Abstract
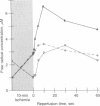
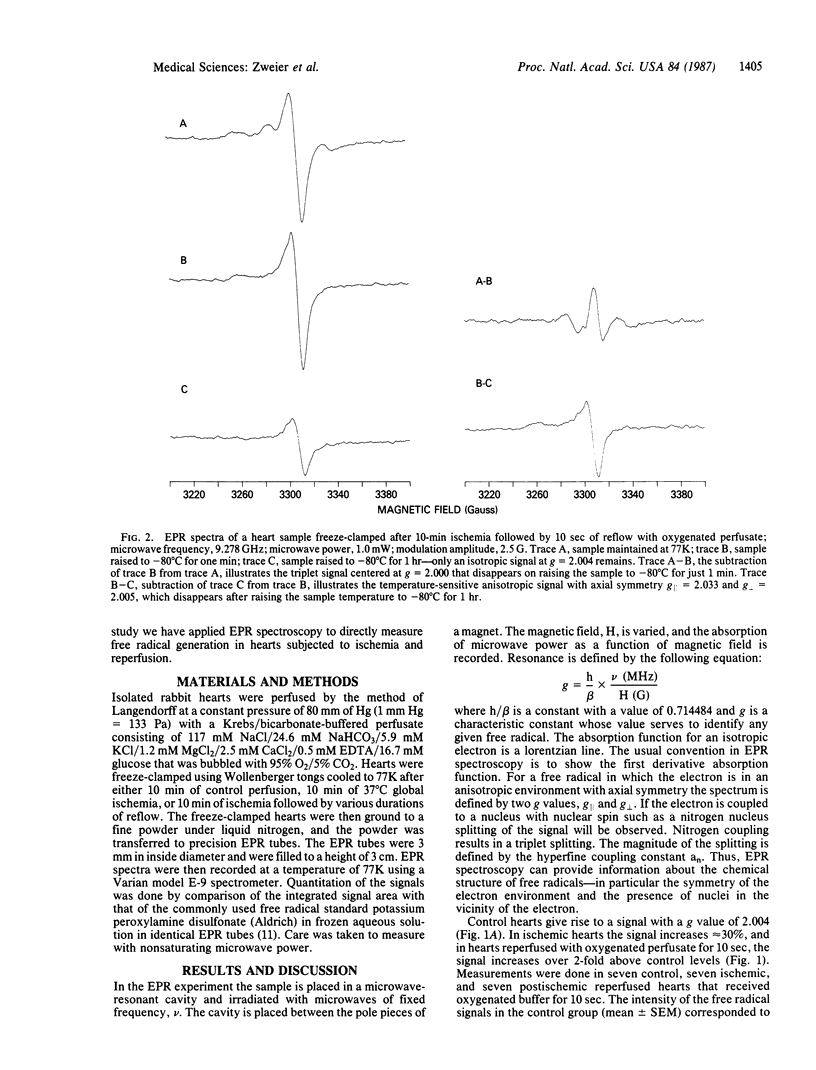
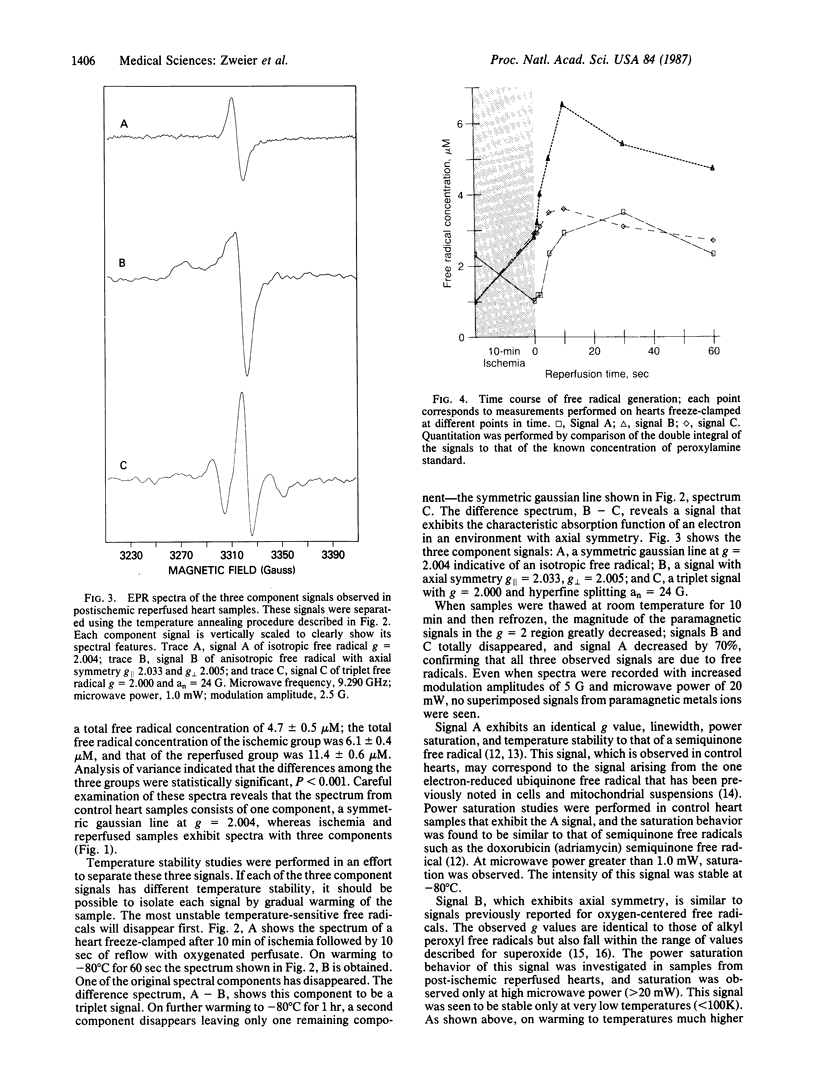
Electron paramagnetic resonance spectroscopy was used to directly measure free radical generation in perfused rabbit hearts. Hearts were freeze-clamped at 77 degrees K during control perfusion, after 10 min of normothermic global ischemia (no coronary flow), or following post-ischemic reperfusion with oxygenated perfusate. The spectra of these hearts exhibited three different signals with different power saturation and temperature stability: signal A was isotropic with g = 2.004; signal B was anisotropic with axial symmetry with g parallel = 2.033 and g perpendicular = 2.005; signal C was an isotropic triplet with g = 2.000 and hyperfine splitting an = 24 G (1 G = 0.1 mT). The g values, linewidth, power saturation, and temperature stability of signal A are identical to those of a carbon-centered semiquinone, whereas those of signal B are similar to alkyl peroxyl or superoxide oxygen-centered free radicals; signal C is most likely a nitrogen-centered free radical. In the control heart samples signal A predominated, whereas in ischemic hearts signal A decreased in intensity, and signals B and C became more intense; with reperfusion all three signals markedly increased. Free radical concentrations derived from the intensities of the B and C signals peaked 10 sec after initiation of reflow. At this time the oxygen-centered free radical concentration derived from the intensity of signal B was increased over six times the concentration measured in control hearts and over two times the concentration measured in ischemic hearts. Hypoxic reperfusion did not increase any of the free radical signals over the levels observed during ischemia. These experiments directly demonstrate that reactive oxygen-centered free radicals are generated in hearts during ischemia and that a burst of oxygen radical generation occurs within moments of reperfusion.
Full text
PDF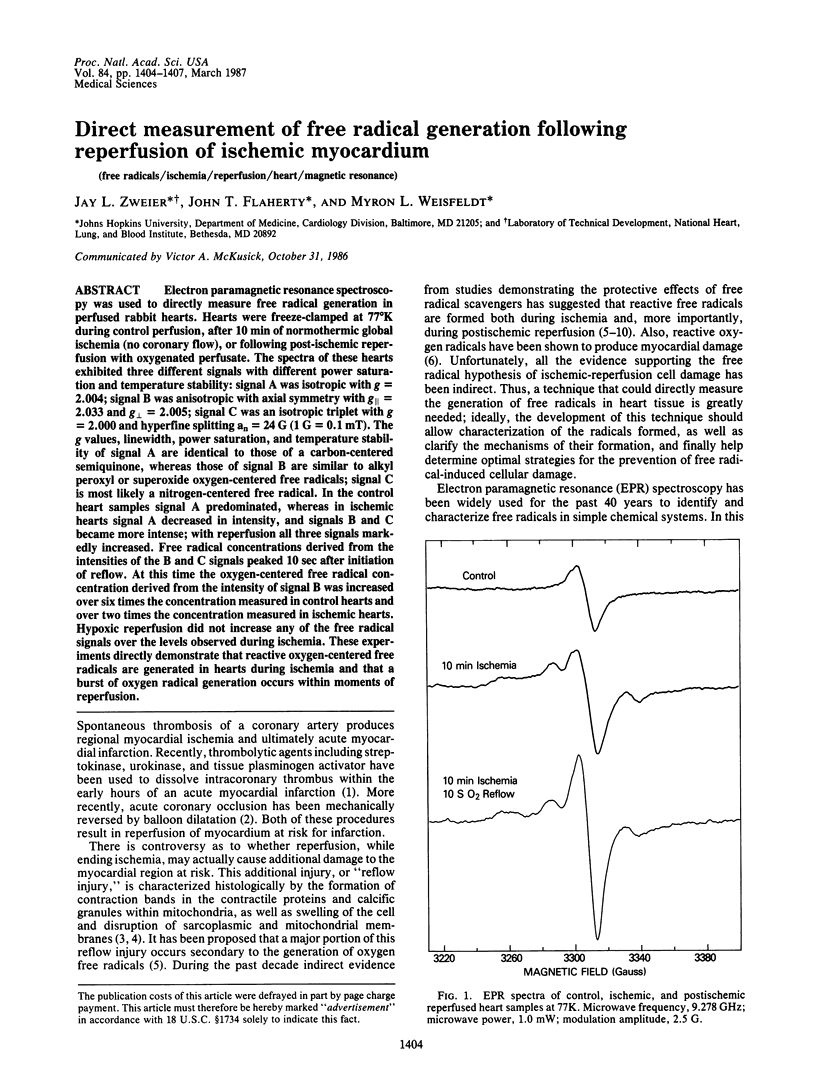

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrosio G., Becker L. C., Hutchins G. M., Weisman H. F., Weisfeldt M. L. Reduction in experimental infarct size by recombinant human superoxide dismutase: insights into the pathophysiology of reperfusion injury. Circulation. 1986 Dec;74(6):1424–1433. doi: 10.1161/01.cir.74.6.1424. [DOI] [PubMed] [Google Scholar]
- Burton K. P., McCord J. M., Ghai G. Myocardial alterations due to free-radical generation. Am J Physiol. 1984 Jun;246(6 Pt 2):H776–H783. doi: 10.1152/ajpheart.1984.246.6.H776. [DOI] [PubMed] [Google Scholar]
- Gianni L., Zweier J. L., Levy A., Myers C. E. Characterization of the cycle of iron-mediated electron transfer from Adriamycin to molecular oxygen. J Biol Chem. 1985 Jun 10;260(11):6820–6826. [PubMed] [Google Scholar]
- Hess M. L., Manson N. H. Molecular oxygen: friend and foe. The role of the oxygen free radical system in the calcium paradox, the oxygen paradox and ischemia/reperfusion injury. J Mol Cell Cardiol. 1984 Nov;16(11):969–985. doi: 10.1016/s0022-2828(84)80011-5. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- Knowles P. F., Gibson J. F., Pick F. M., Bray R. C. Electron-spin-resonance evidence for enzymic reduction of oxygen to a free radical, the superoxide ion. Biochem J. 1969 Jan;111(1):53–58. doi: 10.1042/bj1110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. L., Bolli R., Lekich R. F., Hartley C. J., Roberts R. Enhancement of recovery of myocardial function by oxygen free-radical scavengers after reversible regional ischemia. Circulation. 1985 Oct;72(4):915–921. doi: 10.1161/01.cir.72.4.915. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Trumpower B. L. Differential effects of antimycin on ubisemiquinone bound in different environments in isolated succinate . cytochrome c reductase complex. J Biol Chem. 1980 Apr 25;255(8):3278–3284. [PubMed] [Google Scholar]
- Pepine C. J., Prida X., Hill J. A., Feldman R. L., Conti C. R. Percutaneous transluminal coronary angioplasty in acute myocardial infarction. Am Heart J. 1984 Apr;107(4):820–822. doi: 10.1016/0002-8703(84)90352-1. [DOI] [PubMed] [Google Scholar]
- Shlafer M., Kane P. F., Kirsh M. M. Superoxide dismutase plus catalase enhances the efficacy of hypothermic cardioplegia to protect the globally ischemic, reperfused heart. J Thorac Cardiovasc Surg. 1982 Jun;83(6):830–839. [PubMed] [Google Scholar]
- Whalen D. A., Jr, Hamilton D. G., Ganote C. E., Jennings R. B. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am J Pathol. 1974 Mar;74(3):381–397. [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L. Iron-mediated formation of an oxidized adriamycin free radical. Biochim Biophys Acta. 1985 Apr 17;839(2):209–213. doi: 10.1016/0304-4165(85)90038-8. [DOI] [PubMed] [Google Scholar]