between different types of cells in the immune system.
Allergen-induced pulmonary inflammation is more severe in Tpl2−/− than in Tpl2+/+ mice
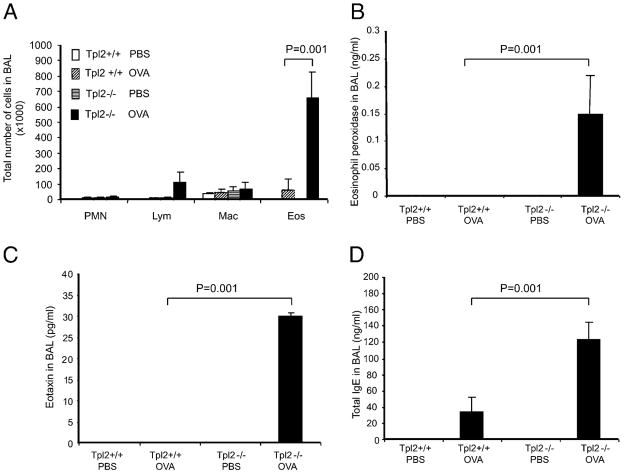
IgE is an important mediator of allergen-induced inflammatory responses. We therefore examined the severity of bronchoalveolar inflammation in OVA immunized Tpl2+/+ and Tpl2−/− mice 1 d after intranasal challenge with OVA. To isolate the bronchoalveolar exudates, we performed BAL as described in Materials and Methods. Only a few inflammatory cells were detected in exudates from Tpl2+/+ mice. In contrast, exudates from Tpl2−/− mice contained significantly higher numbers of inflammatory cells, primarily eosinophils (Fig. 2A). The increased number of eosinophils in Tpl2−/− mice correlated with increased levels of eosinophil peroxidase (Fig. 2B), suggesting that the eosinophils accumulating in the exudates were active. To investigate the mechanisms responsible for the differential recruitment of eosinophils, we proceeded to measure the bronchoalveolar exudate levels of molecules linked to eosinophil recruitment. The results showed that the chemokine eotaxin and IgE were both significantly higher in the exudates of Tpl2−/− mice (Fig. 2C, 2D).
FIGURE 2.
OVA-induced bronchoalveolar inflammation in Tpl2−/− and Tpl2+/+ mice. Tpl2 knockout and WT mice were immunized and challenged with OVA. Age- and sex-matched control mice were inoculated with PBS. Bronchoalveolar exudates were collected 1 d after OVA challenge as described in Materials and Methods. A, Numbers of polymorphonuclear leukocytes (PMN), Lymphocytes (Lym), macrophages (Mac), and eosinophils (Eos) in bronchoalveolar exudates; (B) concentration of eosinophil peroxidase in bronchoalveolar exudates; (C) concentration of eotaxin in bronchoalveolar exudates; (D) concentration of total IgE in bronchoalveolar exudates. Data were presented as mean values ± SD. Each group consisted of three mice. This was 1 of 18 similar experiments. p Values were calculated based on the results of all 18 experiments by the stratified version of the Wilcoxon test. Symbols for different groups are the same as in Fig. 1.
To further explore the mechanism of the enhanced allergic response of Tpl2−/− mice to OVA, we also examined the number of basophils in the blood and spleen and the number of mast cells in the skin and stomach of Tpl2+/+ and Tpl2−/− mice. The results showed no significant differences. Similarly, there were no significant differences between Tpl2+/+ and Tpl2−/− BMMCs development or IgE receptor (FcRI) expression or functionally as measured by β-hexosaminidase release after IgE receptor cross-linking (Supplemental Fig. 1A–C).
OVA challenge induces a stronger Th2 cytokine response in Tpl2−/− than in Tpl2+/+ T cells
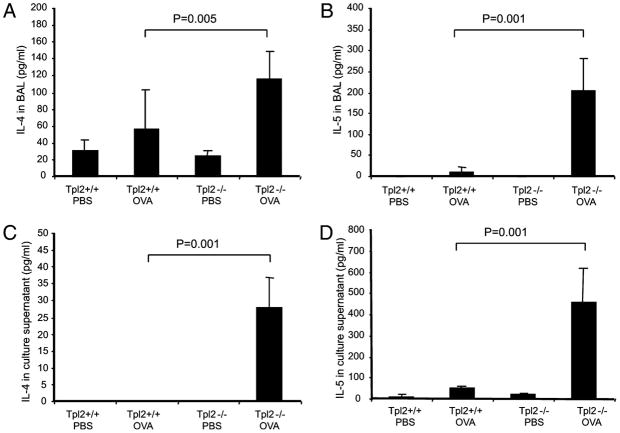
The results of our earlier studies, which were discussed previously, showed that the shift in the relative levels of Ig isotypes in OVA-immunized Tpl2−/− animals are not the result of major defects in lymphoid cell differentiation and distribution or to defects in B cell, basophil, and mast cell function. Given that increased levels of IgE have been linked to cytokines produced by Th2 cells (9–11, 48), the question arose as to whether T cells from OVA-immunized Tpl2−/− mice produce predominantly Th2 cytokines when exposed to OVA. To address this question, we measured the levels of IL-4 and IL-5 (Th2 cytokines) in bronchoalevolar exudates from OVA-immunized or PBS-inoculated control mice. The same cytokines were measured in the supernatants of splenocyte cultures exposed to OVA. The results showed that brochoalveolar exudates from Tpl2−/− mice contain higher concentrations of IL-4 and IL-5 (Fig. 3A, 3B). Culture supernatants of OVA-treated splenocytes from Tpl2−/− mice also contained higher levels of both IL-4 and IL-5 (Fig. 3C, 3D). These data suggested that Tpl2 ablation promotes the Th2 polarization of the immune response to OVA.
FIGURE 3.
Cytokine levels in bronchoalveolar exudates and cultures of splenocytes from OVA-immunized Tpl2+/+ and Tpl2−/− mice challenged with OVA. Tpl2−/− and Tpl2+/+ mice were immunized and challenged with OVA. Age- and sex-matched control mice were inoculated with PBS. A and B, Bronchoalveolar exudates were collected 1 d after OVA challenge. IL-4 (A) and IL-5 (B) levels were measured by ELISA. Data are presented as mean values ± SD. Each group consisted of three mice. This was 1 of 18 similar experiments. p Values were calculated based on the results of all 18 experiments by the stratified version of the Wilcoxon test. Symbols for different groups are the same as in Fig. 1. C and D, Splenocyte cultures were treated with OVA. IL-4 (C) and IL-5 (D) levels were measured 48 h later by ELISA. Data are presented as mean cytokine concentration ± SD. Each group consisted of three mice. This was one of eight similar experiments. p Values were calculated based on the results of all eight experiments by the stratified version of the Wilcoxon test. Symbols for different groups are the same as in Fig. 1.
pl2 ablation gives rise to a T cell-intrinsic defect in Th cell differentiation that favors Th2 polarization
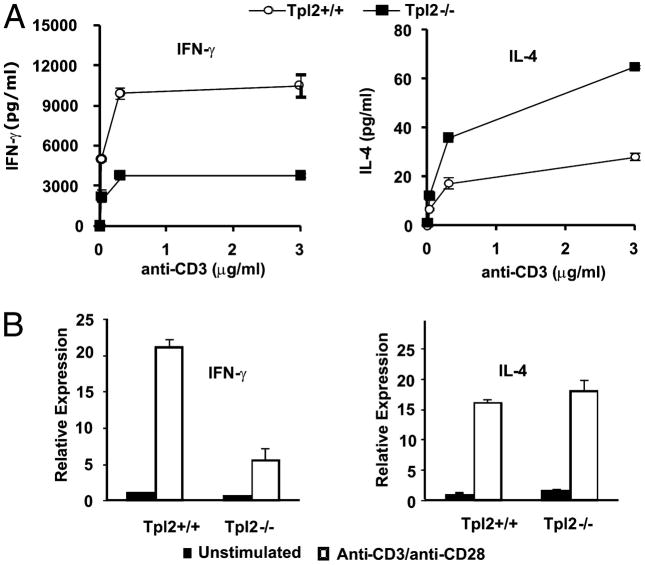
Exposure to Ag promotes T cell proliferation and expression of cytokines and other molecules involved in T cell activation. Theoretically, the polarization of the T cell response could be a T cell autonomous phenomenon, or it may be regulated by signals that originate in the APCs. Alternatively, it may depend on a combination of T cell autonomous and APC-dependent mechanisms. Therefore, Tpl2 ablation may be operating at the T cell level, at the APC level, or both. Experiments presented in this study provide evidence for an intrinsic T cell defect that favors Th2 polarization in Tpl2−/− mice. CD4+ T cells polarized toward the Th1 phenotype express primarily Th1 cytokines, such as IFN-, wheras T cells polarized toward the Th2 phenotype express primarily Th2 cytokines, such as IL-4 and IL-5. Th1 cytokines provide positive feedback signals that promote the differentiation of the Th cells toward the Th1 phenotype. Similarly, Th2 cytokines promote Th cell differentiation toward the Th2 phenotype. Stimulation of splenocytes with soluble anti-CD3 revealed that in the absence of Tpl2, T cells produce less IFN- (a Th1 cytokine), and more IL-4 (a Th2 cytokine) (Fig. 4A). This experiment suggested that the Th2 polarization of the T cell response in Tpl2−/− mice could be mediated by T cell autonomous mechanisms. To confirm this conclusion, purified CD4+ T cells from WT or Tpl2 knockout mice were stimulated with plate-bound anti-CD3 and anti-CD28, which stimulate the T cells in an APC-independent manner. After 18 h, cytokine expression was measured by real-time RT-PCR. Tpl2 knockout CD4+ T cells expressed less IFN- and slightly increased IL-4 compared with WT CD4+ T cells, both basally and after stimulation (Fig. 4B). These data support the hypothesis that Tpl2 ablation gives rise to a T cell-intrinsic defect in Th cell differentiation, which causes a Th1/Th2 imbalance favoring the Th2 cells. Tpl2 therefore may promote T cell differentiation along the Th1 lineage and Tpl2 ablation may give rise to a Th1 differentiation defect.
FIGURE 4.
Tpl2 deficiency leads to a Th2 bias ex vivo. A, Whole splenocytes (5 × 105 in 200 μl media) were stimulated with soluble anti-CD3 for 48 h, and the production of IFN-γ (left plot) and IL-4 (right plot) were measured by cytometric bead array. B, Purified CD4+ T cells were stimulated with plate-bound anti-CD3 and anti-CD28 for 18 h, and cytokine and transcription factor mRNA levels were determined by real-time RT-PCR. Data are representative of two independent experiments.
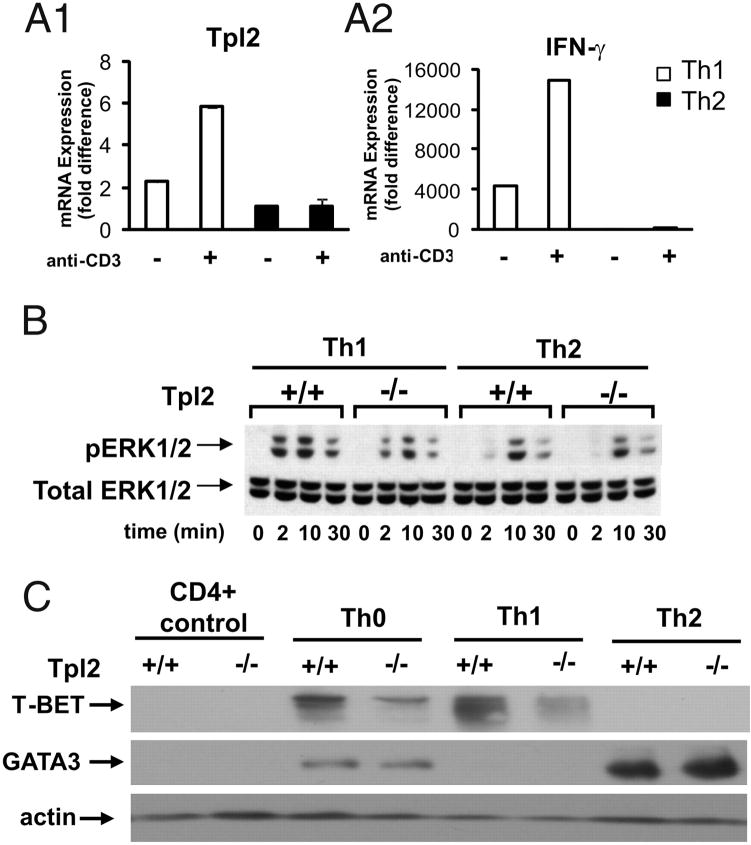
To address the mechanism by which Tpl2 may affect the differentiation of Th cells, we first examined the expression of Tpl2 in cultured Th1 and Th2 cells from WT mice. Analysis of Th1 and Th2 polarized CD4+ T cells revealed that Th1 cells express higher mRNA levels of Tpl2 than Th2 cells that was further enhanced in the presence of TCR ligation (Fig. 5A1). Analysis of IFN- mRNA expression confirmed proper polarization of the Th1 cells (Fig. 5A2). As a result, ERK activation in response to anti-CD3 stimulation is inhibited significantly in Tpl2−/− Th1 cells but not in Tpl2−/− Th2 cells (Fig. 5B). We conclude that TCR signaling depends heavily on Tpl2 in Th1 but not in Th2 cells, making the effects of Tpl2 ablation in Th1 cells more severe than in Th2 cells. Based on these data, we hypothesized that Th1 cells developing in Tpl2−/− mice are functionally defective. To address this hypothesis, we examined the expression of T-bet (a Th1 determinant) and GATA3 (a Th2 determinant) in WT or Tpl2-deficient, fully polarized Th1 and Th2 cells. The results (Fig. 5C) showed that Th1 cells from Tpl2−/− mice express greatly reduced levels of T-bet, whereas Th2 cells from the same mice express only slightly higher levels of GATA3 than Th2 cells of WT mice. These results suggest functional differences between Tpl2−/− and Tpl2+/+ Th1 cells, and they are consistent with the results of earlier studies showing that the phosphorylation of STAT4 and the expression of IFN- in TCR or IL-12–stimulated CD4+ T cells are Tpl2-dependent (42). Overall, these data combined, suggest that Th1 cells developing from Tpl2−/− mice are functionally defective. Given that cytokines produced by Th1 cells promote Th1 cell differentiation, these findings also suggest that the functional defect of the Tpl2−/− Th1 cells contributes to the T cell autonomous Th2 polarization in Tpl2−/− mice.
FIGURE 5.
Tpl2 ablation gives rise to a T cell-intrinsic defect in Th cell differentiation that favors Th2 polarization. A1 and A2, fully polarized murine Th1 and Th2 cells were cultured as described in Materials and Methods. The cells were harvested on day 7 of culture, washed, and restimulated in the presence (+) or absence (−) of immobilized anti-CD3 for 4 h. Tpl2 (A1) and IFN-γ (A2) expression were measured by real-time RT PCR using commercially available primer/probe sets (Applied Biosystems). IFN-γ expression confirmed proper polarization of the cells. Data are expressed as mean ± SD and are representative of 2 independent experiments. B, Th1- and Th2-polarized CD4+ Th cells from WT and Tpl2−/− mice, were stimulated with anti-CD3. Cell lysates harvested at the indicated time points from the start of the stimulation, were probed with total ERK and phosphor-ERK Abs. C, Tpl2−/− Th1 cells express low levels of T-bet, whereas Tpl2−/− Th2 cells express only marginally higher levels of GATA3 than WT Th2 cells. Cell lysates of fully polarized Th0, Th1, and Th2 Tpl2+/+ and Tpl2−/− cells, harvested as described in the text, were probed with the indicated Abs. Data are representative of two experiments yielding identical results.
The effect of Tpl2 ablation on APC function
The Th2 polarization of the Th cell response in Tpl2−/− mice may depend on T cell autonomous mechanisms, as suggested by the data presented previously, or a combination of T cell autonomous and APC-dependent mechanisms. The latter was suggested by a wealth of earlier data showing that the ablation of Tpl2 gives rise to functional defects in both macrophages and dendritic cells (36, 37, 40, 47), also confirmed in the current study by measuring LPS- or zymosan-induced cytokine production in BMDCs (data not shown). To address the role of APC-dependent mechanisms in Th2 polarization in Tpl2 knockout mice, we first examined the effects of Tpl2 ablation on the number of dendritic cells in the spleen of unimmunized animals. To this end, low-density spleen cells enriched in dendritic cells (49) were stained for: (a) CD11c, CD4, and CD8; (b) CD11c and B220; and (c) CD11c, CD11b, and CD8. Flow-cytometric analysis of the stained cells revealed that the numbers of plasmacytoid dendritic cells (B220+CD11clow), CD8+ conventional dendritic cells (CD8+; CD11b+CD11c+CD8+), and CD8− conventional dendritic cells (CD11b+CD11c+CD8−) in the spleen of Tpl2+/+ and Tpl2−/− mice were similar (Supplemental Fig. 2A). Further studies revealed that freshly isolated CD11c-positive dendritic cells from the spleen of Tpl2+/+ and Tpl2−/− mice express similar levels of MHC class II, CD80, CD86, and CD40 (Supplemental Fig. 2B). Finally, the induction of MHC class II, CD80, CD86, and CD40 was also similar in unfractionated WT and Tpl2−/− dendritic cells stimulated with LPS, zymosan, or polyinoinic-polycytidylic acid (Supplemental Fig. 3).
The role of Tpl2 expressed in APCs in the Th1/Th2 differentiation of Ag-stimulated Th cells
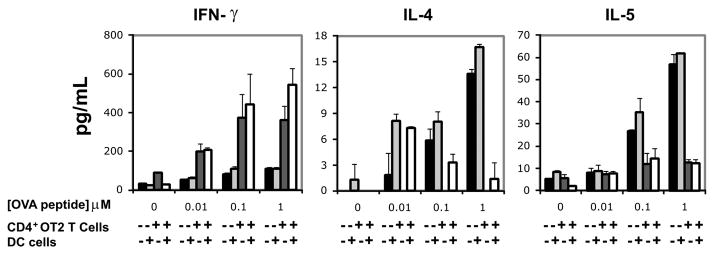
To determine how the TLR signaling defects in Tpl2−/− dendritic cells described previously, affect the differentiation of Th cells responding to Ag stimulation, we first crossed an OVA-TCR transgene (OT2) into the Tpl2−/− genetic background. CD4+ T cells from OT2 transgenic Tpl2+/+ and Tpl2−/− mice and BMDCs from naive Tpl2+/+ or Tpl2−/− mice, were cocultured in all possible combinations, and mixed cultures were exposed to OVA. The results showed that cultures of Tpl2−/− T cells, in combination with either Tpl2+/+ or Tpl2−/− dendritic cells released lower levels of IFNand higher levels of IL-4 and IL-5 than cultures of Tpl2+/+ T cells (Fig. 6), confirming that the expression of Tpl2 in T cells plays a critical role in the Th1 polarization of the Th cell response. CD4+ T cells (of both the Tpl2+/+ and Tpl2−/− genotype) on the other hand, produced similar levels of IFN-, IL-4, and IL-5, when cocultured with Tpl2+/+ and Tpl2−/− DCs (Fig. 6). These data combined, support the hypothesis that T cell-expressed Tpl2 contributes primarily to the Th1 differentiation of Th cells in response to Ag stimulation. Despite the importance of Tpl2 expressed in dendritic cells to dendritic cell function, Tpl2 expressed in these cells contributes minimally or not at all to the differentiation of Th cells toward the Th1 phenotype.
FIGURE 6.
The role of Tpl2 expressed in T cells and APCs in the Th1/Th2 differentiation of Ag-stimulated Th cells. CD4+ T cells from Tpl2+/+/OT2 and Tpl2−/−/OT2 transgenic mice were cocultured with BMDCs cultured from Tpl2+/+ and Tpl2−/− mice in all possible combinations. Mixed cultures were stimulated with different concentrations of the OVA peptide, OVA323–329. The indicated cytokines were measured by ELISA in culture media harvested from the mixed cultures at day 3, as described in the text. Data shown are mean + SD from duplicate samples. Two independent experiments showed similar results.
Discussion
Evidence presented in this paper indicates that Tpl2 not only regulates innate immunity, but also plays an important role in the regulation of adaptive immunity. Thus, OVA-immunized Tpl2−/− mice produce low levels of OVA-specific IgG1 Abs, high levels of OVA-specific IgE Abs and high levels of total IgE. Splenocytes from OVA-immunized Tpl2−/− mice produce high levels of the Th2 cytokines IL-4 and IL-5 when exposed to Ag. Moreover, total splenocytes and purified CD4+ splenic T cells from nonimmunized mice produce low levels of IFN- and high levels of IL-4, upon anti- CD3 or anti-CD3 and anti-CD28 stimulation, respectively. The altered response of Tpl2 knockout T cells to OVA and anti-CD3 was shown to correlate with increased responsiveness to intranasally applied Ag. Thus, intranasal OVA challenge of OVA-immunized Tpl2−/− mice, gave rise to severe bronchoalveolar inflammation with exudates containing increased numbers of eosinophils and increased levels of eosinophil peroxidase, eotaxin, and IgE. The effects of Tpl2 ablation on the polarization of Th cells and on the OVA-mediated allergic responses was not associated with detectable defects in the development of hematopoietic and lymphoid cells. Thus, the histology of hematopoetic and lymphoid organs and the numbers of B cells, T cells, macrophages, basophils, and mast cells in Tpl2−/− mice were normal. Moreover, isolated Tpl2−/− B cells produced low, rather than high levels of IgE on stimulation in culture, and basophils released normal levels of β-hexosaminidase upon IgE cross-linking.
The data presented in this study are in agreement with the data in another recent report showing that Tpl2 ablation renders mice susceptible to T. gondii infection, because it interferes with the mounting of a protective Th1 response (42). However, the data in both these papers differ from the data in another report, showing that Tpl2 ablation promotes the Th1 polarization of the T cell response in OVA-immunized mice (50). The difference between our data and the data in the latter report may be caused by differences in the route of immunization. Thus, whereas our mice were immunized by i.p. injection of OVA mixed with alum, the mice in the latter report were immunized by footpad injection of OVA mixed with complete Freund’s adjuvant, alum, or incomplete Freund’s adjuvant, containing LPS or CpG DNA. Another confounding factor is that there may be differences in the genetic background of the Tpl2−/− mice in the two reports, which has been demonstrated to dramatically affect Th cell differentiation. Whereas the mice used in the current study were backcrossed nine times into the C57BL/6 genetic background, the Tpl2−/− mice in the earlier report were only in the sixth generation of backcrossing into the C57BL/6 background.
The Th1/Th2 polarization of the Th cells depends on a combination of T cell intrinsic and APC-dependent mechanisms (51). To determine the mechanism by which Tpl2 regulates this process, we first examined its contribution to T cell intrinsic mechanisms of Th cell polarization. To this end, we measured the secretion of IL-4 and IFN- by splenocytes from naive Tpl2+/+ and Tpl2−/− mice in response to TCR stimulation with anti-CD3. The results showed that the responding Tpl2−/− cells (presumably T cells) produced lower levels of IFN- and higher levels of IL-4 than the Tpl2+/+ controls. The T cell-intrinsic bias of the Tpl2+/+ Th cells toward Th1 differentiation was confirmed by measuring the expression of IFN- and IL-4 in purified CD4+ T cell cultures stimulated with anti-CD3 and anti-CD28. Further studies revealed that Th1 cells from WT mice express significantly higher levels of Tpl2 than Th2 cells and that the Th1 cells of Tpl2−/− mice express reduced levels of T-bet and exhibit a defect in ERK activation in response to TCR stimulation. As discussed in Results, these functional defects of the Tpl2−/− Th1 cells suggest a T cell autonomous mechanism for the Th2 polarization in Tpl2−/− mice. The preceding experiments did not exclude the possibility that APCs may also have a role in the polarization of the immune response in vivo. APCs include dendritic cells, macrophages, and B cells. Of these, dendritic cells have the ability to present Ag to naive CD4+ T cells and are known as the professional APCs (51, 52). To address their role in regulating the T cell response to Ag, mixed cultures of CD4+ T cells from WT and Tpl2−/− OT2 transgenic mice and BMDCs from naive Tpl2+/+ and Tpl2−/− mice were treated with an OVA peptide specific for the OT2 TCR transgene, and the expression of Th1 and Th2 cytokines was measured by ELISA. These experiments revealed that, whereas Tpl2 expression in T cells is required for the expression of Th1 and the suppression of Th2 cytokines, Tpl2 expression in dendritic cells affects the expression of these cytokines minimally, or not at all. These data confirmed that Tpl2 expression in T cells is critical for the differentiation of Th cells along the Th1 pathway after OVA immunization. In addition, they showed that, despite the fact that Tpl2 expressed in dendritic cells plays a major functional role in these cells, its contribution toward Th cell differentiation in response to OVA is minimal.
In summary, the findings presented in this paper demonstrate that after immunization with T cell-dependent Ags, Tpl2 promotes the differentiation of CD4+ Th cells along the Th1 pathway primarily via T cell intrinsic mechanisms. As a result, Tpl2 ablation promotes the Th2 polarization of the Th cell response and the development of allergy. The results of the current study may facilitate the design of preventive and therapeutic strategies for allergen-induced inflammation.
Supplementary Material
Acknowledgments
We thank Yuri Sykulev, Thomas Jefferson University, for helpful discussions. Part of this work was performed at Thomas Jefferson University before the move of the Tsichlis laboratory to Tufts Medical Center.
This work was supported by the Public Health Service Awards, National Institutes of Health R01 CA38047 and R01 CA095431 (all to P.N.T), National Institutes of Health Training Grant 5-T32-CA09662 (to C.C.W.), a Medical Research Council (UK) Career Development Award (to A.G.E.), and National Institutes of Health Grant 1 K22 AR53953-01 (to W.T.W.).
Abbreviations used in this paper
- BAL
bronchoalveolar lavage
- BMDC
bone marrow-derived dendritic cell
- BMMC
bone marrow-derived mast cell
- WT
wild-type
Footnotes
Disclosures The authors have no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Lee GR, Kim ST, Spilianakis CG, Fields PE, Flavell RA. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity. 2006;24:369–379. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Coffman RL. Origins of the T(H)1-T(H)2 model: a personal perspective. Nat Immunol. 2006;7:539–541. doi: 10.1038/ni0606-539. [DOI] [PubMed] [Google Scholar]
- 3.Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129:33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 6.Lewkowich IP, Rempel JD, HayGlass KT. Antigen-specific versus total immunoglobulin synthesis: total IgE and IgG1, but not IgG2a levels predict murine antigen-specific responses. Int Arch Allergy Immunol. 2004;133:145–153. doi: 10.1159/000076440. [DOI] [PubMed] [Google Scholar]
- 7.Lai WQ, Goh HH, Bao Z, Wong WS, Melendez AJ, Leung BP. The role of sphingosine kinase in a murine model of allergic asthma. J Immunol. 2008;180:4323–4329. doi: 10.4049/jimmunol.180.6.4323. [DOI] [PubMed] [Google Scholar]
- 8.Brusselle G, Kips J, Joos G, Bluethmann H, Pauwels R. Allergen-induced airway inflammation and bronchial responsiveness in wild-type and interleukin-4-deficient mice. Am J Respir Cell Mol Biol. 1995;12:254–259. doi: 10.1165/ajrcmb.12.3.7873190. [DOI] [PubMed] [Google Scholar]
- 9.Tomkinson A, Kanehiro A, Rabinovitch N, Joetham A, Cieslewicz G, Gelfand EW. The failure of STAT6-deficient mice to develop airway eosinophilia and airway hyperresponsiveness is overcome by interleukin-5. Am J Respir Crit Care Med. 1999;160:1283–1291. doi: 10.1164/ajrccm.160.4.9809065. [DOI] [PubMed] [Google Scholar]
- 10.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki M, Zheng X, Zhang X, Li M, Vladau C, Ichim TE, Sun H, Min LR, Garcia B, Min WP. Novel vaccination for allergy through gene silencing of CD40 using small interfering RNA. J Immunol. 2008;180:8461–8469. doi: 10.4049/jimmunol.180.12.8461. [DOI] [PubMed] [Google Scholar]
- 12.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 13.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–1071. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 14.Salvi SS, Babu KS. Treatment of allergic asthma with monoclonal anti-IgE antibody. N Engl J Med. 2000;342:1292–1293. doi: 10.1056/NEJM200004273421715. [DOI] [PubMed] [Google Scholar]
- 15.Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354:2689–2695. doi: 10.1056/NEJMct055184. [DOI] [PubMed] [Google Scholar]
- 16.Coyle AJ, Wagner K, Bertrand C, Tsuyuki S, Bews J, Heusser C. Central role of immunoglobulin (Ig) E in the induction of lung eosinophil infiltration and T helper 2 cell cytokine production: inhibition by a non-anaphylactogenic anti-IgE antibody. J Exp Med. 1996;183:1303–1310. doi: 10.1084/jem.183.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, Fear D, Smurthwaite L. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 18.Cruse G, Kaur D, Yang W, Duffy SM, Brightling CE, Bradding P. Activation of human lung mast cells by monomeric immunoglobulin E. Eur Respir J. 2005;25:858–863. doi: 10.1183/09031936.05.00091704. [DOI] [PubMed] [Google Scholar]
- 19.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 20.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka H, Komai M, Nagao K, Ishizaki M, Kajiwara D, Takatsu K, Delespesse G, Nagai H. Role of interleukin-5 and eosinophils in allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol. 2004;31:62–68. doi: 10.1165/rcmb.2003-0305OC. [DOI] [PubMed] [Google Scholar]
- 22.Van Oosterhout AJ, Ladenius AR, Savelkoul HF, Van Ark I, Delsman KC, Nijkamp FP. Effect of anti-IL-5 and IL-5 on airway hyperreactivity and eosinophils in guinea pigs. Am Rev Respir Dis. 1993;147:548–552. doi: 10.1164/ajrccm/147.3.548. [DOI] [PubMed] [Google Scholar]
- 23.Corrigan CJ, Haczku A, Gemou-Engesaeth V, Doi S, Kikuchi Y, Takatsu K, Durham SR, Kay AB. CD4 T-lymphocyte activation in asthma is accompanied by increased serum concentrations of interleukin-5. Effect of glucocorticoid therapy. Am Rev Respir Dis. 1993;147:540–547. doi: 10.1164/ajrccm/147.3.540. [DOI] [PubMed] [Google Scholar]
- 24.Hamid Q, Azzawi M, Ying S, Moqbel R, Wardlaw AJ, Corrigan CJ, Bradley B, Durham SR, Collins JV, Jeffery PK, et al. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991;87:1541–1546. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalo JA, Lloyd CM, Wen D, Albar JP, Wells TN, Proudfoot A, Martinez-A C, Dorf M, Bjerke T, Coyle AJ, et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188:157–167. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang M, Hogan SP, Mahalingam S, Pope SM, Zimmermann N, Fulkerson P, Dent LA, Young IG, Matthaei KI, Rothenberg ME, et al. Eotaxin-2 and IL-5 cooperate in the lung to regulate IL-13 production and airway eosinophilia and hyperreactivity. J Allergy Clin Immunol. 2003;112:935–943. doi: 10.1016/j.jaci.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Mould AW, Matthaei KI, Young IG, Foster PS. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Invest. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore PE, Church TL, Chism DD, Panettieri RA, Jr, Shore SA. IL-13 and IL-4 cause eotaxin release in human airway smooth muscle cells: a role for ERK. Am J Physiol Lung Cell Mol Physiol. 2002;282:L847–L853. doi: 10.1152/ajplung.00245.2001. [DOI] [PubMed] [Google Scholar]
- 30.Ceci JD, Patriotis CP, Tsatsanis C, Makris AM, Kovatch R, Swing DA, Jenkins NA, Tsichlis PN, Copeland NG. Tpl-2 is an oncogenic kinase that is activated by carboxy-terminal truncation. Genes Dev. 1997;11:688–700. doi: 10.1101/gad.11.6.688. [DOI] [PubMed] [Google Scholar]
- 31.Chiariello M, Marinissen MJ, Gutkind JS. Multiple mitogen-activated protein kinase signaling pathways connect the cot oncoprotein to the c-jun promoter and to cellular transformation. Mol Cell Biol. 2000;20:1747–1758. doi: 10.1128/mcb.20.5.1747-1758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patriotis C, Makris A, Chernoff J, Tsichlis PN. Tpl-2 acts in concert with Ras and Raf-1 to activate mitogen-activated protein kinase. Proc Natl Acad Sci USA. 1994;91:9755–9759. doi: 10.1073/pnas.91.21.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsatsanis C, Patriotis C, Bear SE, Tsichlis PN. The Tpl-2 protooncoprotein activates the nuclear factor of activated T cells and induces interleukin 2 expression in T cell lines. Proc Natl Acad Sci USA. 1998;95:3827–3832. doi: 10.1073/pnas.95.7.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsatsanis C, Patriotis C, Tsichlis PN. Tpl-2 induces IL-2 expression in T-cell lines by triggering multiple signaling pathways that activate NFAT and NF-kappaB. Oncogene. 1998;17:2609–2618. doi: 10.1038/sj.onc.1202460. [DOI] [PubMed] [Google Scholar]
- 35.Belich MP, Salmerón A, Johnston LH, Ley SC. TPL-2 kinase regulates the proteolysis of the NF-kappaB-inhibitory protein NF-kappaB1 p105. Nature. 1999;397:363–368. doi: 10.1038/16946. [DOI] [PubMed] [Google Scholar]
- 36.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, et al. TNF- induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 37.Hatziapostolou M, Polytarchou C, Panutsopulos D, Covic L, Tsichlis PN. Proteinase-activated receptor-1-triggered activation of tumor progression locus-2 promotes actin cytoskeleton reorganization and cell migration. Cancer Res. 2008;68:1851–1861. doi: 10.1158/0008-5472.CAN-07-5793. [DOI] [PubMed] [Google Scholar]
- 38.Eliopoulos AG, Dumitru CD, Wang CC, Cho J, Tsichlis PN. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J. 2002;21:4831–4840. doi: 10.1093/emboj/cdf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das S, Cho J, Lambertz I, Kelliher MA, Eliopoulos AG, Du K, Tsichlis PN. Tpl2/cot signals activate ERK, JNK, and NF-kappaB in a cell-type and stimulus-specific manner. J Biol Chem. 2005;280:23748–23757. doi: 10.1074/jbc.M412837200. [DOI] [PubMed] [Google Scholar]
- 40.Kaiser F, Cook D, Papoutsopoulou S, Rajsbaum R, Wu X, Yang HT, Grant S, Ricciardi-Castagnoli P, Tsichlis PN, Ley SC, et al. TPL-2 negatively regulates interferon-β production in macrophages and myeloid dendritic cells. J Exp Med. 2009;206:1863–1871. doi: 10.1084/jem.20091059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsatsanis C, Vaporidi K, Zacharioudaki V, Androulidaki A, Sykulev Y, Margioris AN, Tsichlis PN. Tpl2 and ERK transduce antiproliferative T cell receptor signals and inhibit transformation of chronically stimulated T cells. Proc Natl Acad Sci USA. 2008;105:2987–2992. doi: 10.1073/pnas.0708381104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watford WT, Hissong BD, Durant LR, Yamane H, Muul LM, Kanno Y, Tato CM, Ramos HL, Berger AE, Mielke L, et al. Tpl2 kinase regulates T cell interfereon-gamma production and host resistance to Toxoplasma gondii. J Exp Med. 2008;205:2803–2812. doi: 10.1084/jem.20081461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based - and β-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 44.Charles N, Watford WT, Ramos HL, Hellman L, Oettgen HC, Gomez G, Ryan JJ, O’Shea JJ, Rivera J. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity. 2009;30:533–543. doi: 10.1016/j.immuni.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP, Pucillo CE. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strath M, Warren DJ, Sanderson CJ. Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J Immunol Methods. 1985;83:209–215. doi: 10.1016/0022-1759(85)90242-x. [DOI] [PubMed] [Google Scholar]
- 47.Eliopoulos AG, Wang CC, Dumitru CD, Tsichlis PN. Tpl2 transduces CD40 and TNF signals that activate ERK and regulates IgE induction by CD40. EMBO J. 2003;22:3855–3864. doi: 10.1093/emboj/cdg386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, Bluethmann H. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994;24:73–80. doi: 10.1111/j.1365-2222.1994.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 49.Yamaoka K, Min B, Zhou YJ, Paul WE, O’shea JJ. Jak3 negatively regulates dendritic-cell cytokine production and survival. Blood. 2005;106:3227–3233. doi: 10.1182/blood-2005-02-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugimoto K, Ohata M, Miyoshi J, Ishizaki H, Tsuboi N, Masuda A, Yoshikai Y, Takamoto M, Sugane K, Matsuo S, et al. A serine/threonine kinase, Cot/Tpl2, modulates bacterial DNA-induced IL-12 production and Th cell differentiation. J Clin Invest. 2004;114:857–866. doi: 10.1172/JCI20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 52.Berg SF, Mjaaland S, Fossum S. Comparing macrophages and dendritic leukocytes as antigen-presenting cells for humoral responses in vivo by antigen targeting. Eur J Immunol. 1994;24:1262–1268. doi: 10.1002/eji.1830240604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.