Abstract
Both RAS and transforming growth factor (TGF)-β signaling cascades are central in tumorigenesis and show synergisms depending on tumor stage and tissue context. In this review we focus on the interaction of RAS subeffector proteins with signaling components of the TGF-β family including those of TGF-βs, activins and bone morphogenic proteins. Compelling evidence indicates that RAS signaling is essentially involved in the switch from tumor-suppressive to tumor-promoting functions of the TGF-β family leading to enhanced cancer growth and metastatic dissemination of primary tumors. Thus, the interface of these signaling cascades is considered as a promising target for the development of novel cancer therapeutics. The current pharmacological anti-cancer concepts combating the molecular cooperation between RAS and TGF-β family signaling during carcinoma progression are critically discussed.
Keywords: Activin, BMP, cancer progression, RAS, Smad, targeted therapy, TGF-β
INTRODUCTION
Carcinoma Progression
The malignant growth and aberrant differentiation of epithelial tissues, collectively designated as carcinoma, develop by a multistep process. Most frequently, carcinoma progression leads to metastatic spread of primary tumor cells and to colonization in distant organs [1, 2]. Six or even seven properties have been defined which allow neoplastic cells to acquire and to increase malignancy [3-5]. Among these mechanisms are the (i) unlimited replicative potential, (ii) evasion of apoptosis, (iii) self-sufficiency in growth signals, (iv) ability to develop blood vessel for neoangiogenesis, (v) insensitivity against anti-growth factors such as transforming growth factor (TGF)-β, (vi) tissue invasion and metastasis, and (vii) cancer-related inflammation supported by the tumor-stroma. The sequence of genetic and epigenetic events resulting in the malignant progression of carcinoma cells appears to be random in a stochastic point of view, but is most frequently accompanied by the loss of functional differentiation and the acquisition of a migratory phenotype. The prototypical conversion of carcinoma cells to fibroblastoid descendants through transdifferentiation has been described as epithelial to mesenchymal transition (EMT), allowing carcinoma cells to infiltrate the tumor-stroma and to enter as well as to exit the circulatory system in order to generate macrometastasis [6-8].
Various stimuli generated by cell autonomous events and the tumor surrounding impinge on carcinoma cells to induce the crosstalk between rat sarcoma (RAS) signaling proteins and components of the TGF-β superfamily. Important downstream signaling events of RAS involved in the cooperation with the TGF-β/Smad family include the (i) RAS-activated factor (RAF)/mitogen activated protein kinase (MAPK) extracellular regulated kinase (ERK) kinase (MEK)/ERK pathway (RAS-RAF-MEK-ERK signaling), (ii) phosphatidyl-inositol-3 kinase (PI3K) pathway activating the serine/threonine kinase AKT-protein kinase B (PKB) and the transcription factor nuclear factor (NF)-κB, and (iii) additional RAS effectors such as RAS-related RAL proteins, phospholipase C (PLC)ε, T cell lymphoma invasion and metastasis-1 (TIAM1), RAS interaction protein-1 (RIN1) and RAS association domain-containing family (RASSF) proteins [9, 10]. The enormous complexity underlying the convergence of these signaling cascades will be summarized and discussed in the light of advanced and novel targeted anti-cancer therapies.
TGF-βSuper Family
With more than 30 members, the TGF-β super family is the largest human cytokine family including TGF-β1-3, activins, nodal, bone morphogenetic proteins (BMPs), growth and differentiation factors (GDFs) and anti-Muellerian hormone (AMH). A phylogenetic tree of all family members and their receptors can be found in a recent review [11]. Structurally, TGF-β cytokines are characterized by cysteine knots which are based on six conserved cysteines forming three intramolecular disulfide bonds. A seventh conserved cysteine is used by most ligands for dimerization via an intermolecular disulfide bond [12]. The canonical signaling cascade for cytokines of the TGF-β family is the Smad pathway, which can be divided into two major branches (Fig. 1). Which branch of the pathway is activated, depends largely on the binding and recruitment of specific ligands to different combinations of type I and II TGF-β family receptors [12, 13]. In total, there are seven type I (activin receptor-like kinase, ALK1-7) and five type II (TGFBRII, ActRII, ActRIIB, BMPRII, AMHRII) receptors, together constituting the only mammalian family of transmembrane serine/threonine kinases [11]. TGF-β1 and activins first bind their type II receptors, which stabilizes interaction between type II and type I receptors and leads to phosphorylation of type I by type II receptors. Some BMPs in contrast bind to pre-formed tetramers of type I and type II receptors [13]. The type I receptors in turn are responsible for phosphorylation of receptor-regulated (R)-Smads at two C-terminal serine residues. ALK4, 5 and 7 phosphorylate R-Smads2 and 3, whereas ALK1, 2, 3, and 6 phosphorylate the R-Smads1, 5, and 8 (Fig. 1). In general, there is a considerable degree of promiscuity in the core Smad activation pathway as (i) several ligands can bind the same type II receptor, (ii) most type II receptors can pair with different type I receptors and (iii) each R-Smad can be phosphorylated by more than one type I receptor [11, 13]. Nevertheless, most ligands activate exclusively either the Smad2/3 branch (activins, nodal, myostatin) or the Smad1/5/8 branch (most BMPs). The most notable exception is TGF-β1, which on the one hand activates Smad2/3 via TGFBRII and TGFBRI-ALK5 in epithelial cells and on the other hand has been shown to activate Smad1/5/8 either dependent on or independent of ALK1 in endothelial cells and additional cell types [14-16]. For relaying the signal into the nucleus, all R-Smads form a complex with the single common mediator (Co-)Smad4. The remaining two members of the Smad family (Smad6, Smad7) are classified as inhibitory (I)-Smads because they block signal propagation of the BMP branch (Smad6) or of all TGF-β family members (Smad7). However, a TGF-β and BMP-independent role of Smad7 has recently been suggested [17]. In the nucleus Smad complexes cooperate with a number of different transcription factors and co-activators or co-repressors to regulate the transcription of target genes [18]. Except for Smad2, all R-Smads as well as Smad4 have direct DNA binding activity. Phospho-Smad2/Smad4 complexes interact for instance with FOXH1 (forkhead box H1, formerly FAST1) and bind to ARE (activin response element) sequences in target gene promoters [19], whereas BMP-activated Smads regulate target genes via BRE (BMP response element) sequences in cooperation with Runx e.g. family transcription factors [20]. In addition to Smad-dependent signals, TGF-β can initiate a Smad-independent signaling pathway via the E3-ligase TRAF6 and subsequent activation of the MAP kinase kinase kinase TAK1 [21]. Activation of the MAPK cascade by TGF-β has also been described to occur via tyrosine kinase activity of the TGF-β type I receptor resulting in receptor autophosphorylation and recruitment of the adaptor protein ShcA [22].
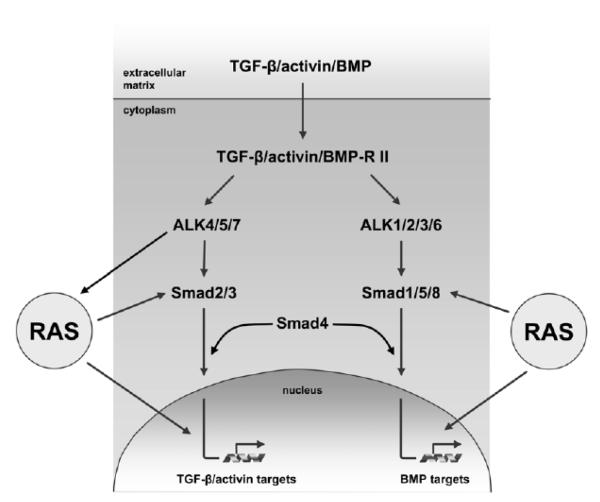
Fig. (1).
Collaboration between RAS signaling and the TGF-β/activin/BMP pathways. Upon binding of cytokines of the TGF-β/activin/BMP family to TGF-β/activin/BMP-specific type II receptors, heterodimerization with type I receptors of activin receptor-like kinases (ALKs) occurs. Type I receptors further phosphorylate receptor-regulated (R)-Smads (Smad1, Smad2, Smad3, Smad5 and Smad8) and cause their nuclear translocation together with the common-partner (Co-)Smad4. In the nucleus, Smad complexes bind to their respective response elements in target gene promoters and act as transcription factors which modulate gene expression. TGF-β/activin/BMP signaling is controlled at the level of (i) ligand mobilization and binding, (ii) receptor heterodimerization and activation, (iii) phosphorylation of R-Smads and nuclear shuttling of R/Co-Smad as well as (iv) Smad-dependent transcription complexes. The crosstalk of RAS and TGF-β family proteins involves (i) modulation of RAS subeffector proteins by TGF-β family receptors, and (ii) the interference of RAS signaling components either with the activation and nuclear translocation of Smad effectors or with Smad-dependent regulation of transcription.
The TGF /Smad pathway cooperates with RAS signaling at three different levels (Fig. 1). First, a crosstalk between TGF-β family receptors and RAS has been demonstrated by phosphorylation of RAS pathway components through TGF-β family receptors. Secondly, Smad proteins are phosphorylated by RAS-regulated kinases (Fig. 2), which controls their nuclear/cytoplasmic distribution and activity. Thirdly, Smad- and RAS-dependent transcription factors interact and cooperatively regulate target gene transcription.
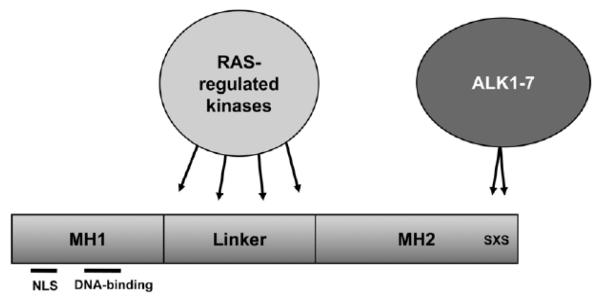
Fig. (2).
Schematic domain structure and phosphorylation pattern of Smad proteins. Smads consist of the N-terminal Mad-homology (MH) 1 region and the C-terminal MH2 domain which is joined by the linker region. The MH1 region is required for DNA-binding and contains a nuclear localization signal (NLS), whereas the MH2 region is involved in protein-protein interactions required for regulation of transcription. In Smad2, direct binding of DNA is blocked by an additional sequence in the MH1 domain. ALK1-7 phosphorylate R-Smads at the two serine residues of the C-terminal SXS motif, which generally leads to hetero-oligomerization with Smad4 and nuclear translocation. Several RAS-regulated kinases such as ERK, JNK and p38MAPK phosphorylate Smad proteins predominantly in the linker region and modulate their activity.
COOPERATION OF RAS WITH THE TGF-B FAMILY
The Synergy of RAS and TGF-β Signaling
TGF-β acts as a potent inhibitor of epithelial cell proliferation through its capability to induce cytostasis by increasing expression of the cyclin-dependent kinase inhibitors (CDKIs) p15Ink4B and p21Cip1 and by repression of c-MYC as well as of inhibitors of differentiation (IDs). These modulations (i) cause differentiation and a block of cell cycle progression, (ii) stimulate cell death, and (iii) repress cytokine and chemokine expression [23]. The tumor-suppressive functions of TGF-β signaling are observed in epithelial tissues under physiological conditions and are mostly active during early stages of tumorigenesis. Importantly, loss of sensitivity to the effects of TGF-β through bypassing the pathway via selective mutations of TGFBRs or Smad4 has a pivotal role in the progression of a variety of carcinomas [24]. In contrast to the tumor suppressor activities of TGF-β, a multitude of studies demonstrated pro-oncogenic functions of TGF-β signaling by dysregulation of CDKIs, altered production of extracellular matrix (ECM) constituents via mobilization of myofibroblasts, autocrine regulation of mitogens, induction of cell motility and EMT, suppression of immunosurveillance, stimulation of angiogenesis and priming of tumor cells towards metastasis [25-27]. Most remarkably, increased expression and secretion of TGF-β1 in hepatocellular carcinoma (HCC), non-small cell lung cancer (NSCLC), gastric, colorectal, breast, and prostate carcinoma correlates with poor prognosis, indicating TGF-β as a tumor promoter which facilitates aggressiveness of carcinoma [25, 28]. The mechanisms of the dichotomous role of the TGF-β pathway and how TGF-β signaling is switched from being tumor-suppressive to tumor-promoting is just beginning to be resolved.
A large body of literature indicates a prominent role of RAS signaling in the conversion from anti- to pro-oncogenic TGF-β signaling, however, the mechanisms of the synergy between oncogenic RAS and TGF-β signaling is still not fully understood [29]. Almost all of these investigations revealed that cancer cells undergo an increase in malignancy and retain a functional TGF-β/Smad core signaling, while tumor-suppressive functions of the canonical TGF-β/Smad pathway are concomitantly inhibited. RAS has been shown to mainly interfere with TGF-β/Smad signaling at the level of Smad2/3 activation and the composition of Smad-dependent transcription factor complexes (Fig. 1).
Involvement of the RAS Subeffector Pathways MAPK, PI3K and RhoA
Previous studies on keratinocyte, mammary, prostate and hepatocyte carcinogenesis models showed a synergistic cooperation of TGF-β and RAS to induce progression to undifferentiated, invasive tumors [30-34]. TGF-β and RAS signaling also cooperates in the intestinal epithelium where overexpression of K-RAS in combination with deletion of TGFRII generated metastatic adenocarcinomas with activated epidermal growth factor (EGF) signaling independent of Wnt/β-catenin [35]. Neither oncogenic K-RAS nor inactivation of TGFRII on its own was able to induce colorectal tumors. In MDCK cells, sustained activation of RAF-MAPK induced EMT and cell invasion dependent on the RAF-mediated autocrine loop of TGF-β [36]. Activation of RAF caused endocytosis and ubiquitin-dependent lysosomal degradation of E-cadherin [37], and inhibited the ability of TGF-β to induce apoptosis without affecting growth retardation. These data demonstrate that the RAF-MAPK pathway synergizes with TGF-β/Smad signaling in promoting malignancy [36]. In particular, hyperactivation of MEK-ERK signaling was responsible for the escape from TGF-β-induced apoptosis in HCC cells by impairing the upregulation of the NADPH oxidase NOX4, which is essential for mitochondrial-dependent apoptosis [38, 39]. Cooperation of hyperactive RAF-MAPK and TGF-β/Smad signaling was required for EMT and metastasis of mammary epithelial cells in vivo, whereas activation of PI3K caused protection against TGF-β-induced apoptosis and scattering of cells leading to tumorigenesis in the absence of metastatic colonization [29, 40]. In malignant hepatocytes, MAPK signaling in collaboration with TGF-β signaling was necessary and sufficient to induce EMT and to promote resistance to TGF-β-mediated cell cycle arrest as well as induction of apoptosis, whereas PI3K activation and TGF-β failed to form tumors [41]. Interestingly, the synergy of RAS and TGF-β signaling induced the secretion and autocrine regulation of platelet-derived growth factor (PDGF) by upregulation of PDGF receptors upon EMT, which resulted in PI3K activation and nuclear β-catenin accumulation [42-44].
Oncogenic K-RAS-ERK signaling in pancreatic adenocarcinoma promoted TGF-β-induced transcriptional downregulation of the tumor suppressor PTEN (phosphatase and tensin homolog) by simultaneous activation of PKB-AKT in a Smad4-independent manner which might provide a switch from growth suppression to growth promotion in pancreatic cancer [45]. Similarly, TGF-β-induced cell migration and EMT of mammary epithelial cells depended on the PI3K-AKT pathway and required Rho GTPase function. This was shown by the pharmacological and genetic interference with the PI3K-AKT pathway which blocked Smad2 phosphorylation and transcriptional responses induced by TGF-β [46]. TGFBRI-ALK5-dependent activation of the p38MAPK pathway was required for TGF-β-mediated EMT of breast carcinoma cells which associated with Rac1 activation and upregulation of tropomyosins (TPM1) necessary for TGF-β-induced stress fiber formation during EMT [47, 48]. Interestingly, the upregulation of TPM1 is delayed by several hours as compared to immediate-early and intermediate response genes of TGF-β [49, 50]. RAS appears to inhibit late-response but not immediate-early response TGF-β target genes as it did not affect the immediate phosphorylation of R-Smads and their nuclear translocation, but it reduced nuclear presence of Smad4 at later times [49]. Noteworthy, RAS-ERK signaling suppressed Smad-dependent expression of TPM1 and inhibited stress fibers in metastatic breast cancer cells which allowed invasion of EMT transformed cells. H-RAS-V12 prevented binding of Smads to the TPM1 promoter by forcing CRM1-dependent nuclear export of Smad4 [51]. Animal studies demonstrated that H-RAS-V12 confers the metastatic potential in epithelial cells, whereas tropomyosin suppresses tumor growth and metastases. Thus, these data suggest that (i) TPM1 represents a convergent target of the synergism of RAS and TGF-β, and (ii) TGF-β-induced EMT is not sufficient for acquisition of invasive features, while activated RAS alters the TGF-β response and confers metastatic potential [52]. In this line, activation of RhoA through continuous stimulation of TGFBRI-ALK5 was required for efficient H-RAS-V12, V-RAF and V600E-BRAF mediated malignant transformation [53]. Smad3 was found to be critical for v-H-RAS-mediated transformation in murine embryonic fibroblasts through activation of Jun N-terminal kinase (JNK) and MAPK-ERK signaling [54]. In addition, Smad3 both suppressed and promoted a RAS-driven cancer phenotype. Whereas expression of Smad3-dependent cytostatic genes of TGF-β1 were not altered by v-H-RAS along with an intact TGF-β1 growth arrest, expression of proteases and migration of keratinocytes was induced [55].
Smad Activation by Linker Phosphorylation
Kretzschmar et al. described that the linker region of Smad2 and Smad3 is phosphorylated at several serine/threonine sites through growth factor-mediated MAPK-ERK activation which results in cytoplasmic retention of Smad2/Smad3 and attenuation of TGF-β signaling [56]. In contrast, Smad2/Smad3 with phosphorylated linker regions have been reported to be predominantly localized in the nucleus in invasive late-stage colorectal carcinoma [57, 58]. Mutations of the Smad3 linker region preventing JNK-dependent phosphorylation resulted in a preserved tumor-suppressive function of TGF-β and inhibited tumor cell invasion. The distinct phosphorylation patterns of the Smad2/Smad3 linker region or C-terminal region were characterized using selective antibodies. TGFBRI-ALK5 and hepatocyte growth factor (HGF) induced JNK-dependent phosphorylation of Smad2 and Smad3 at the same sites in the linker region allowing their translocation into the nucleus together with Smad4 [59]. JNK inhibition reduced TGF-β or HGF-mediated cell invasion. However, TGF-β but not HGF treatment induced C-terminal Smad2/Smad3 phosphorylation, whereas HGF treatment reduced TGF-β-dependent elevation of p15Ink4B, which is mediated by Smad3 phosphorylation at the C-terminal region. Other studies, however, have reported C-terminal phosphorylation of Smad2/Smad3 proteins in response to HGF and nocodazole, the latter mediated by the mitotic checkpoint kinase Mps1 in a TGF-β-independent fashion [60, 61]. In colorectal cancer, Smad2/Smad3 is phosphorylated at C-terminal serine residues by TGFBRI-ALK5 while JNK and CDK4 differentially phosphorylated linker regions and C-termini [62]. Dependent on CDK4, TGF-β generated pSmad2C/L (C/L, phosphorylation at C-terminus and linker region) and pSmad3C/L, which enhanced cell growth by upregulation of c-MYC. TGFBRI-ALK5 signaling together with JNK stimulated cell invasion through pSmad2L/C mediated matrix metalloproteinase (MMP)-9 expression. Clinical samples confirmed nuclear localization of pSmad2L/C and pSmad3L/C at the invasion front of TGF-β-producing, metastatic colon carcinomas. These data suggest that CDK4 together with JNK alters TGF-β signaling from tumor-suppressive to tumor-promoting at late stages of colorectal cancer.
In the liver, tumor suppressive functions of TGF-β were mediated by pSmad3C (phosphorylation at C-terminus), while oncogenic activities such as cell proliferation and invasion were promoted by pSmad3L (phosphorylation at linker region) [63]. pSmad3L-mediated signaling induced ECM deposition by mesenchymal liver cells, and hepatitis B virus infected hepatocytes showed a transition from the anti-oncogenic pSmad3C to the fibrogenic/oncogenic pSmad3L pathway, accelerating liver fibrosis and increasing risk of HCC. Importantly, RAS-associated activation of JNK through pro-inflammatory cytokines such as interleukin-1β mediated this perturbed hepatocytic TGF-β signaling [64, 65].
K-RAS and TGF-β signaling cooperated in the induction of SNAI1 (Snail), a transcriptional repressor of E-cadherin, in pancreatic cancer cells. This occured in a Smad-dependent manner independently of MAPK-ERK and JNK activation as well as in the absence of phosphorylation at the linker region of R-Smads [66]. SNAI1 also acts as a transcriptional repressor of RIN1, a RAB5 guanine nucleotide exchange factor (GEF), which downregulates receptor tyrosine kinases (RTKs) and promotes TGFBR signaling through enhanced endocytosis [67]. RIN1 integrates RAS and TGF-β signaling since persistent RTK-RAS activation stabilized TGF-β/Smad induced SNAI1 protein by decreased glycogen synthase kinase (GSK)-3β, resulting in silencing of RIN1 and stabilization of RTKs by the concomitant reduction in RAB5-mediated TGFBR internalization.
Smad-Independent Mechanisms
R-RAS transformed EpH4 mammary epithelial cells were insensitive to TGF-β-mediated growth inhibition along with increased proliferation and malignancy in response to exogenous TGF-β. The effects of TGF-β were mediated through Smad-independent mechanisms and required the activation of TGF-β-associated kinase 1 (TAK1) and its downstream effectors JNK/p38MAPK/PI3K/AKT and mTOR pathways [68]. Independent of Co-Smad4, TGF-β signaling resulted in PI3K-mediated tyrosine phosphorylation of α-and β-catenin leading to the dissociation of the E-cadherin/β-catenin complexes from actin cytoskeleton and reduced cell adhesion. Both PI3K and PTEN were associated with E-cadherin/β-catenin complexes and TGF-β decreased the level of PTEN enhanced β-catenin phosphorylation [69].
RAS Interferes with TGF-β/Smad at the Level of Transcription
A mechanistic link between RAS, p53 and TGF-β has been demonstrated, as RAS-MAPK activity induced p53 N-terminal phosphorylation through CK1ε/δ enabling the interaction of p53 with TGF-β-activated Smads and promoting TGF-β-dependent cytostasis [70]. Interestingly, the growth-promoting effects of activated RAS are balanced by the wild type p53/Smad cooperation that sustains TGF-β growth control and thus limits neoplastic transformation. A more recent study in breast carcinoma showed insights into the enigma of the pro-metastatic switches of mutant-p53 and TGF-β by demonstrating that RAS-activated mutant-p53 and TGF-β cooperate to counteract the activity of the p53 family member p63. The p63 protein is a master regulator for maintaining normal epithelial stem cells by protecting them from apoptosis and coordinating their differentiation [71]. Noteworthy, oncogenic H-RAS/CK1ε/δ induced mutant-p53 phosphorylation and subsequent assembly of a mutant-p53/p63 protein complex in which Smads serve as essential platforms. Normal p63 functions are antagonized within this ternary complex of mutant-p53/p63 and TGF-β activated Smads, allowing the gain of metastatic properties. In addition, Sharp1 and Cyclin G2 have been identified as metastasis suppressors that mediate p63 effects. Downregulation of Sharp1 and Cyclin G2 in response to the TGF-β/mutant-p53/p63 pathway promoted the pro-oncogenic and invasive TGF-β responses. These data provide novel insights into the mechanism of the interplay between RAS and TGF-β, since RAS signaling promotes mutant-p53 phosphorylation and is required for the formation of the mutant-p53/Smad complex. RAS signaling is therefore suggested to play a significant regulatory role on the composition of co-activators and co-repressors of Smad transcriptional complexes.
RAS Interacts with the Activin and BMP Branches of the TGF-β Family
A role in tumor development and progression has also been demonstrated for several other TGF-β family members including activin A, several BMPs, GDF15/MIC-1, and nodal, to name just some prominent examples. As described for most TGF-β family members, promotion or suppression of tumorigenesis mostly depends on the stage and type of tumor. For activin, an anti-oncogenic activity has been described in breast [72, 73], colon [74] and HCC [75], whereas pro-oncogenic effects have been demonstrated in lung [76], prostate [77], esophageal cancer [78, 79] and oral cancer [80].
Activin A uses a different set of receptors than TGF-β but depends on the same Smad proteins (Smad2/3/4) for most of its reported activities and relies on similar mechanisms for tumor suppression namely induction of apoptosis and cell cycle arrest via p15Ink4B [81, 82]. Specific loss of activin signals in the course of tumor development has been connected to inactivating mutations of activin receptors in colon, pancreas, and prostate cancer [74, 83, 84] as well as overexpression of antagonistic proteins such as follistatin and follistatin-related gene (FLRG) in breast cancer and HCC [73, 75]. In those malignancies with pro-tumorigenic roles of activin A, the signaling pathways are only beginning to be explored. Recently it has been shown that activin A, which inhibits growth and induces apoptosis in prostate cancer cell lines, is overexpressed and enhances cell migration in bone metastatis [77]. This oncogenic effect was dependent on the interaction of Smad3 with androgen receptor (AR), demonstrating that activin A can switch to a tumor-promoting function similar to TGF-β depending on the stage of cancer progression. The activin antagonist follistatin suppressed metastasis in small cell lung cancer indicating a contribution of activin A to tumor dissemination [85]. In addition, ERK and p38MAPK-dependent signal transduction by activin A has been shown to be involved in the transcriptional control of Pit-1 and tyrosine hydroxylase [86, 87]. In keratinocytes, activin induced stress fiber formation and migration in a Smad-independent fashion that includes RhoA and MEKK1 signaling resulting in phosphorylation of JNK and c-Jun [88]. This pathway was suggested to regulate epithelial morphogenesis and might contribute to pro-tumorigenic mechanisms of activin A. The RAS-GAP binding protein Dok-1, which signals downstream of tyrosine kinase pathways, was found to act as an adaptor linking activin receptors with Smad3 and Smad4 activation [89]. Overexpression of Dok-1 enhanced activin-induced apoptosis and inhibited the RAS-ERK pathway in a mouse B-cell line. As reported for TGF-β signal transduction, an impact of RAS-MAPK-dependent phosphorylation in the Smad 2/3 linker region might also affect activin signaling, however, clear evidence needs to be demonstrated. A recent study on transcriptional cooperation showed that activin A increases vascular endothelial growth factor expression via a physical interaction of Smad2 with the MAPK-regulated transcription factor SP1 in HCC cell lines [90]. The synergistic cooperation of activin A with fibroblast growth factor (FGF)2, which mediates tube formation of bovine aortic endothelial cells, could be inhibited by follistatin, Smad7 or inhibition of ERK [91].
The cooperation of the Smad2/3-dependent activin/nodal pathway and RAS-MAPK-activating FGF2 signals including expression of Nanog was also required for maintaining pluripotency of human embryonic stem cells [92, 93]. Since both the activin/nodal and the FGF/FGFR axis are hyperactivated in lung cancer and melanoma [76, 94-96], it will be interesting to find possible implications of this cooperation on the stem cell-like properties of tumor cells.
In contrast to TGF-β and activins, the BMP subfamily signals via the Smad1/5/8 of the canonical TGF-β pathway. Beside phosphorlylation at the C-terminus by ALK1/2/3/6, the linker region of Smad1 is phosphorylated by MAPKs (ERK, p38, JNK) as well as by GSK-3β [97-99], which has been connected to the inhibition of nuclear translocation and ubiquitin-mediated degradation, thus reducing Smad transcriptional activity. In other studies, however, RAS-ERK signals enhanced the transcriptional activity of Smad1 in response to BMP [100]. Smad-independent activities of BMPs include signaling via direct interaction of the cytoplasmic tail of BMPRII with LIM kinase 1 (LIMK1), a regulator of actin dynamics, and suppression of PTEN via RAS-ERK signaling in Smad4-negative colon cancer cells [101, 102]. With respect to the role of BMPs in tumor progression, the picture is diverse. Frequent inactivation of the BMP signaling pathway by mutation of BMP receptors or Smad proteins was found in colon cancer [103, 104]. In lung cancer, an association between RAS mutations and silencing of BMP expression has been demonstrated. NSCLC patients with K-RAS codon 12 mutations were six times more likely to have epigenetically silenced BMP3b/GDF10 or BMP6 than those with wild type K-RAS [105]. The molecular mechanism behind this crosstalk, however, remains to be elucidated. BMP2 and BMP4 have been implicated in enhanced invasion and bone metastasis of breast cancer cells as well as in EMT of ovarian cancer cells [106, 107]. BMP7 on the other hand has been demonstrated to counteract TGF-β1 induced EMT in renal tubular epithelial cells [108]. While induction of EMT by BMP4 was associated with Rho GTPase activation, inhibition of EMT by BMP7 involved Smad1-mediated signal transduction. Thus, context-dependent modulation of BMP signals by the cell is crucial for functional outcomes. Future studies need to work out the mechanisms how RAS-dependent signals contribute to pro- versus anti-tumorigenic effects of BMP-activated Smad signals.
IMPLICATIONS FOR TARGETED CANCER THERAPY
Pathways of the RAS and TGF-β family are attractive targets for cancer therapy since they are activated in numerous carcinomas. Yet, the success of specifically targeting either RAS or TGF-β signaling has been limited due to the toxicity of drugs or to vast pleiotropic effects leading to physiological imbalances. As comprehensively outlined, experimental cancer research revealed that both RAS and TGF-β signaling pathways are promising targets to combat tumorigenesis, however, much remains to be investigated to understand the dual anti- and pro-oncogenic roles of TGF-β signaling and to develop proper anti-cancer concepts targeting TGF-β. Determination of deleterious or beneficial TGF-β signaling in cancer development and the complexity of the interrelationship between RAS and TGF-β signaling, which depends on the cell type, tumor stage and tissue context, complicates therapeutic interference with TGF-β signaling [109]. The aim of targeted therapy must be directed against the double-edge sword of TGF-β through inhibition of the pro-oncogenic aspects at advanced stages of cancer progression and the proper restoration of anti-oncogenic effects that are observed during early stages of tumor development.
Various strategies have been pursued to accomplish inhibition of TGF-β signaling including (i) sequence-specific anti-sense oligonucleotides for degradation of TGF-β mRNA, (ii) isoform-selective, neutralizing antibodies or soluble TGFBRII fragments blocking the binding of TGF-β ligands to the heteromeric receptor complex, and (iii) low molecular weight inhibitors antagonizing the intracellular kinase activity of TGFBRs [110-112]. For instance, the small molecule inhibitor LY2109761 targeting TGFBRI-ALK5 and TGFBRII induced a complete abrogation of Smad-dependent and - independent signaling in human colon carcinoma cells harboring activated K-RAS, resulting in reduced tumor cell invasion and liver metastasis [113]. Remarkably, TGF-β antisense approaches showed reactivation of tumor-specific immune responses, rendering patients more susceptible to a number of different therapeutic measures [110]. Inhibitors in clinical settings such as human monoclonal antibodies against TGF-β2 (CAT-152) and TGF-β1 (CAT-192) successfully completed phase I/II trials. A TGF-β2 antisense oligonucleotide (AP 12009) has begun phase II/III testing to treat high grade gliomas and phase I/II trials against pancreatic cancer. However, these approaches in clinical trials focus on the inhibition of non-canonical and canonical TGF-β signaling rather than on the molecular crosstalk between TGF-β signaling and the RAS pathway.
Recent strategies further involve targeting of TGF-β signaling in the tumor microenvironment, and in particular, the immune system which is critical for carcinoma progression. Importantly, TGF-β provided by bone marrow-derived Gr-1+CD11b+ immature myeloid cells interfered with the immune system by suppression of natural killer cells, cytotoxic T cell and B cell function [28, 114]. Infiltration of Gr-1+CD11b+ into breast carcinoma resulted in increased MMP and TGF-β production enforcing tumor cell invasion and metastasis [114]. Thus, Gr-1+CD11b+ cells act as an additional component causing the switch from tumor-suppressive to tumor-promoting functions which is of paramount importance for the timing of therapeutic TGF-β intervention. Recent studies suggested Gr-1+CD11b+ cells as biomarkers for patient selection in ongoing phase I/II clinical trials of TGF-β therapy which is supported by recent findings in HCC and colorectal cancer [26, 115, 116].
Efforts to target hyperactive RAS (H-RAS, N-RAS or K-RAS) signaling emanating from mutations by employing GTP analogues or inhibitors of post-translational modifications such as farnesylation failed to prove successful so far [117]. However, inhibition of RTKs upstream of RAS like EGF-R or ErbB2/Her2 is successfully used in the clinic in selected malignancies and patient collectives [118]. Moreover, inhibition of downstream effectors of RAS like RAF and MEK is currently being tested in clinical trials [119]. Recent studies showed that NF-κB, which is a master regulator of the inflammatory response, might be a more promising target for interfering with cancer development than RAS itself [120]. TBK1, activated by the RAS-RAL pathway and inducing NF-κB by phosphorylation of IκB (an inhibitor of NF-κB), has been described to be essential for mutated RAS signaling [121, 122]. Targeting of NF-κB might be particularly promising, since RAS and TGF-β together induce NF-κB signaling associated with EMT and metastasis of mammary epithelial cells in vivo, which is reversible by NF-κB inhibition [123]. This therapeutic approach would favor the interference with the deleterious consequences of the RAS-TGF-β cooperation by the concomitant maintenance of tumor-suppressive TGF-β functions.
CONCLUDING REMARKS
RAS signaling is an essential determinant in the establishment of the vicious cycle of the TGF-β family, which is fundamentally involved in tumor progression. It seems, however, that most carcinomas are not addicted to the oncogenic activity of RAS as its abatement by pharmacological intervention is insufficient to combat the disease. Yet, RAS activation with respect to its crosstalk with TGF-β and the concomitant onset of the pro-oncogenic aspects of the TGF-β family appears to comprehensively overcome physiological programs and to induce a state of cellular amnesia [124]. This state of cellular amnesia rather than addiction to a single oncogene like RAS results in bypassing checkpoint mechanisms which is essential for (i) EMT, cell invasion and metastasis (ii) immune suppression, (iii) neo-angiogenesis, (iv) multiple drug resistance, and (v) self renewal and cancer stemness during carcinoma progression. Pharmacological interference with TGF-β signaling and its modulation by RAS activation could be most effective, when it succeeds in targeting the pro-oncogenic aspects by simultaneously restoring the anti-oncogenic functions of TGF-β. Thus, future studies should focus on targeting the ambiguous role of TGF-β signaling in carcinoma progression, and should take combination therapy with inhibition of RTK-RAS signaling into consideration.
ACKNOWLEDGEMENTS
The authors apologize to those investigators whose experimental work has only been cited indirectly because of space limitations. M.G. is supported by the Herzfelder’sche Family Foundation and by the Fund of the City of Vienna for Interdisciplinary Cancer Research. The work of the corresponding author W.M. is supported by grants from the Austrian Science Fund, FWF, grant numbers SFB F28, P20905-B13 and P19598-B13, the Herzfelder’sche Family Foundation, and the European Union, FP7 Health Research, project number HEALTH-F4-2008-202047.
ABBREVIATIONS
- ActRII
activin type II receptor
- ActRIIB
activin type IIB receptor
- ALK
activin receptor-like kinase
- AMH
anti-Muellerian hormone
- AMHRII
anti-Mullerian hormone type II receptor
- AR
androgen recptor
- ARE
activin response element
- BMP
bone morphogenic protein
- BMPRII
BMP type II receptor
- BRE
BMP response element
- CDK
cyclin-dependet kinase
- CDKI
cyclin-dependet kinase CDK inhibitor
- Co-Smad
common mediator Smad
- Dok-1
docking protein 1
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EGF-R
epidermal growth factor receptor
- EMT
epithelial to mesenchymal transition
- ERK
extracellular regulated kinase
- FGF
fibroblast growth factor
- FLRG
follistatin-related gene
- FOXH1
forkhead box H1
- GDF
growth and differentiation factor
- GEF
guanine nucleotide exchange factor
- GSK-3β
glycogen synthase kinase 3β
- HCC
hepatocellular carcinoma
- HGF
hepatocyte growth factor
- H-RAS
Harvey rat sarcoma viral oncogene homolog
- ID
inhibitor of differentiation
- I-Smad
inhibitory Smad
- JNK
c-Jun N-terminal kinase
- K-RAS
Kirsten rat sarcoma viral oncogene homolog
- LIMK1
LIM kinase 1
- MAPK
mitogen activated protein kinase
- MDCK
Madin-Darby canine kidney cells
- MEK
mitogen activated protein kinase kinase
- MH
Mad homology
- MMP
matrix metalloprotease
- Mps1
monopolar spindle 1
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor κB
- NLS
nuclear localization signal
- N-RAS
neuroblastoma RAS viral oncogene homolog
- NSCLC
non-small lung cell carcinoma
- PDGF
platelet-derived growth factor
- PI3K
phosphatidyl-inositol-3 kinase
- PKB
protein kinase B
- PLCε
phosholipase Cε
- PTEN
phosphatase and tensin homolog
- RAF
rat sarcoma activated factor
- RAS
rat sarcoma
- RASSF
RAS association domain containing family
- RIN1
RAS interaction protein 1
- R-Ras
related RAS viral oncogene
- R-Smad
receptor-regulated Smad
- RTK
receptor tyrosine kinase
- TAK1
TGF-β associated kinase 1
- TGFBRI
TGF-β type I receptor
- TGFBRII
TGF-β type II receptor
- TGF-β
transforming growth factor β
- TIAM1
T cell lymphoma invasion and metastasis 1
- TPM1
tropomyosin 1
- TRAF6
tumor necrosis factor receptor-associated factor 6
- v-RAS
rat sarcoma viral oncogene
REFERENCES
- [1].Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- [2].Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- [3].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [5].Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- [6].Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat. Rev. Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- [9].Downward J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- [10].Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat. Rev. Mol. Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- [12].Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- [13].Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- [14].Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol. Cell. 2003;12:817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- [15].Liu IM, Schilling SH, Knouse KA, Choy L, Derynck R, Wang XF. TGFbeta-stimulated Smad1/5 phosphorylation requires the ALK5 L45 loop and mediates the pro-migratory TGFbeta switch. EMBO J. 2009;28:88–98. doi: 10.1038/emboj.2008.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wrighton KH, Lin X, Feng XH. Phospho-control of TGF-beta superfamily signaling. Cell Res. 2009;19:8–20. doi: 10.1038/cr.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Krampert M, Chirasani SR, Wachs FP, Aigner R, Bogdahn U, Yingling JM, Heldin CH, Aigner L, Heuchel R. Smad7 regulates the adult neural stem/progenitor cell pool in a transforming growth factor beta-and bone morphogenetic protein-independent manner. Mol. Cell Biol. 2010;30:3685–3694. doi: 10.1128/MCB.00434-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ross S, Hill CS. How the Smads regulate transcription. Int. J. Biochem. Cell Biol. 2008;40:383–408. doi: 10.1016/j.biocel.2007.09.006. [DOI] [PubMed] [Google Scholar]
- [19].Chen X, Rubock MJ, Whitman M. A transcriptional partner for MAD proteins in TGF-beta signalling. Nature. 1996;383:691–696. doi: 10.1038/383691a0. [DOI] [PubMed] [Google Scholar]
- [20].Wang Q, Wei X, Zhu T, Zhang M, Shen R, Xing L, O’Keefe RJ, Chen D. Bone morphogenetic protein 2 activates Smad6 gene transcription through bone-specific transcription factor Runx2. J. Biol. Chem. 2007;282:10742–10748. doi: 10.1074/jbc.M610997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Heldin CH, Landstrom M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr. Opin. Cell Biol. 2009;21:166–176. doi: 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- [22].Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, Derynck R. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007;26:3957–3967. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Padua D, Massague J. Roles of TGFbeta in metastasis. Cell Res. 2009;19:89–102. doi: 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]
- [26].Yang L, Moses HL. Transforming growth factor beta: tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 2008;68:9107–9111. doi: 10.1158/0008-5472.CAN-08-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Barcellos-Hoff MH, Akhurst RJ. Transforming growth factor-beta in breast cancer: too much, too late. Breast Cancer Res. 2009;11:202. doi: 10.1186/bcr2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat. Rev. Mol. Cell Biol. 2003;4:657–665. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- [30].Gotzmann J, Huber H, Thallinger C, Wolschek M, Jansen B, Schulte-Hermann R, Beug H, Mikulits W. Hepatocytes convert to a fibroblastoid phenotype through the cooperation of TGF-beta1 and Ha-Ras: steps towards invasiveness. J. Cell Sci. 2002;115:1189–1202. doi: 10.1242/jcs.115.6.1189. [DOI] [PubMed] [Google Scholar]
- [31].Iglesias M, Frontelo P, Gamallo C, Quintanilla M. Blockade of Smad4 in transformed keratinocytes containing a Ras oncogene leads to hyperactivation of the Ras-dependent Erk signalling pathway associated with progression to undifferentiated carcinomas. Oncogene. 2000;19:4134–4145. doi: 10.1038/sj.onc.1203764. [DOI] [PubMed] [Google Scholar]
- [32].Oft M, Heider KH, Beug H. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr. Biol. 1998;8:1243–1252. doi: 10.1016/s0960-9822(07)00533-7. [DOI] [PubMed] [Google Scholar]
- [33].Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E. TGF-β beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996;10:2462–2477. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- [34].Park BJ, Park JI, Byun DS, Park JH, Chi SG. Mitogenic conversion of transforming growth factor-beta1 effect by oncogenic Ha-Ras-induced activation of the mitogen-activated protein kinase signaling pathway in human prostate cancer. Cancer Res. 2000;60:3031–3038. [PubMed] [Google Scholar]
- [35].Trobridge P, Knoblaugh S, Washington MK, Munoz NM, Tsuchiya KD, Rojas A, Song X, Ulrich CM, Sasazuki T, Shirasawa S, Grady WM. TGF-beta receptor inactivation and mutant Kras induce intestinal neoplasms in mice via a beta-catenin-independent pathway. Gastroenterology. 2009;136:1680–1688. e1687. doi: 10.1053/j.gastro.2009.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lehmann K, Janda E, Pierreux CE, Rytomaa M, Schulze A, McMahon M, Hill CS, Beug H, Downward J. Raf induces TGFbeta production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells. Genes Dev. 2000;14:2610–2622. doi: 10.1101/gad.181700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Janda E, Nevolo M, Lehmann K, Downward J, Beug H, Grieco M. Raf plus TGFbeta-dependent EMT is initiated by endocytosis and lysosomal degradation of E-cadherin. Oncogene. 2006;25:7117–7130. doi: 10.1038/sj.onc.1209701. [DOI] [PubMed] [Google Scholar]
- [38].Caja L, Sancho P, Bertran E, Iglesias-Serret D, Gil J, Fabregat I. Overactivation of the MEK/ERK pathway in liver tumor cells confers resistance to TGF-{beta}-induced cell death through impairing up-regulation of the NADPH oxidase NOX4. Cancer Res. 2009;69:7595–7602. doi: 10.1158/0008-5472.CAN-09-1482. [DOI] [PubMed] [Google Scholar]
- [39].Fabregat I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J. Gastroenterol. 2009;15:513–520. doi: 10.3748/wjg.15.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, Beug H, Grunert S. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 2002;156:299–313. doi: 10.1083/jcb.200109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fischer AN, Herrera B, Mikula M, Proell V, Fuchs E, Gotzmann J, Schulte-Hermann R, Beug H, Mikulits W. Integration of Ras subeffector signaling in TGF-{beta} mediated late stage hepatocarcinogenesis. Carcinogenesis. 2005:931–942. doi: 10.1093/carcin/bgi043. [DOI] [PubMed] [Google Scholar]
- [42].Fischer AN, Fuchs E, Mikula M, Huber H, Beug H, Mikulits W. PDGF essentially links TGF-beta signaling to nuclear beta-catenin accumulation in hepatocellular carcinoma progression. Oncogene. 2007;26:3395–3405. doi: 10.1038/sj.onc.1210121. [DOI] [PubMed] [Google Scholar]
- [43].Gotzmann J, Fischer AN, Zojer M, Mikula M, Proell V, Huber H, Jechlinger M, Waerner T, Weith A, Beug H, Mikulits W. A crucial function of PDGF in TGF-beta-mediated cancer progression of hepatocytes. Oncogene. 2006;25:3170–3185. doi: 10.1038/sj.onc.1209083. [DOI] [PubMed] [Google Scholar]
- [44].Jechlinger M, Sommer A, Moriggl R, Seither P, Kraut N, Capodiecci P, Donovan M, Cordon-Cardo C, Beug H, Grunert S. Autocrine PDGFR signaling promotes mammary cancer metastasis. J. Clin. Invest. 2006;116:1561–1570. doi: 10.1172/JCI24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chow JY, Quach KT, Cabrera BL, Cabral JA, Beck SE, Carethers JM. RAS/ERK modulates TGFbeta-regulated PTEN expression in human pancreatic adenocarcinoma cells. Carcinogenesis. 2007;28:2321–2327. doi: 10.1093/carcin/bgm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- [47].Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic trans-differentiation and cell migration. J. Cell Sci. 2002;115:3193–3206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- [48].Bakin AV, Safina A, Rinehart C, Daroqui C, Darbary H, Helfman DM. A critical role of tropomyosins in TGF-beta regulation of the actin cytoskeleton and cell motility in epithelial cells. Mol. Biol. Cell. 2004;15:4682–4694. doi: 10.1091/mbc.E04-04-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc. Natl. Acad. Sci. USA. 2001;98:6686–6691. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- [51].Safina AF, Varga AE, Bianchi A, Zheng Q, Kunnev D, Liang P, Bakin AV. Ras alters epithelial-mesenchymal transition in response to TGFbeta by reducing actin fibers and cell-matrix adhesion. Cell Cycle. 2009;8:284–298. doi: 10.4161/cc.8.2.7590. [DOI] [PubMed] [Google Scholar]
- [52].Zavadil J. New TGF-beta and Ras crosstalk in EMT. Cell Cycle. 2009;8:184. [PubMed] [Google Scholar]
- [53].Fleming YM, Ferguson GJ, Spender LC, Larsson J, Karlsson S, Ozanne BW, Grosse R, Inman GJ. TGF-beta-mediated activation of RhoA signalling is required for efficient (V12)HaRas and (V600E)BRAF transformation. Oncogene. 2009;28:983–993. doi: 10.1038/onc.2008.449. [DOI] [PubMed] [Google Scholar]
- [54].Arany PR, Rane SG, Roberts AB. Smad3 deficiency inhibits v-ras-induced transformation by suppression of JNK MAPK signaling and increased farnesyl transferase inhibition. Oncogene. 2008;27:2507–2512. doi: 10.1038/sj.onc.1210889. [DOI] [PubMed] [Google Scholar]
- [55].Bae DS, Blazanin N, Licata M, Lee J, Glick AB. Tumor suppressor and oncogene actions of TGFbeta1 occur early in skin carcinogenesis and are mediated by Smad3. Mol. Carcinog. 2009;48:441–453. doi: 10.1002/mc.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGFbeta/ Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Oft M, Akhurst RJ, Balmain A. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat. Cell Biol. 2002;4:487–494. doi: 10.1038/ncb807. [DOI] [PubMed] [Google Scholar]
- [58].Sekimoto G, Matsuzaki K, Yoshida K, Mori S, Murata M, Seki T, Matsui H, Fujisawa J, Okazaki K. Reversible Smad-dependent signaling between tumor suppression and oncogenesis. Cancer Res. 2007;67:5090–5096. doi: 10.1158/0008-5472.CAN-06-4629. [DOI] [PubMed] [Google Scholar]
- [59].Mori S, Matsuzaki K, Yoshida K, Furukawa F, Tahashi Y, Yamagata H, Sekimoto G, Seki T, Matsui H, Nishizawa M, Fujisawa J, Okazaki K. TGF-beta and HGF transmit the signals through JNK-dependent Smad2/3 phosphorylation at the linker regions. Oncogene. 2004;23:7416–7429. doi: 10.1038/sj.onc.1207981. [DOI] [PubMed] [Google Scholar]
- [60].de Caestecker MP, Parks WT, Frank CJ, Castagnino P, Bottaro DP, Roberts AB, Lechleider RJ. Smad2 transduces common signals from receptor serine-threonine and tyrosine kinases. Genes Dev. 1998;12:1587–1592. doi: 10.1101/gad.12.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhu S, Wang W, Clarke DC, Liu X. Activation of Mps1 promotes transforming growth factor-beta-independent Smad signaling. J. Biol. Chem. 2007;282:18327–18338. doi: 10.1074/jbc.M700636200. [DOI] [PubMed] [Google Scholar]
- [62].Matsuzaki K, Kitano C, Murata M, Sekimoto G, Yoshida K, Uemura Y, Seki T, Taketani S, Fujisawa J, Okazaki K. Smad2 and Smad3 phosphorylated at both linker and COOH-terminal regions transmit malignant TGF-beta signal in later stages of human colorectal cancer. Cancer Res. 2009;69:5321–5330. doi: 10.1158/0008-5472.CAN-08-4203. [DOI] [PubMed] [Google Scholar]
- [63].Matsuzaki K. Modulation of TGF-beta signaling during progression of chronic liver diseases. Front. Biosci. 2009;14:2923–2934. doi: 10.2741/3423. [DOI] [PubMed] [Google Scholar]
- [64].Matsuzaki K, Murata M, Yoshida K, Sekimoto G, Uemura Y, Sakaida N, Kaibori M, Kamiyama Y, Nishizawa M, Fujisawa J, Okazaki K, Seki T. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology. 2007;46:48–57. doi: 10.1002/hep.21672. [DOI] [PubMed] [Google Scholar]
- [65].Murata M, Matsuzaki K, Yoshida K, Sekimoto G, Tahashi Y, Mori S, Uemura Y, Sakaida N, Fujisawa J, Seki T, Kobayashi K, Yokote K, Koike K, Okazaki K. Hepatitis B virus X protein shifts human hepatic transforming growth factor (TGF)-beta signaling from tumor suppression to oncogenesis in early chronic hepatitis B. Hepatology. 2009;49:1203–1217. doi: 10.1002/hep.22765. [DOI] [PubMed] [Google Scholar]
- [66].Horiguchi K, Shirakihara T, Nakano A, Imamura T, Miyazono K, Saitoh M. Role of Ras signaling in the induction of snail by transforming growth factor-beta. J. Biol. Chem. 2009;284:245–253. doi: 10.1074/jbc.M804777200. [DOI] [PubMed] [Google Scholar]
- [67].Hu H, Milstein M, Bliss JM, Thai M, Malhotra G, Huynh LC, Colicelli J. Integration of transforming growth factor beta and RAS signaling silences a RAB5 guanine nucleotide exchange factor and enhances growth factor-directed cell migration. Mol. Cell Biol. 2008;28:1573–1583. doi: 10.1128/MCB.01087-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Erdogan M, Pozzi A, Bhowmick N, Moses HL, Zent R. Transforming growth factor-beta (TGF-beta) and TGF-beta-associated kinase 1 are required for R-Ras-mediated transformation of mammary epithelial cells. Cancer Res. 2008;68:6224–6231. doi: 10.1158/0008-5472.CAN-08-0513. [DOI] [PubMed] [Google Scholar]
- [69].Vogelmann R, Nguyen-Tat MD, Giehl K, Adler G, Wedlich D, Menke A. TGFbeta-induced downregulation of E-cadherin-based cell-cell adhesion depends on PI3-kinase and PTEN. J. Cell Sci. 2005;118:4901–4912. doi: 10.1242/jcs.02594. [DOI] [PubMed] [Google Scholar]
- [70].Cordenonsi M, Montagner M, Adorno M, Zacchigna L, Martello G, Mamidi A, Soligo S, Dupont S, Piccolo S. Integration of TGF-beta and Ras/MAPK signaling through p53 phosphorylation. Science. 2007;315:840–843. doi: 10.1126/science.1135961. [DOI] [PubMed] [Google Scholar]
- [71].Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, Parenti AR, Rosato A, Bicciato S, Balmain A, Piccolo S. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- [72].Jeruss JS, Sturgis CD, Rademaker AW, Woodruff TK. Down-regulation of activin, activin receptors, and Smads in high-grade breast cancer. Cancer Res. 2003;63:3783–3790. [PubMed] [Google Scholar]
- [73].Razanajaona D, Joguet S, Ay AS, Treilleux I, Goddard-Leon S, Bartholin L, Rimokh R. Silencing of FLRG, an antagonist of activin, inhibits human breast tumor cell growth. Cancer Res. 2007;67:7223–7229. doi: 10.1158/0008-5472.CAN-07-0805. [DOI] [PubMed] [Google Scholar]
- [74].Jung B, Doctolero RT, Tajima A, Nguyen AK, Keku T, Sandler RS, Carethers JM. Loss of activin receptor type 2 protein expression in microsatellite unstable colon cancers. Gastroenterology. 2004;126:654–659. doi: 10.1053/j.gastro.2004.01.008. [DOI] [PubMed] [Google Scholar]
- [75].Grusch M, Drucker C, Peter-Vorosmarty B, Erlach N, Lackner A, Losert A, Macheiner D, Schneider WJ, Hermann M, Groome NP, Parzefall W, Berger W, Grasl-Kraupp B, Schulte-Hermann R. Deregulation of the activin/follistatin system in hepatocarcinogenesis. J. Hepatol. 2006;45:673–680. doi: 10.1016/j.jhep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- [76].Seder CW, Hartojo W, Lin L, Silvers AL, Wang Z, Thomas DG, Giordano TJ, Chen G, Chang AC, Orringer MB, Beer DG. Upregulated INHBA expression may promote cell proliferation and is associated with poor survival in lung adenocarcinoma. Neoplasia. 2009;11:388–396. doi: 10.1593/neo.81582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kang HY, Huang HY, Hsieh CY, Li CF, Shyr CR, Tsai MY, Chang C, Chuang YC, Huang KE. Activin A enhances prostate cancer cell migration through activation of androgen receptor and is overexpressed in metastatic prostate cancer. J. Bone Miner. Res. 2009;24:1180–1193. doi: 10.1359/jbmr.090219. [DOI] [PubMed] [Google Scholar]
- [78].Seder CW, Hartojo W, Lin L, Silvers AL, Wang Z, Thomas DG, Giordano TJ, Chen G, Chang AC, Orringer MB, Beer DG. INHBA overexpression promotes cell proliferation and may be epigenetically regulated in esophageal adenocarcinoma. J. Thorac. Oncol. 2009;4:455–462. doi: 10.1097/JTO.0b013e31819c791a. [DOI] [PubMed] [Google Scholar]
- [79].Yoshinaga K, Yamashita K, Mimori K, Tanaka F, Inoue H, Mori M. Activin a causes cancer cell aggressiveness in esophageal squamous cell carcinoma cells. Ann. Surg. Oncol. 2008;15:96–103. doi: 10.1245/s10434-007-9631-1. [DOI] [PubMed] [Google Scholar]
- [80].Chang KP, Kao HK, Liang Y, Cheng MH, Chang YL, Liu SC, Lin YC, Ko TY, Lee YS, Tsai CL, Wang TH, Hao SP, Tsai CN. Overexpression of activin A in oral squamous cell carcinoma: association with poor prognosis and tumor progression. Ann. Surg. Oncol. 2010;17:1945–1956. doi: 10.1245/s10434-010-0926-2. [DOI] [PubMed] [Google Scholar]
- [81].Hully JR, Chang L, Schwall RH, Widmer HR, Terrell TG, Gillett NA. Induction of apoptosis in the murine liver with recombinant human activin A. Hepatology. 1994;20:854–862. doi: 10.1002/hep.1840200413. [DOI] [PubMed] [Google Scholar]
- [82].Ho J, de Guise C, Kim C, Lemay S, Wang XF, Lebrun JJ. Activin induces hepatocyte cell growth arrest through induction of the cyclin-dependent kinase inhibitor p15INK4B and Sp1. Cell Signal. 2004;16:693–701. doi: 10.1016/j.cellsig.2003.11.002. [DOI] [PubMed] [Google Scholar]
- [83].Hempen PM, Zhang L, Bansal RK, Iacobuzio-Donahue CA, Murphy KM, Maitra A, Vogelstein B, Whitehead RH, Markowitz SD, Willson JK, Yeo CJ, Hruban RH, Kern SE. Evidence of selection for clones having genetic inactivation of the activin A type II receptor (ACVR2) gene in gastrointestinal cancers. Cancer Res. 2003;63:994–999. [PubMed] [Google Scholar]
- [84].Rossi MR, Ionov Y, Bakin AV, Cowell JK. Truncating mutations in the ACVR2 gene attenuates activin signaling in prostate cancer cells. Cancer Genet. Cytogenet. 2005;163:123–129. doi: 10.1016/j.cancergencyto.2005.05.007. [DOI] [PubMed] [Google Scholar]
- [85].Ogino H, Yano S, Kakiuchi S, Muguruma H, Ikuta K, Hanibuchi M, Uehara H, Tsuchida K, Sugino H, Sone S. Follistatin suppresses the production of experimental multiple-organ metastasis by small cell lung cancer cells in natural killer cell-depleted SCID mice. Clin. Cancer Res. 2008;14:660–667. doi: 10.1158/1078-0432.CCR-07-1221. [DOI] [PubMed] [Google Scholar]
- [86].de Guise C, Lacerte A, Rafiei S, Reynaud R, Roy M, Brue T, Lebrun JJ. Activin inhibits the human Pit-1 gene promoter through the p38 kinase pathway in a Smad-independent manner. Endocrinology. 2006;147:4351–4362. doi: 10.1210/en.2006-0444. [DOI] [PubMed] [Google Scholar]
- [87].Bao YL, Tsuchida K, Liu B, Kurisaki A, Matsuzaki T, Sugino H. Synergistic activity of activin A and basic fibroblast growth factor on tyrosine hydroxylase expression through Smad3 and ERK1/ERK2 MAPK signaling pathways. J. Endocrinol. 2005;184:493–504. doi: 10.1677/joe.1.05978. [DOI] [PubMed] [Google Scholar]
- [88].Zhang L, Deng M, Parthasarathy R, Wang L, Mongan M, Molkentin JD, Zheng Y, Xia Y. MEKK1 transduces activin signals in keratinocytes to induce actin stress fiber formation and migration. Mol. Cell Biol. 2005;25:60–65. doi: 10.1128/MCB.25.1.60-65.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yamakawa N, Tsuchida K, Sugino H. The rasGAP-binding protein, Dok-1, mediates activin signaling via serine/threonine kinase receptors. EMBO J. 2002;21:1684–1694. doi: 10.1093/emboj/21.7.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wagner K, Peters M, Scholz A, Benckert C, Ruderisch HS, Wiedenmann B, Rosewicz S. Activin A stimulates vascular endothelial growth factor gene transcription in human hepatocellular carcinoma cells. Gastroenterology. 2004;126:1828–1843. doi: 10.1053/j.gastro.2004.03.011. [DOI] [PubMed] [Google Scholar]
- [91].Hayashi Y, Maeshima K, Goto F, Kojima I. Activin A as a critical mediator of capillary formation: interaction with the fibroblast growth factor action. Endocr. J. 2007;54:311–318. doi: 10.1507/endocrj.k06-222. [DOI] [PubMed] [Google Scholar]
- [92].Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- [93].Vallier L, Mendjan S, Brown S, Chng Z, Teo A, Smithers LE, Trotter MW, Cho CH, Martinez A, Rugg-Gunn P, Brons G, Pedersen RA. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development. 2009;136:1339–1349. doi: 10.1242/dev.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Fischer H, Taylor N, Allerstorfer S, Grusch M, Sonvilla G, Holzmann K, Setinek U, Elbling L, Cantonati H, Grasl-Kraupp B, Gauglhofer C, Marian B, Micksche M, Berger W. Fibroblast growth factor receptor-mediated signals contribute to the malignant phenotype of non-small cell lung cancer cells: therapeutic implications and synergism with epidermal growth factor receptor inhibition. Mol. Cancer Ther. 2008;7:3408–3419. doi: 10.1158/1535-7163.MCT-08-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat. Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- [96].Nesbit M, Nesbit HK, Bennett J, Andl T, Hsu MY, Dejesus E, McBrian M, Gupta AR, Eck SL, Herlyn M. Basic fibroblast growth factor induces a transformed phenotype in normal human melanocytes. Oncogene. 1999;18:6469–6476. doi: 10.1038/sj.onc.1203066. [DOI] [PubMed] [Google Scholar]
- [97].Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- [98].Sapkota G, Alarcon C, Spagnoli FM, Brivanlou AH, Massague J. Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol. Cell. 2007;25:441–454. doi: 10.1016/j.molcel.2007.01.006. [DOI] [PubMed] [Google Scholar]
- [99].Wrighton KH, Lin X, Yu PB, Feng XH. Transforming Growth Factor {beta} Can Stimulate Smad1 Phosphorylation Independently of Bone Morphogenic Protein Receptors. J. Biol. Chem. 2009;284:9755–9763. doi: 10.1074/jbc.M809223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Suzawa M, Tamura Y, Fukumoto S, Miyazono K, Fujita T, Kato S, Takeuchi Y. Stimulation of Smad1 transcriptional activity by Ras-extracellular signal-regulated kinase pathway: a possible mechanism for collagen-dependent osteoblastic differentiation. J. Bone Miner. Res. 2002;17:240–248. doi: 10.1359/jbmr.2002.17.2.240. [DOI] [PubMed] [Google Scholar]
- [101].Beck SE, Carethers JM. BMP suppresses PTEN expression via RAS/ERK signaling. Cancer Biol. Ther. 2007;6:1313–1317. doi: 10.4161/cbt.6.8.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Foletta VC, Lim MA, Soosairajah J, Kelly AP, Stanley EG, Shannon M, He W, Das S, Massague J, Bernard O. Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J. Cell Biol. 2003;162:1089–1098. doi: 10.1083/jcb.200212060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kodach LL, Bleuming SA, Musler AR, Peppelenbosch MP, Hommes DW, van den Brink GR, van Noesel CJ, Offerhaus GJ, Hardwick JC. The bone morphogenetic protein pathway is active in human colon adenomas and inactivated in colorectal cancer. Cancer. 2008;112:300–306. doi: 10.1002/cncr.23160. [DOI] [PubMed] [Google Scholar]
- [104].Kodach LL, Wiercinska E, de Miranda NF, Bleuming SA, Musler AR, Peppelenbosch MP, Dekker E, van den Brink GR, van Noesel CJ, Morreau H, Hommes DW, Ten Dijke P, Offerhaus GJ, Hardwick JC. The bone morphogenetic protein pathway is inactivated in the majority of sporadic colorectal cancers. Gastroenterology. 2008;134:1332–1341. doi: 10.1053/j.gastro.2008.02.059. [DOI] [PubMed] [Google Scholar]
- [105].Kraunz KS, Nelson HH, Liu M, Wiencke JK, Kelsey KT. Interaction between the bone morphogenetic proteins and Ras/MAP-kinase signalling pathways in lung cancer. Br. J. Cancer. 2005;93:949–952. doi: 10.1038/sj.bjc.6602790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, Ogata E, Ehata S, Miyazono K, Imamura T. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene. 2008;27:6322–6333. doi: 10.1038/onc.2008.232. [DOI] [PubMed] [Google Scholar]
- [107].Theriault BL, Shepherd TG, Mujoomdar ML, Nachtigal MW. BMP4 induces EMT and Rho GTPase activation in human ovarian cancer cells. Carcinogenesis. 2007;28:1153–1162. doi: 10.1093/carcin/bgm015. [DOI] [PubMed] [Google Scholar]
- [108].Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- [109].Javelaud D, Mauviel A. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene. 2005;24:5742–5750. doi: 10.1038/sj.onc.1208928. [DOI] [PubMed] [Google Scholar]
- [110].Iyer S, Wang ZG, Akhtari M, Zhao W, Seth P. Targeting TGFbeta signaling for cancer therapy. Cancer Biol. Ther. 2005;4:261–266. doi: 10.4161/cbt.4.3.1566. [DOI] [PubMed] [Google Scholar]
- [111].Pennison M, Pasche B. Targeting transforming growth factor-beta signaling. Curr. Opin. Oncol. 2007;19:579–585. doi: 10.1097/CCO.0b013e3282f0ad0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-beta signalling inhibitors for cancer therapy. Nat. Rev. Drug Discov. 2004;3:1011–1022. doi: 10.1038/nrd1580. [DOI] [PubMed] [Google Scholar]
- [113].Zhang B, Halder SK, Zhang S, Datta PK. Targeting transforming growth factor-beta signaling in liver metastasis of colon cancer. Cancer Lett. 2009;277:114–120. doi: 10.1016/j.canlet.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, Wang XW. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- [116].Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- [117].Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat. Rev. Drug Discov. 2007;6:541–555. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- [118].Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr. Opin. Cell Biol. 2009;21:177–184. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- [119].Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, Hanson LJ, Gore L, Chow L, Leong S, Maloney L, Gordon G, Simmons H, Marlow A, Litwiler K, Brown S, Poch G, Kane K, Haney J, Eckhardt SG. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J. Clin. Oncol. 2008;26:2139–2146. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Downward J. Cancer: A tumour gene’s fatal flaws. Nature. 2009;462:44–45. doi: 10.1038/462044a. [DOI] [PubMed] [Google Scholar]
- [121].Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, Schinzel AC, Sandy P, Meylan E, Scholl C, Frohling S, Chan EM, Sos ML, Michel K, Mermel C, Silver SJ, Weir BA, Reiling JH, Sheng Q, Gupta PB, Wadlow RC, Le H, Hoersch S, Wittner BS, Ramaswamy S, Livingston DM, Sabatini DM, Meyerson M, Thomas RK, Lander ES, Mesirov JP, Root DE, Gilliland DG, Jacks T, Hahn WC. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, Jacks T. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature. 2009;462:104–107. doi: 10.1038/nature08462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Felsher DW. Oncogene addiction versus oncogene amnesia: perhaps more than just a bad habit? Cancer Res. 2008;68:3081–3086. doi: 10.1158/0008-5472.CAN-07-5832. [DOI] [PubMed] [Google Scholar]