Abstract
The direct effector mechanisms of CD4 T cells during γ-herpesvirus 68 (γHV68)-persistent infection are less well understood than those of their CD8 T cell counterparts, although there is substantial evidence that CD4 T cells are critical for the control of persistent γ-herpesvirus infection. Our results show that in γHV68-persistently infected mice, CD4 T cells are not cytokine polyfunctional, but there is a division of labor in the CD4 T cell compartment in which CD4 T cells polarize toward two distinct populations with different effector functions: IFN-γ producers and CD107+ cytolytic effectors. These two CD4 T cell effector populations degranulate and produce IFN-γ during steady state without need for exogenous antigenic restimulation, which is fundamentally different from that observed with γHV68-specific CD8 T cells. By using anti–IFN-γ Ab depletions and IFN-γ–deficient mice, we show that CD4 T cell-mediated cytotoxicity in vivo is not dependent on IFN-γ activity. In addition, our data show that purified CD4 T cells isolated from γHV68-latently infected mice have the capacity to inhibit γHV68 reactivation from latency. Our results support the concept that CD4 T cells are critical effectors for the control of γ-herpesvirus latent infection, and they mediate this effect by two independent mechanisms: IFN-γ production and cytotoxicity.
CD4 T cells are an essential component of the immune system that coordinate adaptive immune responses at multiple levels. They are very heterogeneous in phenotype and function, and a broad spectrum of CD4 T cell responses has been observed in different settings. CD4 T cells can play a critical role, via IFN-γ production, in mediating killing against a variety of intracellular pathogens (1). In addition, CD4 T cells with a polyfunctional phenotype, defined by simultaneous production of IFN-γ and IL-2, appear to be good correlates of protective immunity during chronic viral infections (2, 3).
Murine γ-herpesvirus 68 (γHV68) has important biological similarities to its human counterparts and is a good in vivo model to dissect γ-herpesvirus–specific immune responses in the host (4, 5). Human studies using peripheral blood leukocytes from EBV-infected individuals have shown that CD4 T cells are responsive to latent and lytic EBV proteins (6), whereas studies in mice go on to show that CD4 T cells can temporally control γ-herpesvirus infection in the absence of CD8 and B cells (7). Genetic disruption of MHC class II results in death during persistent infection (8). Taken together, these data indicate that CD4 T cells are an important component for the control γ-herpesvirus infection during the latent phase of infection.
Human studies demonstrate that EBV-specific CD4 T cell clones have direct cytolytic activities against EBV-associated tumor cells. These clones target mainly latent cycle proteins (EBV-encoded nuclear Ag [EBNA]1, EBNA2, and latent membrane protein 2) but also lytic Ags (6, 9–11). In addition, EBNA1-specific cytolytic CD4 T cell clones inhibited EBV-induced B cell proliferation (12, 13). When γHV68-positive S11 lymphoma B cells were injected into nude mice, adoptively transferred CD4 T cells were most effective in preventing tumor formation (14). Using adoptive transfer experiments with RAG mice, it has been shown that CD4 T cells can temporarily control γHV68 latency (7). IFN-γ is critical in regulating γHV68 infection (15) and blocks virus reactivation from latency (16). Furthermore, the analysis of γHV68 infection has shown that CD4 T cell-mediated virus control is mediated by and requires IFN-γ (17, 18). We have previously shown that CD4 T cells elicit γHV68-specific cytolytic activity in vivo and in vitro (19). Thus, although CD4 T cells produce IFN-γ and possess cytotolytic activity during γHV68-persistent infection, the relationship between these two effector mechanisms remains unclear. The current study was initiated to analyze the multifunctional effector mechanisms that CD4 T cells use to mediate antiviral control during the latent phase of γHV68 infection.
Our data indicate that during γHV68-persistent infection, there is a division of labor in the CD4 T cell compartment in which CD4 T cells polarize toward two distinct populations with direct effector functions: IFN-γ producers and CD107+ cytolytic effectors. Furthermore, we show that CD4 T cells purified from persistently infected mice inhibit γHV68 reactivation. These results highlight the importance of effector CD4 T cells for the direct control of γ-herpesvirus–persistent infection.
Materials and Methods
Mice and viral infection
C57BL/6J, BALB/c, and IFN-γ−/− mice (BALB/c background) were obtained from The Jackson Laboratory (Bar Harbor, ME) or Harlan (Indianapolis, IN), or were bred at the Research Institute at Nationwide Children’s Hospital (Columbus, OH). γHV68, clone WUMS, was propagated and titered on monolayers of NIH3T3 fibroblasts. Mice were housed in BL2 containment under pathogen-free conditions. The Institutional Animal Care and Use Committee at the Research Institute at Nationwide Children’s Hospital approved all of the animal studies described in this study. Mice were anesthetized with 2,2,2,-tribromoethanol and intranasally inoculated with 1000 PFU γHV68 in 30 μl HBSS.
Flow cytometry analysis
Splenocytes were isolated, RBCs were lysed, and the number of cells per spleen was determined. Cells were stained with Fc block (CD16/32) and then washed. The cells were then stained with anti-CD4 (clone GK1.5) or anti-CD8 (clone 53-6.7). All Abs were purchased from eBioscience (San Diego, CA). Flow cytometry data were acquired on BD LSR II (BD Biosciences, San Jose, CA) and analyzed using FlowJo software. Gates were set using negative controls, isotype controls, and following the staining pattern of each marker on the bulk lymphocyte and CD4 population. For any given marker combination, all of the analysis gates are identical in size and position.
Intracellular cytokine staining
Single-cell suspensions were obtained as indicated above. A total of 2 × 106 cells/sample were incubated in tissue culture medium in the presence of IL-2 (10 U/ml), brefeldin A (10 μg/ml), and 1 μg/ml gp15067–83 I-Ab or whole γHV68 virus (multiplicity of infection [MOI] 10) for 5 h at 37°C. As a positive control, cells were stimulated with PMA/ionomycin. After Fc blocking, the cells were stained with Abs anti-CD4 and anti-CD8, fixed, permeabilized, and stained with anti–IFN-γ (clone XMG1.2), anti–IL-2 (clone JES6-5H4), anti–TNF-α (clone MP6-XT22), or isotype control Abs. When indicated, CD4 T cells were purified by negative selection with Mouse T cell CD4 Subset Column Kit (R&D Systems, Minneapolis, MN), and purification was confirmed by FACS analysis of CD4 expression (clone RM4-5) as >90% pure.
Degranulation assay
Single-cell suspensions obtained from spleens of naive or long-term γHV68-infected mice were generated as described above. A total of 106 splenocytes were incubated with 1 μg/ml each of anti-CD28 (clone 37.51), anti-CD49d (clone 9C10 [MRF4.B]), anti-CD107a (clone 1D4B), and anti-CD107b (clone ABL-93) and 1 μg/ml gp15067–83 I-Ab peptide or whole γHV68 virus (MOI 10) in 1 ml complete medium. PMA/ionomycin was used as a positive control. In every experiment, a negative control (anti-CD28, anti-CD49d, anti-CD107a, and anti-CD107b) was included to control for spontaneous production of cytokine and/or expression of CD107a/b. The cultures were incubated for 1 h at 37°C in 5% CO2, followed by an additional 5 h in the presence of the secretion inhibitor monensin. Intracellular IFN-γ production was determined as described above.
In vivo cytotoxicity assay
In vivo cytotoxicity was performed as described previously (19). Briefly, single-cell suspensions obtained from naive spleens were pulsed with whole γHV68 virus (MOI 10) or left unpulsed for 2 h at 37°C. The unpulsed control cells were stained with 50 nM CFSE and the relevant targets with 0.5 μM CFSE for 15 min at 37°C. The cells were thoroughly washed and combined in a 1:1 ratio, and a total of 5 × 106 cells were injected i.v. into recipient mice. The presence of CFSE-positive cells was analyzed in spleen cell suspensions from the recipient mice 40 h later. Percent-specific killing was calculated according to the formula percent-specific killing = (1 − [ratio of infected recipients/ratio of naive recipients] × 100), where ratio = (number of CFSEhigh/number of CFSElow).
IFN-γ ELISA
In vitro IFN-γ concentration was determined per manufacturer’s instructions using Mouse IFN-γ ELISA Kit II (BD Biosciences). Splenocytes from C57BL/6 mice at 3 mo postinfection (mpi) were processed into single-cell suspensions as described above and stained with anti-CD4 and anti-CD107 Abs. Cells were purified using a FACSVantage with Diva option. A total of 8 × 104 CD107+ or CD107− cells were plated in a 96-well plate and left unstimulated or stimulated with PMA/ionomycin for 22 h at 37°C. IFN-γ depletion was confirmed using sera from anti–IFN-γ–treated mice.
γHV68-lysate production and dendritic cell stimulation
NIH3T3 fibroblasts were grown to confluency and infected or not with γHV68 (MOI 2) for 3 h. Excess virus was removed, and cells were incubated 3 d until cytopathic effect was obvious. Then, 3T3 cells were resuspended in glycine buffer, sonicated, and stored at −80°C as 3T3-control fibroblasts or 3T3 cells containing γHV68 Ags (termed 3T3/γHV68). DC2.4 cells (20) were pulsed overnight with the supernatant of 3T3-control (dendritic cell [DC] control) or 3T3/γHV68 (DC γHV68). CD4 splenocytes were isolated from naive or long-term γHV68-infected mice and purified by negative selection with Mouse T cell CD4 Subset Column Kit (R&D Systems) to eliminate APCs carrying γHV68 Ags. Next, CD4 T cells were used directly ex vivo (unrested sample) or rested overnight at 37°C in the absence of other contaminating cell populations (rested sample). Unrested or rested cells were left unstimulated or stimulated with DC control or DC γHV68 for 4 h, following an intracellular cytokine staining protocol as described above.
Viral reactivation assay
On day 0, 105 NIH3T3 fibroblasts were plated on a 12-well plate. On day 1, CD4 splenocytes were positively selected from noninfected control C57BL/6 mice and from 3 mpi γHV68-infected mice using columns from Miltenyi Biotec (Auburn, CA). As a source of latently infected cells, splenocytes isolated from 14 or 21 d postinfection γHV68-infected mice were depleted of T cells by negative selection (CD90.2−) using columns from Miltenyi Biotec. Control wells included 3T3 cells only or 3T3 cells plus 2 × 106 latently infected splenocytes. A total of 2 × 106 CD4 T cells from control or from 3 mpi mice were then added to experimental wells and overlaid with carboxymethyl cellulose after overnight incubation. In some experiments, 10 μg/ml anti-mouse IFN-γ (clone R4-6A2) or rat IgG1 isotype control Abs (eBioscience) were added to the experimental wells. After 6 d of culture, cells were fixed and stained with Giemsa (Sigma-Aldrich, St. Louis, MO). Reactivable latent virus levels were calculated by counting the number PFUs in triplicate wells.
Statistics
The Student t test was used to determine statistical significance. All error bars represent SD.
Results
CD4 T cells produce steady state IFN-γ during γHV68-latent infection
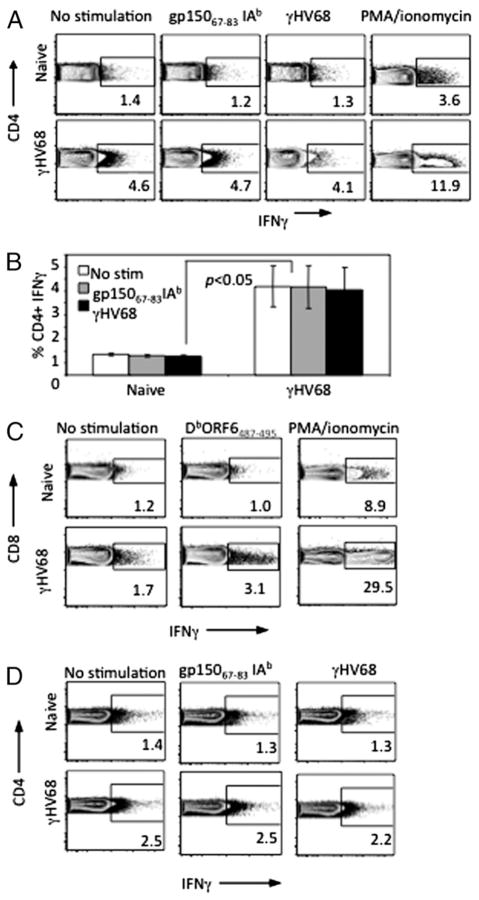
To investigate the functional properties of the CD4 T cell population during long-term (>3 mpi) infection with γHV68, we measured IFNγ production by intracellular cytokine staining. After in vitro stimulation with γHV68 peptide (gp15067–83 IAb) or whole virus, we observed a significant increase in IFN-γ production by CD4 T cells isolated from latently infected mice relative to CD4 T cells from naive controls (p < 0.05) (Fig. 1A, 1B). This increased level of IFN-γ production was also observed in the nonstimulated sample isolated from latently infected mice, and the response was not significantly different between these cells and those stimulated with peptide or whole virus. These data suggested that there was increased steady-state production of IFN-γ by CD4 T cells in γHV68-persistently infected mice when compared with their noninfected control counterparts. We used PMA/ionomycin as a positive control to demonstrate the potential of cells from both control and experimental groups to produce IFNγ (Fig. 1A, right column). A similar assay was used to examine the pattern of intracellular IFN-γ production by CD8 T cells. The γHV68-specific peptide DbORF6487–495 was used to stimulate virus-specific CD8 T cells. The data show that CD8 T cells require in vitro restimulation to generate an IFN-γ response (Fig. 1C). These results indicated that continuous IFN-γ production during steady-state persistent γHV68 infection is specific to CD4 T cells and not to CD8 T cells.
FIGURE 1.
CD4 T cells continuously produce IFN-γ during γ-herpesvirus infection. A, Intracellular cytokine staining of CD4 T cell splenocytes from naive or long-term (>3 mpi) γHV68-infected mice. Cells were left unstimulated, stimulated with gp15067–83 IAb peptide, whole virus, or PMA/ionomycin. Five hours later, intracellular IFN-γ production was measured by FACS. B, Bar diagram of one representative experiment measuring CD4/IFN-γ–positive cells from three mice per group. C, Intracellular cytokine staining of CD8 cells from naive or long-term γHV68-infected mice left unstimulated or stimulated with the class I-specific peptide DbORF6487–495 or PMA/ionomycin. D, CD4 T cells isolated from naive or long-term γHV68-infected mice were purified by negative selection and left unstimulated or stimulated with gp15067–83 IAb peptide or whole virus. Five hours later, intracellular IFN-γ production was measured by FACS. Error bars represent SD.
It remained possible that our findings may be explained by the presence of latently infected cells or APCs presenting viral Ags in the context of class II molecules in our latently infected samples. Therefore, to eliminate APCs that may carry endogenous Ag/virus, we purified CD4 T cells from naive and long-term γHV68-infected mice and incubated them in vitro with APCs isolated from naive mice. We found that purified CD4 T cells incubated with naive APCs in the absence of viral Ags showed the same pattern of continuous IFN-γ production as described above with bulk splenocytes isolated from latently infected mice (Fig. 1D). These data demonstrate that CD4 T cells isolated from mice latently infected with γHV68 produce IFN-γ ex vivo without requiring Ag restimulation. Altogether, our results indicate that during the latency phase of γHV68 infection a population of CD4 T cells is constantly producing IFN-γ.
Steady-state CD4 T cell production of IFN-γ is γHV68 specific and regulated by mechanisms different from IL-2/TNF-α
So far we have shown a significant difference between IFN-γ production by CD4 T cells isolated from noninfected and long-term γHV68-infected mice. Next, we sought to confirm that our observations were virus specific. Few MHC class II epitopes have been described during γHV68 infection of mice (21), and the frequency of each Ag-specific CD4 T cell population during the persistent phase of infection is too low to perform functional assays. Therefore, we used the lysate from γHV68-infected 3T3 fibroblasts as a source of virus-specific Ags following a similar approach to that used to analyze CMV-specific CD4 T cell responses (22). As described in Materials and Methods, 3T3 fibroblasts were infected with γHV68 (3T3/γHV68) or not (3T3 control), and the cell pellet was sonicated and used to pulse bone marrow-derived DCs. The 3T3 control-pulsed (DC control) or 3T3/γHV68-pulsed (DC γHV68) DC 2.4 cells were then used to stimulate CD4 T cells that had previously been isolated from the spleen of naive or long-term γHV68-infected mice and purified by negative selection. The purified CD4 T cells were incubated directly ex vivo (unrested sample) with DC control or DC γHV68 (Fig. 2A, 2B) or rested 24 h in the absence of APCs (Fig. 2C, 2D). We rested the CD4 T cells to eliminate endogenous IFN-γ production prior to restimulation. The rested CD4 T cells were subsequently restimulated with DC control or DC γHV68 APCs. The data show that in vitro stimulation of unrested CD4 T cells with DC control or DC γHV68 produces similar levels of intracellular IFN-γ. This pattern is consistent with that observed in Fig. 1 demonstrating steady-state IFN-γ with little change between control and virus stimulation. In contrast, we observed that first resting CD4 T cells eliminates the steady-state production of IFN-γ noted by reduced levels of intracellular IFN-γ after stimulation with DC control supernatant. However, we detected a significant increase in IFN-γ production (p < 0.005) following stimulation with DC γHV68 APCs (Fig. 2C, 2D). Taken together, these findings confirm the existence of a population of γHV68-specific effector CD4 T cells that continuously produce IFN-γ during long-term latent infection.
FIGURE 2.
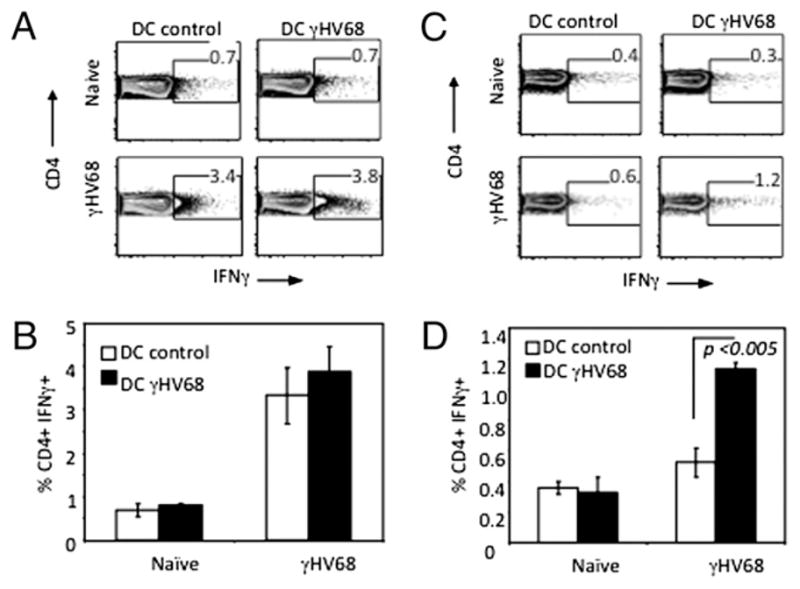
CD4 T cell production of IFN-γ is γHV68 specific. CD4 T cells were isolated from spleens of noninfected controls or long-term γHV68-infected mice and purified by negative selection. The purified CD4 cells were stimulated immediately with DCs that had been pulsed with control 3T3 lysates (DC control) or pulsed with lysates of 3T3 fibroblasts infected with γHV68 (DC γHV68) (A, B) or rested overnight in culture media and then stimulated as described above (C, D). Five hours after stimulation, intracellular cytokine staining of IFN-γ production by the CD4 splenocytes was measured by FACS. Error bars represent SD.
CD4 T cells with a polyfunctional phenotype are thought to be good correlates of protective immunity during virus persistence (2, 3). However, the polyfunctionality of CD4 T cells during γHV68-persistent infection remains poorly characterized. To examine the polyfunctional response of CD4 T cells during latent γHV68 infection, we analyzed TNF-α and IL-2 production by intracellular cytokine staining. Our data show that CD4 T cells from γHV68-latently infected mice do not produce TNF-α (Fig. 3A) or IL-2 (Fig. 3B) under short-term stimulatory conditions similar to those that revealed steady-state production of IFN-γ. Stimulation with PMA/ionomycin demonstrated that CD4 T cells from latently infected had retained the potential to produce TNF-α and IL-2. These results suggest the absence of polyfunctional CD4 T cells as measured by their capacity to produce IFN-γ/TNF-α/IL-2 during γHV68-persistent infection, which is fundamentally different from other persistent viral infections, such as CMV, HSV, and non-progressive HIV (2, 3). Our results indicate that IFN-γ, TNF-α, and IL-2 production by γHV68-specific CD4 T cells is differentially regulated, and polyfuctional CD4 T cells are not a hallmark of γHV68-persistent infection.
FIGURE 3.
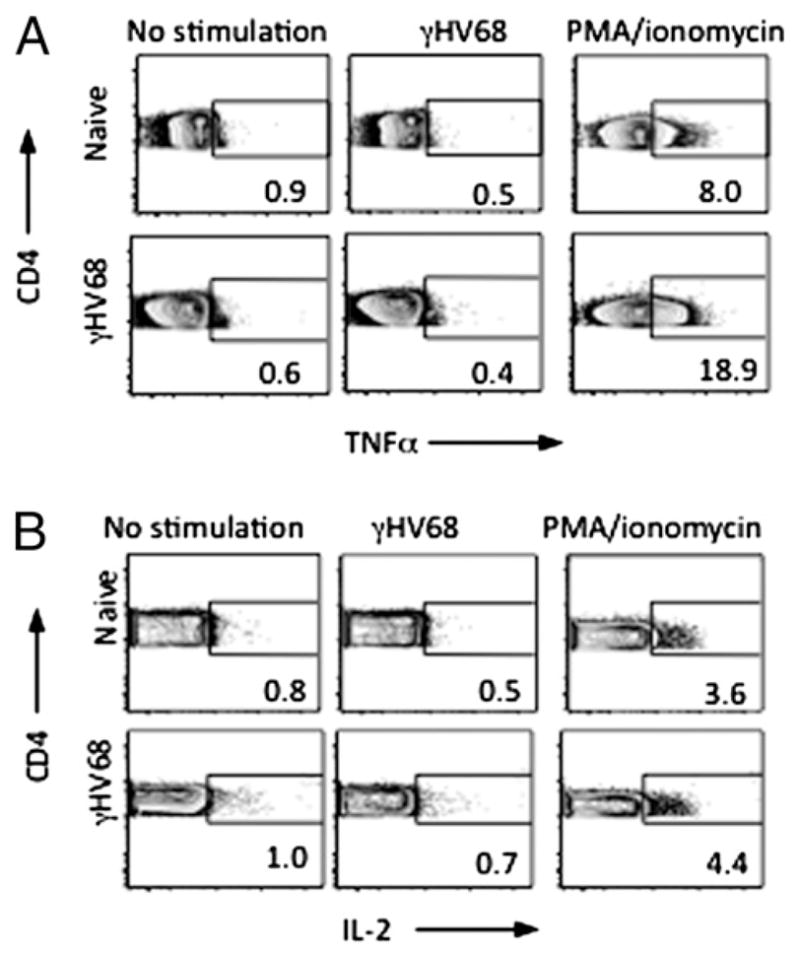
CD4 T cells are not cytokine polyfunctional during long-term γHV68 infection. Intracellular cytokine staining of TNF-α (A) and IL-2 (B) production by CD4 T cell splenocytes was performed with naive or long-term (>3 mpi) γHV68-infected mice. Cells were left unstimulated or stimulated with whole virus or PMA/ionomycin. Five hours later, intracellular TNF-α or IL-2 production was measured by FACS.
Division of labor among CD4 T cells during γHV68 persistence: cytotoxicity and IFN-γ production
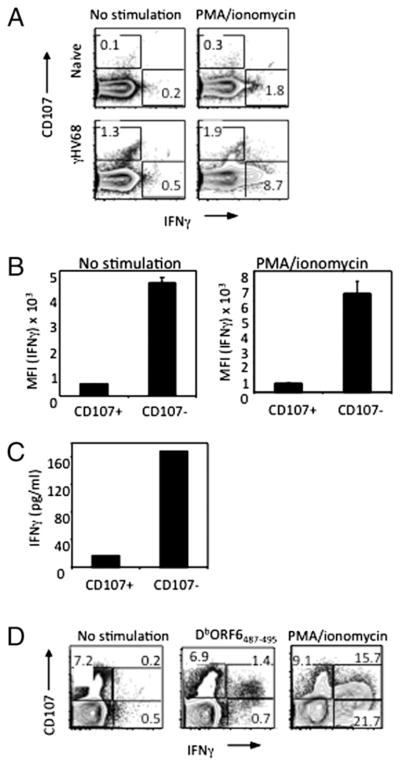
We have previously identified a cytolytic CD4 T cell population during latent γHV68 infection capable of killing γHV68-loaded targets in vitro and in vivo (19). Our studies further demonstrated steady-state expression of CD107, a surrogate marker of cytolytic T cells (23), on CD4 T cells similar to the pattern observed with intracellular IFN-γ production (Figs. 1, 2). Therefore, we sought to investigate whether CD4 T cells during γHV68-latent infection are multifunctional with regard to IFN-γ production and CD107 degranulation or whether they polarize toward one of those two different effector mechanisms. First, we performed in vitro degranulation assays in conjunction with an intracellular IFN-γ staining. Bulk splenocytes were isolated from naive or long-term γHV68-infected mice and left unstimulated or stimulated with PMA/ionomycin. The results in Fig. 4A show the existence of two distinct CD4 T cell populations, one capable of degranulating (CD107+IFNγ−) and the other producing IFN-γ (CD107−IFNγ+). Analysis of the mean IFN-γ fluorescence intensity further supports the conclusion that the CD107+ population is distinct from the CD107−population in terms of IFN-γ production (Fig. 4B). Second, we purified CD107+ and CD107− CD4 T cell populations by flow cytometry cell sorting. Purified subsets were left unstimulated or stimulated with PMA/ionomycin for 48 h, and IFN-γ secretion was measured in the supernatant by ELISA. Our results show increased secretion of IFN-γ protein by purified CD107− cells relative to CD107+ cells (Fig. 4C). Importantly, the results obtained with CD4 T cells are distinct from those of CD8 T cells in which the cytolytic CD107+ cells are also IFN-γ+ after stimulation with the class I-restricted epitope DbORF6487–495 (Fig. 4D).
FIGURE 4.
Cytotoxic CD4 T cells do not produce IFN-γ. A, Intracellular expression of IFN-γ and cell surface mobilization of CD107 on CD4 T cell splenocytes isolated from naive or long-term (>3 mpi) γHV68-infected mice after 5 h in vitro restimulation with or without PMA/ionomycin. B, Mean fluorescent intensity of IFN-γ expression on CD107+ and CD107− CD4 T cells. C, ELISA analysis measuring IFN-γ protein in the supernatant of FACS-purified CD107+ and CD107− CD4 T cells. D, Intracellular expression of IFN-γ and cell surface expression of CD107 on CD8 T cell splenocytes isolated from naive or long-term γHV68-infected mice analyzed as described above. Error bars represent SD.
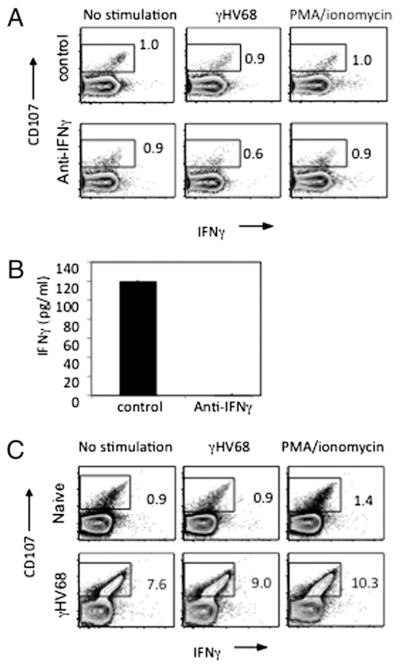
Having identified two different effector CD4 T cell populations, we sought to determine whether IFN-γ was necessary for the acquisition of cytotoxic capabilities by CD4 T cells. To do so, we used two complementary approaches: in vivo neutralization of IFN-γ by Ab administration and the use of transgenic mice deficient in IFN-γ production (IFN-γ−/−). In these two complementary models, we determined the capacity of CD4 T cells to mobilize CD107 and to elicit in vivo cytotoxicity of γHV68-loaded targets in the absence of IFN-γ stimulation. As shown in Fig. 5A, CD4 T cells isolated from long-term γHV68-infected mice are capable of CD107 cell surface mobilization in the presence or absence of Abs specific for IFN-γ. IFN-γ depletion was confirmed using the serum of mice treated with isotype control or anti–IFN-γ Abs (Fig. 5B). A similar assay was performed to measure CD107 cell surface expression in IFN-γ−/− mice during long-term infection. When compared with noninfected control IFN-γ−/− mice, increased CD107 expression was observed in CD4 T cells isolated from persistently infected IFN-γ−/− mice (Fig. 5C). These differences in expression are consistent with those previously observed by us in wild-type mice containing normal levels of IFN-γ (19).
FIGURE 5.
CD4 T cells degranulate in the absence of IFN-γ. A, IFN-γ was neutralized in long-term (>3 mpi) γHV68-infected mice by in vivo administration of anti–IFN-γ Ab for 1 wk prior to a degranulation assay using isotype-treated γHV68-infected mice as controls. CD4 T cell splenocytes were left unstimulated or stimulated with virus or PMA/ionomycin. Five hours later, surface CD107 and intracellular IFN-γ was measured by FACS. B, IFN-γ neutralization was confirmed by ELISA in the serum of anti–IFN-γ–treated mice relative to mice treated with isotype control Ab. C, Analysis of CD107 degranulation on long-term (>3 mpi) γHV68-infected IFN-γ−/− mice compared with noninfected IFN-γ−/− controls. Error bars represent SD.
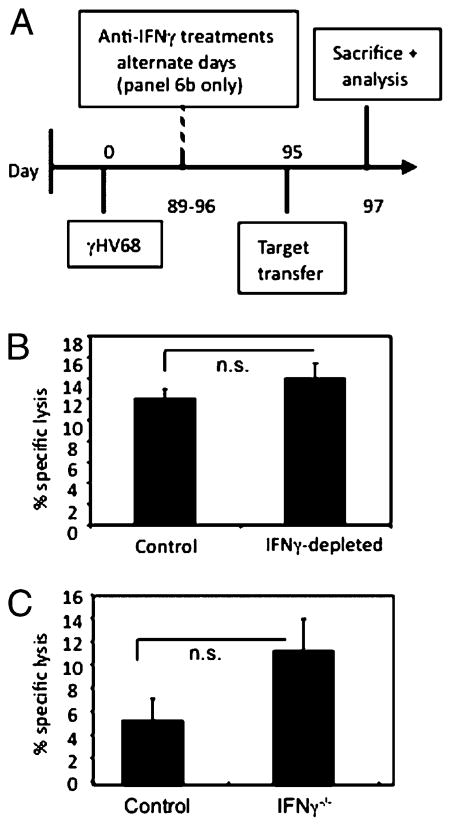
To determine the ability of CD4 T cells to elicit direct control by cytotoxicity in the absence of IFN-γ, we performed in vivo cytotoxicity assays during IFN-γ neutralization or using IFN-γ−/− mice as described before (19). As shown in Fig. 6A, CFSE-labeled target cells were transferred into γHV68-infected mice at 3 mpi in which IFN-γ was neutralized by Ab administration. Forty hours later, recipient mice were sacrificed, and percentage-specific lysis was calculated as described in Materials and Methods. As shown in Fig. 6B, CD4 cells maintain a consistent level of cytotoxicity after in vivo treatment with anti–IFN-γ or isotype control. These findings suggest that in the absence of IFN-γ, CD4 T cells do not significantly change their level of cytotoxic activity. In vivo cytotoxicity was also measured using IFN-γ−/− recipient mice (Fig. 6C). Similar to the protocol described in Fig. 6A, CFSE-labeled target cells were transferred into naive or 3 mpi IFN-γ−/− recipient mice. Using the calculation described in Materials and Methods, we compared the percentage-specific lysis of naive and 3 mpi wild-type or IFN-γ−/− recipient mice and observed no significant difference between the two groups. The degranulation and in vivo cytotoxicity analyses suggest that CD4 T cells maintain cytotoxic ability in vivo in the absence of IFN-γ.
FIGURE 6.
CD4 T cells maintain cytotoxic activity in the absence of IFN-γ. A, Experimental design of the in vivo cytotoxicity assay. IFN-γ neutralization was confirmed by ELISA. Forty hours after target cell transfer, recipient mice were sacrificed, and the percentage-specific lysis was calculated as described in Materials and Methods. B, Bar diagram showing the percentage of specific lysis after in vivo IFN-γ neutralization or isotype control treatment. C, Bar diagram shows the percentage of specific lysis of long-term infected IFN-γ−/− mice relative to long-term infected wild-type controls. Error bars represent SD.
CD4 T cells directly impair γHV68 reactivation
To confirm our hypothesis that CD4 T cells are capable of direct control of latent γ-herpesvirus infection, we performed a viral reaction assay in the presence of CD4 T cells. Enriched APCs (CD90.2−) isolated from day 14 γHV68-infected mice were plated on a confluent 3T3 fibroblast monolayer. Purified CD4 T cells isolated from non-infected or from long-term γHV68-infected mice (3 mpi) were then added to the assay, and formation of viral plaques was monitored 1 wk later. The results show that the samples with control CD4 T cells isolated from noninfected mice developed a similar number of viral plaques to that observed in the wells containing only infected APCs (Fig. 7A). In contrast, addition of CD4 T cells isolated from persistently infected mice abrogated the ability of γHV68 to reactivate and form plaques in the 3T3 monolayer. These results demonstrate that during latent γ-herpesvirus infection, CD4 T cells have the ability to directly inhibit γHV68 reactivation and the production of infectious virus. To explore the mechanism responsible for CD4 T cell inhibition of γHV68 reactivation, we performed similar viral reaction assays in the presence of CD4 T cells isolated from long-term γHV68-infected mice by adding or not adding anti–IFN-γ blocking Abs (Fig. 7B). The data show that the presence of anti–IFN-γ blocking Abs abrogated the capacity of long-term CD4 T cells to suppress γHV68 reactivation. Thus, our results indicate that IFN-γ produced by long-term CD4 T cells is a mechanism directly responsible for the inhibition of γHV68 reactivation.
FIGURE 7.
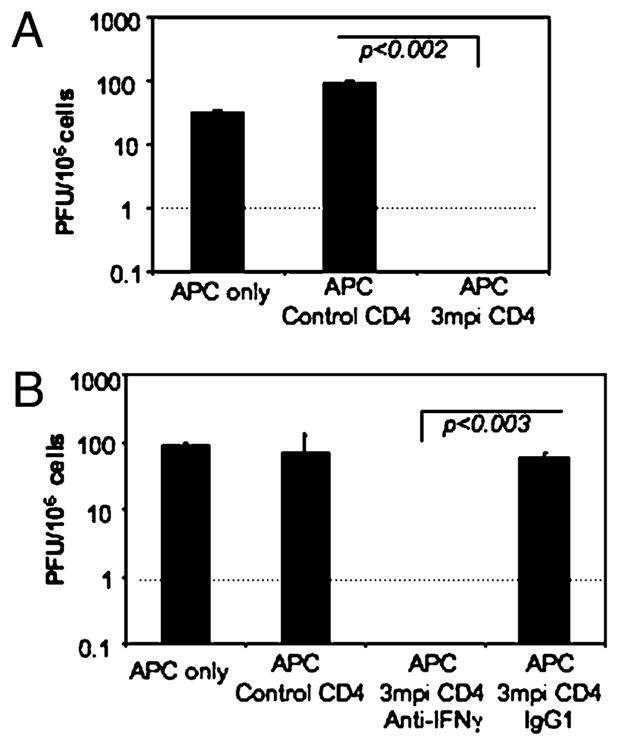
CD4 T cells control viral reactivation of γHV68-infected APCs. A, A modified infectious center assay was used to determine the capacity of CD4 T cells for controlling γHV68 reactivation from latency. γHV68-latently infected APCs were purified (CD90.2−) from mice at 14 d postinfection and plated on a fibroblast monolayer. Purified CD4 T cells from noninfected (control CD4) or long-term γHV68-infected (3 mpi CD4) mice were then added to the assay. PFUs were determined 6 d later. The dotted line represents the limit of detection of the assay. Error bars represent SD. B, During a similar reactivation experiment, anti–IFN-γ blocking Ab or IgG1 isotype control was added at 10 μg/ml to determine the effect of IFN-γ produced by CD4 T cells on γHV68 reactivation. The dotted line represents the limit of detection of the assay. Error bars represent SD.
Discussion
Our findings add to the growing information indicating that CD4 T cells play a major role in the direct control of γHV68-persistent infection. We have shown that CD4 T cells continuously degranulate or produce IFN-γ in γHV68-latently infected hosts. We identified two distinct populations of effector CD4 T cells during γHV68 persistence: a population of CD4 T cells capable of degranulating that does not simultaneously produce IFN-γ, which is distinct from a population that produces high levels of IFN-γ but fails to degranulate. The steady-state effector activity of CD4 T cells during γHV68 persistence is fundamentally different from that of CD8 T cells, which need exogenous Ag restimulation to elicit effector function, and suggests that CD4 T cells are continuously recognizing viral Ags in the mouse.
CD4 T cells with a polyfunctional phenotype, defined by simultaneous production of IFN-γ and IL-2, appear to be good correlates of protective immunity (2, 3). It is interesting to note that we found that neither IL-2 nor TNF-α production by CD4 T cells follow the same steady-state production pattern observed with IFN-γ. In fact, neither cytokine is produced after short-term stimulation with γHV68, although CD4 T cells maintain the potential to produce IL-2 and TNF-α as shown in the samples stimulated with PMA/ionomycin. Thus, these results suggest differential regulation of distinct cytokines and argue against a polyfunctional cytokine phenotype of CD4 T cells during persistent γHV68 infection.
Our analysis identified two populations of CD4 T cells with effector function. Cytolytic CD107+ CD4 T cells do not produce IFN-γ (IFN-γ−) relative to the IFN-γ+ noncytolytic CD107− CD4 population isolated from the same mice. These findings were corroborated when CD107+ and CD107− CD4 T cells were purified and stimulated in vitro. Intracellular cytokine staining and ELISA analysis demonstrated lower IFN-γ protein produced by CD107+ CD4 T cells relative to CD107− CD4 T cells. These two effector populations degranulate and produce IFN-γ during steady state without need for exogenous antigenic restimulation. Two observations indicate that this steady-state effector activity is unique to CD4 T cells. First, CD8 T cells require restimulation in vitro to produce IFN-γ (our data and Ref. 24). The steady-state production of IFN-γ in CD4 T cells (4.5%) is higher than in CD8 T cells (1.7%) isolated from the spleens of persistently infected mice. Second, simultaneous analysis of CD107 and IFN-γ shows that among CD8 T cells there is a subpopulation of dual-functional CD107+IFN-γ+ effector cells that respond to antigenic stimulation that is not detected in the CD4 T cell compartment. Collectively, these results suggest that in γHV68-latently infected mice CD4 T cells are continuously exposed to persistent Ag stimulation, which results in continuous steady-state degranulation and IFN-γ production by two distinct populations of effector CD4 T cells. Importantly, our data demonstrate that this effector activity is γHV68 specific because it wanes in the absence of APCs isolated from persistently infected mice, and it is subsequently triggered by exposure to γHV68 Ags obtained from infected cell lysates.
Our current findings are in accord with our previous work in which we demonstrated steady-state degranulation of CD4 T cells during long-term γHV68 infection (CD107+) (19). The similarities between the patterns of degranulation and IFN-γ production by two independent effector populations were intriguing. Therefore, we investigated the interdependence of these two viral control mechanisms. Using IFN-γ Ab neutralization in vivo and an IFN-γ–deficient mouse model, we found that in the absence of IFN-γ CD4 T cells remained cytolytic in vivo during latent infection. These data corroborate that one subpopulation of CD4 T cells is responsible for degranulation, whereas a separate produces IFN-γ, and that these two populations are not functionally dependent on one another as CD4 T cells maintain their cytolytic activity in vivo in the absence of IFN-γ. Thus, the CD4 T cell response during γHV68 persistence that is not polyfunctional in terms of one population producing several cytokines but heterogeneous in that distinct CD4 T cell populations acquire different effector functions with the potential to engage in viral control.
Our data are consistent with other studies showing that CD4 T cells play a critical role in regulating persistent γHV68 by direct antiviral functions that are independent of CD8 T cells and B cells (7). CD4 T cells control γHV68 infection by IFN-γ (17, 18), and we have recently shown that γHV68-specific CD4 T cells are cytotoxic during latent infection (19). In this study, we show that during γHV68 persistence continuous IFN-γ production and cytotoxicity are mediated by two distinct subsets of effector CD4 T cells. The ability of γHV68-specific CD4 T cells to elicit direct antiviral functions is corroborated by the capacity of purified CD4 T cells isolated from latently infected mice to inhibit γHV68 reactivation by an IFN-γ–dependent process during an infectious center assay in which other components of the immune system are lacking. Nevertheless, it should be noted that these virus reactivation assays use splenocytes at the peak of the latency phase, which are expected to be quite antigenic and where γHV68 is highly reactivatable. It is still formally possible that long-term latency may be controlled by different means.
Recent studies of the immune response against latent EBV Ags have revealed consistent glycoprotein-, EBNA1-, and latent membrane protein 1-specific recognition by CD4 T cells of healthy carriers. Importantly, these class II-restricted responses are more frequent than those of any class I-restricted Ag (6, 9, 11, 25). Human EBV-specific CD4 T cells can be cytotoxic and secrete IFN-γ (6, 11, 12, 26, 27). Altogether, the information provided by the γHV68 mouse model demonstrating that CD4 T cells elicit direct antiviral effector functions (7, 17–19, and this paper) and the evidence from EBV studies highlight the concept that CD4 T cells are critical effectors for the control of γ-herpesvirus–latent infection. It is tempting to speculate that this CD4 T cell-mediated effector control found during EBV and γHV68 persistence may be an adaptation of the immune system to better control pathogens with marked tropism for MHC class II-positive B cells.
Acknowledgments
We thank C. McAllister and D. Dunaway of the Nationwide Children’s Hospital Flow Cytometry Core Laboratory.
This work was supported in part by National Institutes of Health Grant AI-59603 and by The Research Institute.
Abbreviations used in this paper
- DC
dendritic cell
- EBNA
EBV-encoded nuclear Ag
- γHV68
γ-herpesvirus 68
- MOI
multiplicity of infection
- mpi
month postinfection
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Foulds KE, Wu CY, Seder RA. Th1 memory: implications for vaccine development. Immunol Rev. 2006;211:58–66. doi: 10.1111/j.0105-2896.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 2.Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA, Pantaleo G. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol Rev. 2006;211:236–254. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 3.Pantaleo G, Harari A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat Rev Immunol. 2006;6:417–423. doi: 10.1038/nri1840. [DOI] [PubMed] [Google Scholar]
- 4.Doherty PC, Christensen JP, Belz GT, Stevenson PG, Sangster MY. Dissecting the host response to a γ-herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356:581–593. doi: 10.1098/rstb.2000.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virgin HW. Immune regulation of viral infection and vice versa. Immunol Res. 2005;32:293–315. doi: 10.1385/IR:32:1-3:293. [DOI] [PubMed] [Google Scholar]
- 6.Münz C, Bickham KL, Subklewe M, Tsang ML, Chahroudi A, Kurilla MG, Zhang D, O’Donnell M, Steinman RM. Human CD4+ T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J Exp Med. 2000;191:1649–1660. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sparks-Thissen RL, Braaten DC, Kreher S, Speck SH, Virgin HW., IV An optimized CD4 T-cell response can control productive and latent gammaherpesvirus infection. J Virol. 2004;78:6827–6835. doi: 10.1128/JVI.78.13.6827-6835.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a γ-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munz C. Immune response and evasion in the host-EBV interaction. In: Robertson ER, editor. Epstein-Barr Virus. Caister Academic Press; Norfolk, U.K: 2005. pp. 197–231. [Google Scholar]
- 10.Landais E, Saulquin X, Scotet E, Trautmann L, Peyrat MA, Yates JL, Kwok WW, Bonneville M, Houssaint E. Direct killing of Epstein-Barr virus (EBV)-infected B cells by CD4 T cells directed against the EBV lytic protein BHRF1. Blood. 2004;103:1408–1416. doi: 10.1182/blood-2003-03-0930. [DOI] [PubMed] [Google Scholar]
- 11.Adhikary D, Behrends U, Moosmann A, Witter K, Bornkamm GW, Mautner J. Control of Epstein-Barr virus infection in vitro by T helper cells specific for virion glycoproteins. J Exp Med. 2006;203:995–1006. doi: 10.1084/jem.20051287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paludan C, Bickham K, Nikiforow S, Tsang ML, Goodman K, Hanekom WA, Fonteneau JF, Stevanović S, Münz C. Epstein-Barr nuclear antigen 1-specific CD4+ Th1 cells kill Burkitt’s lymphoma cells. J Immunol. 2002;169:1593–1603. doi: 10.4049/jimmunol.169.3.1593. (PubMed) [DOI] [PubMed] [Google Scholar]
- 13.Nikiforow S, Bottomly K, Miller G. CD4+ T-cell effectors inhibit Epstein-Barr virus-induced B-cell proliferation. J Virol. 2001;75:3740–3752. doi: 10.1128/JVI.75.8.3740-3752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson KA, Usherwood EJ, Nash AA. Regression of a murine gammaherpesvirus 68-positive B-cell lymphoma mediated by CD4 T lymphocytes. J Virol. 2001;75:3480–3482. doi: 10.1128/JVI.75.7.3480-3482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weck KE, Dal Canto AJ, Gould JD, O’Guin AK, Roth KA, Saffitz JE, Speck SH, Virgin HW. Murine γ-herpesvirus 68 causes severe large-vessel arteritis in mice lacking interferon-γ responsiveness: a new model for virus-induced vascular disease. Nat Med. 1997;3:1346–1353. doi: 10.1038/nm1297-1346. [see comments] [DOI] [PubMed] [Google Scholar]
- 16.Steed AL, Barton ES, Tibbetts SA, Popkin DL, Lutzke ML, Rochford R, Virgin HW., IV γ Interferon blocks gammaherpesvirus reactivation from latency. J Virol. 2006;80:192–200. doi: 10.1128/JVI.80.1.192-200.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen JP, Cardin RD, Branum KC, Doherty PC. CD4+ T cell-mediated control of a γ-herpesvirus in B cell-deficient mice is mediated by IFN-γ. Proc Natl Acad Sci USA. 1999;96:5135–5140. doi: 10.1073/pnas.96.9.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sparks-Thissen RL, Braaten DC, Hildner K, Murphy TL, Murphy KM, Virgin HW., IV CD4 T cell control of acute and latent murine gammaherpesvirus infection requires IFNγ. Virology. 2005;338:201–208. doi: 10.1016/j.virol.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Stuller KA, Flaño E. CD4 T cells mediate killing during persistent gammaherpesvirus 68 infection. J Virol. 2009;83:4700–4703. doi: 10.1128/JVI.02240-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–2730. (PubMed) [PubMed] [Google Scholar]
- 21.Flaño E, Woodland DL, Blackman MA, Doherty PC. Analysis of virus-specific CD4+ T cells during long-term gammaherpesvirus infection. J Virol. 2001;75:7744–7748. doi: 10.1128/JVI.75.16.7744-7748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walton SM, Wyrsch P, Munks MW, Zimmermann A, Hengel H, Hill AB, Oxenius A. The dynamics of mouse cytomegalovirus-specific CD4 T cell responses during acute and latent infection. J Immunol. 2008;181:1128–1134. doi: 10.4049/jimmunol.181.2.1128. (PubMed) [DOI] [PubMed] [Google Scholar]
- 23.Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004;75:497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- 24.Cush SS, Anderson KM, Ravneberg DH, Weslow-Schmidt JL, Flaño E. Memory generation and maintenance of CD8+ T cell function during viral persistence. J Immunol. 2007;179:141–153. doi: 10.4049/jimmunol.179.1.141. (PubMed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heller KN, Gurer C, Münz C. Virus-specific CD4+ T cells: ready for direct attack. J Exp Med. 2006;203:805–808. doi: 10.1084/jem.20060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bickham K, Münz C, Tsang ML, Larsson M, Fonteneau JF, Bhardwaj N, Steinman R. EBNA1-specific CD4+ T cells in healthy carriers of Epstein-Barr virus are primarily Th1 in function. J Clin Invest. 2001;107:121–130. doi: 10.1172/JCI10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heller KN, Upshaw J, Seyoum B, Zebroski H, Münz C. Distinct memory CD4+ T-cell subsets mediate immune recognition of Epstein Barr virus nuclear antigen 1 in healthy virus carriers. Blood. 2007;109:1138–1146. doi: 10.1182/blood-2006-05-023663. [DOI] [PMC free article] [PubMed] [Google Scholar]