Abstract
Despite significant advances in our understanding of the pathophysiology of acute lung injury, a lung-protective strategy of mechanical ventilation remains the only therapy with a proven survival advantage. Numerous pharmacologic therapies have failed to show benefit in multicenter clinical trials. The paradigm of early, goal-directed therapy of sepsis suggests greater clinical benefit may derive from initiating therapy prior to the onset of respiratory failure that requires mechanical ventilation. Thus, there is heightened interest in more accurate and complete characterization of high-risk patient populations and identification of patients in the early stage of acute lung injury, prior to the need for mechanical ventilation. This article discusses the growing literature on clinical predictors of acute lung injury (including risk factors for specific subgroups) with an emphasis on transfusion-related risk factors and recent research targeting the early identification of high-risk patients and those with early acute lung injury prior to the onset of respiratory failure.
Keywords: acute lung injury, acute respiratory distress syndrome, mechanical ventilation, noninvasive ventilation, pulmonary edema, risk factors, surgery, transfusion-related lung injury, trauma
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) represent a spectrum of acute hypoxemic respiratory failure disorders, characterized by bilateral airspace consolidation with high permeability and protein-rich edema fluid. ARDS was first described by Ashbaugh and colleagues in a series of 12 patients in 1967 [1]. They recognized a common pattern of severe respiratory distress, refractory cyanosis, loss of lung compliance and diffuse alveolar infiltrates in a variety of clinical disorders, including sepsis, pneumonia, aspiration and major trauma. Previously, similar syndromes of acute respiratory failure were recognized only as distinct conditions named for their specific inciting etiology (e.g., Da Nang lung, shock lung, post-traumatic lung and respirator lung) [2]. However, lack of consistent definitions and appropriately powered clinical trials diluted the impact of early research efforts and constrained improvements in clinical outcomes. As recently as 1990, the mortality rate was estimated to be as high as 67% [3].
In 1994, the American and European Consensus Conference (AECC) established more specific clinical criteria for ALI and ARDS, providing standardization for clinical research and multicenter clinical trials [4]. However, despite our improved understanding of the etiologies and pathophysiology of ALI, in the nearly 20 years since the AECC [5], a lung-protective strategy of mechanical ventilation is the only supportive therapy that clearly improves survival [6]. Other ventilatory strategies, including prone positioning [7,8], high levels of positive end-expiratory pressure (PEEP) [9–11] and a conservative fluid strategy [12], have shown potential benefit in terms of a reduction in the duration of mechanical ventilation, but none have significantly reduced mortality. While these studies represent advances in the supportive care of patients with ALI, no disease-specific treatments targeting the pathogenesis of the underlying lung injury can currently be recommended. Numerous pharmacologic therapies have shown promise in early phase studies but failed to demonstrate benefit in multicenter clinical trials [13,14]. The apparent benefit of early goal-directed therapy for sepsis [15] suggests that greater clinical benefit may derive from initiating therapy prior to the onset of mechanical ventilation-dependent respiratory failure. Early intervention to limit tidal volumes and transfusions in at-risk patients may prevent ALI [16,17]. Multiple pharmacologic therapies that have either failed to show benefit in ALI after progression to mechanical ventilation, such as aerosolized albuterol (NCT00434993 [201]), or that are currently being evaluated, such as statins (SAILS Trial [NCT00979121] [202] and HARP study [18]) or antiplatelet agents [19], may yield additional benefit if initiated earlier in the progression of lung injury. This focus has led to greater interest in earlier identification of ALI and better characterization of high-risk patient populations prior to the onset of lung injury.
Improved understanding of cellular pathways of injury and genomic and proteinomic signatures of ALI offer potential for more accurate and early detection but are not currently sufficiently validated for use in clinical practice [5,20–24]. These topics have been reviewed elsewhere and are beyond the scope of this article, which will focus on the growing literature on clinical risk factors and strategies for early identification of ALI.
Limitations of current consensus criteria
The AECC criteria have allowed standardization of patients for clinical trial and epidemiologic purposes. However, controversies with the criteria still exist.
Correlation between clinical criteria and the accepted histopathalogic correlate of diffuse alveolar damage (DAD) is less than perfect. Esteban et al. found a clinical diagnosis of ALI was 75% sensitive and 84% specific for the presence of DAD on autopsy of patients in an intensive care unit (ICU) [25], while de Hemptinne et al. found DAD was present at autopsy in only 50% of patients with clinical criteria for ALI [26]. However, autopsy findings may not conform temporally to the constellation of clinical symptoms, and histopathologic data are rarely available in clinical settings. Until more accurate physiologic, laboratory or imaging assessments are routinely available, current clinical criteria will need to continue to define the syndrome.
While a syndrome defined by physiologic parameters fosters conceptualization of ALI as a final common pathway of a broad array of potential etiologies of lung injury (a major strength of Ashbaugh's series and of the AECC criteria), it also results in inclusion of heterogeneous patient populations with potentially different pathophysiologies and prognoses. Better characterization and risk stratification of subgroups of ALI may be important for the success of future clinical trials. Furthermore, criteria based on physiologic parameters, as opposed to disease entities, may not be intuitive for clinicians. A recent survey of ICU clinicians still identified ‘physician under-recognition’ of the syndrome as a barrier to implementing lung-protective ventilation [27].
In addition, the AECC criteria include acute respiratory failure and require calculation of the PaO2/FiO2 ratio [4]. Traditionally, this has limited the diagnosis to patients receiving mechanical ventilation. In the most rigorous prospective study to date of the epidemiology of ALI/ARDS in the USA, Rubenfeld et al. interpreted respiratory failure to include mechanical ventilation via a noninvasive facemask or endotracheal tube [28]. However, other authors have expanded interpretation of the consensus criteria to include nonmechanically ventilated patients and patients outside of the ICU [29–32]. In a pediatric study of ALI, 85 out of 328 children were not intubated at the time of diagnosis and 46 never required intubation or mechanical ventilation [30]. A second pediatric study retrospectively identified emergency department patients with acute hypoxic respiratory failure defined as a PaO2/FiO2 ratio of less than 300 (using a PaO2 derived from recorded saturations and charted FiO2) [31]. However, only 5% of these patients were intubated during the follow-up period. Ferguson et al. prospectively followed 815 patients who were admitted to a hospital ward or an ICU with at least one predefined risk factor for ALI [29]. Overall, 53 patients (7%) developed ALI; 17 patients were diagnosed with ALI outside of the ICU (15 were never admitted to an ICU) and 24 were not receiving mechanical ventilation at the time of the diagnosis.
Expanding the definition of ALI to outside the ICU may contribute to earlier recognition but it raises several issues that may jeopardize standardization of study populations. First, measuring a PaO2/FiO2 ratio assumes a reasonably accurate estimation of the inspired FiO2, which can be problematic in patients who are spontaneously breathing with an indeterminate inspired concentration of oxygen (i.e., not an endotracheal tube or tight-fitting noninvasive mask). Still, high oxygen flow rates delivered by a facemask likely result in an inspired FiO2 of at least 50% [33] so only a PaO2 less than 150 mmHg would be required to meet the PaO2/FiO2 criteria for ALI (PaO2/FiO2 <300). However, quantifying lung injury by a PaO2/FiO2 ratio in spontaneously breathing patients ignores the beneficial effects of positive pressure ventilation on lung recruitment and oxygenation, and is likely not directly comparable to mechanically ventilated patients to whom this criterion has traditionally been applied. Furthermore, expanding the criteria to include spontaneously breathing patients focuses the definition of respiratory failure to merely a need for supplemental oxygen without regard for respiratory distress or impending respiratory arrest. If a PaO2/FiO2 ratio less than 300 is the sole criterion for respiratory failure, then any patient with a PaO2 less than 63 mmHg (arterial saturation of 92%) on room air (21% FiO2) could meet criteria for ALI. Finally, and most relevant to this article, this expanded definition blurs the distinction between early recognition of ALI versus identifying high-risk patients with clinical predictors of developing ALI. For example, a recent study compared ALI (defined by bilateral infiltrates and hypoxemia) to patients without one or both in patients admitted to respiratory isolation rooms outside the ICU [32]. Respiratory distress was higher in the ALI group but mortality was low and similar between groups (12 vs 10%). If ALI by these criteria does correlate with important differences in outcomes and healthcare utilization, is it a meaningful distinction? While convenient, direct application of ALI criteria to spontaneously breathing patients outside the ICU may not provide clinical relevance without more rigorous validation in these cohorts.
Early identification of at-risk patients
Inside the ICU
Early studies prior to standardized criteria and management for ALI/ARDS and sepsis identified the incidence of ARDS associated with several predisposing conditions [34–36]. Hudson et al. prospectively evaluated 695 ICU patients at risk for development of ARDS [35]. The presence of one or more of seven clinical risk factors (e.g., sepsis, aspiration or trauma) was 79% sensitive and 26% specific for developing ARDS. Sepsis (43%), massive transfusion (40%) and multiple trauma (25%) carried the greatest risk of developing ARDS. Increasing age, Acute Physiologic and Chronic Health Evaluation II (APACHE II) and Injury Severity Scores (ISS) were also associated with an increased incidence of ARDS. Previously, Fowler et al. found pneumonia (12%), aspiration (36%) and disseminated intravascular coagulation (22%) were the strongest predictors but the overall incidence of ALI was only 7% [34]. The presence of multiple risk factors compounded the risk of ARDS [34–36] and was more predictive than the ISS and the degree of impaired oxygenation [36].
More recently, Gong et al. prospectively evaluated a cohort of 688 ICU patients with a predisposing factor of sepsis, trauma, hypertransfusion or aspiration. Overall, 32% developed ARDS. A pulmonary etiology of injury (pneumonia, aspiration or pulmonary contusion), transfusion of more than eight units of packed red blood cells and transfer from another hospital increased the risk of ARDS, while trauma and a history of diabetes were associated with a lower risk [37]. A respiratory rate above 33 breaths/min, platelets less than 80,000/μl, albumin less than 2.3 g/dl, hematocrit above 37.5% and pH less than 7.33 were also associated with an increased risk of ARDS [37]. The apparent protective effect of diabetes was also observed in a cohort of septic patients at risk for ARDS [38]. In septic patients, additional factors have been associated with increased risk of developing ALI including a history of alcohol abuse, delayed initiation of early goal-directed therapy or appropriate antibiotics, treatment with chemotherapy, an increased respiratory rate [38] and hypoproteinemia [39].
Outside the ICU
In a prospective study of patients in an acute-care setting, primarily the emergency department, we found that patients without clinical evidence of left atrial hypertension and without chronic lung disease who had bilateral opacities on the chest radiograph and required more than 2 l/min nasal cannula oxygen, progressed to require mechanical ventilation and meet the standard consensus definition of ALI [40]. On multivariate analysis, only the level of supplemental oxygen was independently associated with progression to ALI (Table 1). Thus, a clinical definition of early ALI, defined by hospital admission with bilateral opacities on the chest radiograph in the absence of left atrial hypertension and a supplemental oxygen requirement greater than 2 l/min to maintain a saturation above 90%, identified patients who progressed to ALI with 73% sensitivity and 79% specificity [40]. When clinical data for the first 72 h of admission (or up to 6 h prior to onset of ALI) were analyzed, only the APACHE II and supplemental oxygen requirement were independent predictors of progression to ALI [Levitt JE, Unpublished Data]. Cutoffs of above 2 l/min and more than 6 l/min of supplemental oxygen had similar discrimination (AUC 0.76 for both) with more than 2 l/min being more sensitive (81 vs 61%) and more than 6 l/min more specific (91 vs 71%).
Table 1. Multivariate analysis of risk factors for progression to acute lung injury in patients with evidence of existing lung injury on chest radiograph.
| Risk factor | OR | 95% CI | p-value |
|---|---|---|---|
| >2 l/min supplemental O2† | 8.1 | 2.7–24.5 | 0.0002 |
| Modified REMS (per 1 point increase) | 1.2 | 1.0–1.4 | 0.07 |
| Immunosuppression‡ | 2.6 | 0.9–7.4 | 0.07 |
| Airspace opacity beyond bases§ | 1.3 | 0.4–3.8 | 0.68 |
| SIRS | 0.9 | 0.2–4.0 | 0.88 |
>2 l/min nasal cannula: amount of supplemental oxygen required to maintain an oxygen saturation >90%.
Immunosuppression: active immunosuppression as defined by APACHE II.
Airspace opacity beyond bases: airspace opacities on chest radiograph beyond bases compared with bibasilar opacities or diffuse interstitial edema.
APACHE II: Acute Physiologic and Chronic Health Evaluation II; OR: Odds ratio; REMS: Rapid Emergency Medicine Score; SIRS: Systemic inflammatory response syndrome.
Reprinted from [40] with permission of the American College of Chest Physicians.
In 815 ward patients with at least one risk factor for ALI, Ferguson et al. found pulmonary risk conditions had a higher rate of progression to ALI than nonpulmonary conditions (15 vs 5%; p < 0.0001), but shock was the strongest predictor (shock 36%, pneumonia without shock 10% and nonpulmonary sepsis 1%; p < 0.001) [29].
In the largest prospective multicenter study to date, Gajic et al. recently reported validation of their previously derived Lung Injury Prediction Score (LIPS) [41,42]. Using a 2500 patient training cohort and a 3084 patient validation cohort recruited from 22 centers in the USA, the investigators modeled the development of ALI from previously published risk factors present at the time of hospital admission [41]. Risk factors were divided into predisposing conditions (sepsis, shock, pneumonia, aspiration, trauma and high-risk surgery) and risk modifiers (obesity, alcohol abuse, diabetes, hypoalbuminemia, acidosis, tachypnea and oxygen supplementation). The overall incidence of ALI in the cohort was low at 7%. The incidence of ALI varied depending on risk factor, with smoke inhalation (26%) and shock (18%) carrying the highest risk and spine surgery and pancreatitis (3% each) the lowest risk of developing ALI. Overall, nonmutually exclusive diagnoses of shock, sepsis and/or pneumonia occurred in 298 (79%) of 377 cases of ALI. On multivariate analysis, shock, aspiration, traumatic brain injury, high-risk (acute abdomen, cardiac and aortic vascular) and emergent surgery were independent predictors for developing ALI. Obesity (BMI >30), tachypnea (respiratory rate >30/min), supplemental oxygen greater than 4 l/min, acidosis (pH <7.35) and hypoalbuminemia were modifiers that increased risk of ALI (Table 2). The AUC of their model was 0.80 and it outperformed the APACHE II (AUC 0.67). A LIPS of 4 provided the best discrimination with an associated sensitivity of 69%, specificity of 78% and a positive predictive value of 18%.
Table 2. Multivariate regression analysis of risk factors for acute lung injury.
| Predisposing conditions | OR† | 95% CI† | p-value |
|---|---|---|---|
| Shock | 2.2 | 1.2–3.7 | 0.008 |
| Aspiration | 2.2 | 1.1–4.3 | 0.02 |
| Sepsis | 1.4 | 0.9–2.4 | 0.14 |
| Pneumonia | 0.3 | 0.02–1.7 | 0.3 |
| High-risk surgery | |||
| Thoracic (noncardiac) | 0.9 | 0.1–3.2 | 0.9 |
| Orthopedic spine | 2.1 | 0.9–4.6 | 0.07 |
| Acute abdomen | 2.5 | 1.1– 5.6 | 0.03 |
| Cardiac | 3.7 | 2.0–7.1 | <0.001 |
| Aortic vascular | 5.9 | 2.5–13.0 | <0.001 |
| High-risk trauma | |||
| Traumatic brain injury | 3.6 | 2.0–6.8 | <0.001 |
| Smoke inhalation | 2.5 | 0.8–4.1 | 0.44 |
| Near drowning | 5.4 | 0.06–6.6 | 0.50 |
| Lung contusion | 1.5 | 0.6–3.4 | 0.36 |
| Multiple fractures | 1.9 | 0.8–4.1 | 0.12 |
| Risk modifiers | |||
| Male gender | 1.0 | 0.7–1.5 | 0.91 |
| Alcohol abuse | 1.7 | 0.9–2.9 | 0.08 |
| Obesity (BMI >30) | 1.8 | 1.2–2.5 | 0.004 |
| Chemotherapy | 1.6 | 0.6–3.6 | 0.32 |
| Diabetes mellitus‡ | 0.6 | 0.2–1.2 | 0.14 |
| Smoking | 1.1 | 0.7–1.5 | 0.4 |
| Emergency surgery | 3.1 | 1.6–5.9 | <0.01 |
| Tachypnea (RR >30/min) | 2.0 | 1.1–3.5 | 0.02 |
| SpO2 <95% | 1.4 | 1.0–2.1 | 0.08 |
| FiO2 >0.35 (>4 l/min) | 2.8 | 1.9–4.1 | <0.001 |
| Hypoalbuminemia | 1.6 | 1.0–2.4 | 0.03 |
| Acidosis (pH <7.35) | 1.7 | 1.1–2.7 | 0.02 |
Logistic regression coefficients presented in original table converted to ORs in this reproduction.
Only in sepsis.
OR: Odds ratio.
Reprinted from [41] with permission of the American Thoracic Society.
The incidence of ALI (33%) in the study by Levitt et al. [40] was substantially higher than the 7% in the two studies by Ferguson et al. [29] and Gajic et al. [41]. In Gajic's cohort, even a LIPS above 4 had a positive predictive value of only 18%. This difference likely highlights differences between strategies identifying at-risk patients prior to the onset of lung injury versus identification of existing (defined by bilateral chest radiograph opacities in Levitt et al.) but early ALI prior to onset of respiratory failure. For some pulmonary-specific risk factors (i.e., pneumonia, aspiration and so on), this distinction may be semantic while for others (nonpulmonary sepsis, high-risk elective surgery) the distinction is real, affecting not only the prevalence but also the time of progression to ALI. In the cohort by Levitt et al., median time to progression was less than 24 h [40]. Similarly rapid rates of progression were reported by Ferguson et al. (median 0 days, interquartile range [IQR] 0–2) [29] and Pepe et al. (76% within 24 h) [36]. Gajic et al. included elective admissions for high-risk surgeries and found progression to ALI occurred over a median of 2 days (IQR 1–4 days) [41]. These differences may have important implications when selecting strategies to identify patients within an adequate window to initiate therapeutic or preventative therapies.
Important subgroups of at-risk patients
Mechanically ventilated patients
The tidal volumes in mechanically ventilated patients without ALI are likely an important contributor to the development of lung injury. In a 2004 review of non-ALI patients mechanically ventilated for more than 48 h, Gajic et al. found that tidal volumes were large (11.4 and 10.4 ml/kg predicted bodyweight for women and men, respectively) and were independently (along with transfusions of blood products, acidemia and a history of interstitial lung disease) associated with the development of ALI [43]. In a similar review of 789 patients, Jia et al. found tidal volumes and peak airway pressures along with plasma transfusion, sepsis and a high positive fluid balance independently predicted ARDS [44]. In a prospective trial, Determann et al. randomized 150 critically ill patients requiring mechanical ventilation to receive tidal volumes of 6 or 10 ml/kg predicted bodyweight [45]. The study was stopped early owing to a greater rate of ALI in the higher tidal volume group (14 vs 3%; p = 0.01). In addition, lower tidal volumes led to lower plasma (but not lavage fluid) levels of IL-6 consistent with the results of lower tidal volumes in patients with ALI [6].
By contrast, an early clinical trial of mechanically ventilated patients at risk for ARDS found no difference in rates of ARDS between patients randomized to PEEP of 8 cm H2O versus no PEEP. However, a subsequent trial randomized 131 patients with normal chest radiographs and a PaO2/FiO2 above 250 to receive 5–8 cm H2O of PEEP versus no PEEP and reported reduced rates of ventilator-associated pneumonia (25 vs 9%; p = 0.017) and a number of patients who developed hypoxemia (54 vs 19%; p < 0.001) with a trend toward a reduced rate of ARDS (14 vs 5%; p = 0.08) in patients ventilated with PEEP [46]. In addition, ventilator strategies to reduce atelectasis attenuated bacterial growth and translocation in an animal model of pneumonia [47]. The ideal ventilation strategies for non-ALI patients or for specific subgroups of patients at risk for ALI remain to be established. However, the routine use of modest tidal volumes (6–8 ml/kg predicted bodyweight) and PEEP (5–10 cm H2O) seems a reasonable approach.
Noninvasively ventilated patients
The role of noninvasive mechanical ventilation in the treatment of ALI remains uncertain. A small clinical trial showed reduced rates of endotracheal intubation and mortality in immunocompromised patients with pulmonary infiltrates and respiratory failure who were randomized to receive noninvasive ventilation versus standard treatment with supplemental oxygen [48]. However, in a study of 123 ICU patients with acute hypoxic respiratory failure and pulmonary edema (102 with ALI and 21 with cardiac disease) randomized to receive continuous positive airway pressure (CPAP) versus standard oxygen therapy, CPAP did not reduce rates of endotracheal intubation or mortality despite improved PaO2/FiO2 ratios and subjective improvement at 1 h of therapy. Furthermore, there were more adverse events in the CPAP group suggesting potential detriment with delayed intubation. In a 2006 meta-analysis of randomized clinical trials, Agarwal et al. found noninvasive ventilation did not reduce the rate of endotracheal intubation or mortality in patients with ARDS [49]. However, the number of patients with ARDS in these trials was small and there was significant heterogeneity among the trials. In a subsequent review of noninvasive ventilation at a single center, the same authors found 12 out of 21 patients (57%) with ALI successfully avoided intubation, but there was no difference in response to noninvasive ventilation between patients with ALI and other causes of acute hypoxemic respiratory failure [50]. Only a lower baseline PaO2/FiO2 ratio independently predicted the failure of noninvasive ventilation. Another single-center review found 33 of 47 patients (70%) with ALI avoided intubation with treatment with noninvasive ventilation [51]. An APACHE II score above 17 and a respiratory rate above 25 after 1-h of noninvasive ventilation predicted failure. Similarly, in a multicenter review of ARDS patients treated with noninvasive ventilation, Antonelli et al. found a 1 h Simplified Acute Physiology Score II above 34 and a PaO2/FiO2 ratio of less than 175 predicted failure [52]. In this review, 79 of 147 patients (54%) avoided intubation, and avoidance of intubation led to lower rates of ventilator-associated pneumonia and mortality. However, in a series of 79 patients with ALI treated with noninvasive ventilation, 70% (including all 19 patients with shock) required endotracheal intubation [53]. In patients without shock, metabolic acidosis and severe hypoxemia independently predicted failure of noninvasive ventilation. In addition, patients who failed noninvasive ventilation had higher-than-predicted mortality, again suggesting potential detriment with delayed intubation. Heterogeneity in settings (noninvasive ventilation vs CPAP), patient selection and clinician expertise may explain the different results across centers. However, noninvasive ventilation needs further validation in prospective randomized trials before it can be routinely recommended in patients with ALI or ARDS.
Transfusion-related lung injury
There is growing evidence that transfusion of blood products plays an important role in the pathogenesis of ALI in at-risk patients. Numerous authors have reported increased rates of ALI with transfusion of red blood cells [37,54–60], platelets [60–63] and fresh frozen plasma (FFP) in adults [60–62,64–66] and children [67]. However, quantification of the risk of transfusion is challenged by the complex pathophysiology and current criteria for transfusion-related acute lung injury (TRALI). Improved understanding of the pathophysiology of TRALI has identified the importance of the ‘two-hit’ model [68]. First, the pulmonary vascular endothelium is activated by one or more endogenous stimuli (i.e., sepsis or surgery) resulting in priming and adherence of neutrophils [69,70]. A second event, such as the transfusion of antibodies to leukocyte antigens or the infusion of bioactive lipids, results in activation and neutrophil-mediated cytotoxicity of the vascular endothelium, resulting in capillary leak and ALI [68,71].
However, despite this enhanced understanding of the importance of a ‘first hit’, current clinical criteria for TRALI limit the diagnosis in the setting of existing risk factors for ALI. The 2004 National Heart, Lung and Blood Institute (NHLBI) and Canadian Consensus Conference criteria exclude pre-existing ALI and require progression within 6 h of transfusion [72,73]. The NHLBI criteria [73] allow coexisting risk factors if clinical deterioration is appropriately temporally related to transfusion, while the Canadian criteria [72] only allow a diagnosis of ‘possible TRALI’ in the setting of a known risk factor for ALI. Past estimates, dependent on recognition of overt cases in low-risk patients, place the incidence of TRALI at a fraction of a percent and likely underestimate the true incidence [74,75]. In the largest prospective trial to date enrolling 901 consecutively transfused medical ICU patients over a 2-year period, Gajic et al. found an incidence of TRALI of 8% [60]. Even this estimate likely underestimates the true contribution of transfusions to the development or worsening of lung injury in critically ill patients. Expanding the definition to include delayed TRALI (development of ALI 6–72 h after transfusion, regardless of existing risk factors) increases the incidence to 25% with an associated mortality of 40% [76]. A prospective trial of 225 patients admitted to an ICU owing to gastrointestinal bleeding found a TRALI incidence of 17%, which increased to 29% in patients with end-stage liver disease [77]. In patients with existing ALI, Gong et al. found a dose-dependent increase in mortality with an increasing number of red blood cell transfusions [37]. In the prospective trial by Gajic et al., 12% of patients had worsening of their oxygenation following transfusion [60].
To complicate the diagnostic complexity even more, TRALI must be distinguished from the related entities of transfusion-related circulatory overload, which may be three-times as prevalent as TRALI [63], anaphylactic transfusion reactions, and transfusion of contaminated blood products. In addition, we need to shift the paradigm of TRALI from the patient developing ALI after receiving a massive red blood cell transfusion. Gajic et al. showed a higher incidence of TRALI with plasma-rich products (FFP and platelets) compared with packed red blood cells [60]. Many older studies [34,35,78] failed to control for FFP, which was routinely given along with massive red blood cell transfusion while more recent studies in medical, trauma and surgical populations have found plasma containing blood products were independent predictors of ALI, while packed red blood cells were not [57,60,62,63,79].
Important additional donor and host factors exist. Gajic et al. found a history of chronic alcohol abuse or sepsis in transfusion recipients increased the risk of ALI, while female gender and a higher number of pregnancies among donors increased rates of post-transfusion ALI. Higher rates of ALI were also associated with a higher number of units with anti-granulocyte and anti-HLA class II antibodies and increased concentrations of lysophosphatidylcholine in the donor products [60]. In addition, in a case–control study of ICU patients transfused with more than 2 units of FFP or apheresis platelets, Gajic et al. found a deterioration in oxygenation (PaO2/FiO2) in patients receiving female only but not male only products compared with controls [64]. Recipients of male only products had more ventilator-free days and a strong trend toward improved survival. The increased incidence of TRALI with female and multiparous donors has led to removal of females from the plasma donor pool in the UK, The Netherlands and many parts of the USA [68]. The move to male only plasma products led to a reduction in the observed incidence of TRALI and plasma product-associated TRALI in the UK [80]. The role of other donor factors such as the age (shorter storage time) and leukocyte reduction of packed red blood cells are less well established [57,68].
Trauma
Multiple authors have confirmed the association of transfusions in this subgroup of at-risk patients [54,55,59,66,81]. Using multivariate regression models, Watson et al. found a 2.5% increase in ARDS and 2.1% increase in multiple organ failure per unit of FFP received in 1175 blunt trauma patients with hemorrhagic shock [66]. Navarrete-Navarro et al. reported an incidence of ARDS of 7% in 693 patients prospectively identified with severe trauma (ISS ≥16). Chest trauma (sternal fracture or pneumothorax), femur fracture, number of long bone fractures, APACHE II, ISS and amount of blood and colloid transfusions were associated with ARDS, but on multivariate analysis, only chest trauma, APACHE II and blood transfusions independently predicted the development of ARDS [82]. Miller et al. reported an incidence of ARDS of 5% in 4397 patients receiving blunt (nonpenetrating) trauma [83]. Age above 65 years, ISS greater than 25, hypotension and transfusion of more than 10 units of packed red blood cells in the first 24 h predicted ARDS but metabolic acidosis, femur fracture, infection or severe brain injury did not. However, in studies of smaller cohorts, the severity of metabolic acidosis was associated with developing ALI [81,84]. Rainer et al., using classification and regression tree analysis, found an ISS above 27 and a hematocrit less than 37, or a hematocrit less than 36 and a white blood cell count less than 15 predicted ALI with good sensitivity and specificity (classification rate of 96.7%) [85]. Pallister et al. reported that elevated levels of urinary albumin excretion rate were a good predictor of early post-traumatic ALI [86].
Mascia et al. found higher tidal volumes and respiratory rates were associated with developing ALI in patients intubated for severe brain injury [87]. Kahn et al. reported an incidence of ALI of 27% in 626 patients with subarachnoid hemorrhage indentified retrospectively by CT scan report [88]. Severity of illness, clinical grade of hemorrhage, severe sepsis and transfusion of packed red blood cells all predicted ALI on multivariate analysis.
In analysis of a prospective database of 897 trauma patients collected from 1997 to 2004, Ciesla et al. found that PaO2/FiO2 ratios at 24 h were unchanged throughout the study period on both univariate and multivariate analysis controlling for age, severity of injury and blood transfusion in the first 12 h [17]. However, the adjusted PaO2/FiO2 at 72 h improved significantly during the study period and rates of ARDS and multiple organ failure decreased from 43 to 25% and 33 to 12%, respectively. The authors conclude that baseline severity of injury or ‘systemic priming’ as a risk for lung injury did not change over the study period, but that improvements in supportive care (lung-protective ventilation, judicious transfusions, tight glycemic control and treatment of adrenal insufficiency) attenuated the inflammatory response and reduced rates of ARDS and multiple organ failure. This study highlights the potential of early and accurate identification of at-risk patients to prevent or reduce the severity of ALI.
Surgical patients
The important perioperative risk factors for ALI, including the role of lung-protective ventilation, are not as well characterized in surgical patients. There is evidence that the type of anesthesia may impact postoperative lung function through suppression of the adrenal axis and impaired resolution of pulmonary edema [89]. Recently, Gajic et al. (in a prospective cohort of >5000 patients with at least one previously published risk factor for ALI) documented the incidence of ALI in five high-risk surgeries [41]. In decreasing order, incidence of ALI was 17% (21 out of 127) for aortic surgery, 10% (55 out of 541) for cardiac surgery, 9% (27 out of 295) for an acute abdomen, 4% (7 out of 175) for thoracic surgery and 3% (16 out of 486) for spine surgery. In multivariate analysis, acute abdominal, cardiac and aortic vascular surgery were independent predictors of ALI and spine surgery showed a trend for an increased risk (p = 0.07).
Hughes et al. found that intraoperative fluid resuscitation but not tidal volumes or number of packed red blood cell transfusions predicted postoperative ARDS [90]. In a subgroup of patients undergoing major abdominal surgery, an open lung strategy of intraoperative mechanical ventilation (6 ml/kg tidal volume with high PEEP of 15 cm H2O and recruitment maneuvers) improved intraoperative PaO2/FiO2 and lung mechanics but not postoperative PaO2/FiO2 or plasma levels of IL-6 or IL-8 compared with standard practice (10 ml/kg tidal volume with no PEEP or recruitment maneuvers) [91]. In general, lung-protective modes of mechanical ventilation are not routinely used intraoperatively, even in patients who might otherwise meet criteria for ARDS. In a recent survey of noncardiac or thoracic surgery cases, Blum et al. compared the intraoperative ventilation strategies among patients grouped by their intraoperative PaO2/FiO2 ratio (>300, 200–300, 100–200 or <100) [92]. Tidal volumes ranged from 8.6 to 9.2 ml/kg predicted bodyweight and PEEP from 2.5 to 5.5 cm H2O. There were only minor statistical but no clinically relevant differences in tidal volumes or PEEP between patients based on their PaO2/FiO2 ratio. In an early clinical trial (published 10 years prior to publication of the ARDS Network trial of lower tidal volumes), Lee et al. randomized 103 noncardiac and non-neurosurgical surgical ICU patients to receive tidal volumes of 6 versus 12 cc/kg [93]. There was a trend toward lower pulmonary infections (p = 0.06) and duration of intubation (p = 0.07) and ICU stay (p = 0.06) in the lower tidal volume group despite a low baseline severity of illness (mean APACHE II of 13) in both groups. The lack of routine use of perioperative lung-protective ventilation may increase the risk of developing postoperative ALI. Multicenter prospective trials evaluating the effects of perioperative tidal volumes, airway pressures and fluid and blood product transfusion strategies are called for [89].
Surgical subgroups
Cardiopulmonary bypass represents a special class of surgery. Mechanisms and interventions to prevent lung injury after cardiopulmonary bypass have been reviewed by Clark [94]. While the cytokine release and complement activation due to the bypass circuit may predispose to ALI, studies comparing off-pump to onpump coronary artery bypass grafting have failed to demonstrate consistent improved postoperative lung function with off-pump procedures. Other interventions such as maintaining ventilation during the procedure, heparin coating of the extracorporeal circuit, leukocyte reducing filters and treatment with aproprotinin, pentoxyphylline or aspirin have shown variable improvements in biologic markers and lung function, but none have been validated to improve outcomes in a prospective clinical trial [94].
In a subgroup of patients undergoing pneumonectomy for lung cancer, Jeon et al. found higher tidal volumes (odds ratio: 3.37 per 1 ml/kg predicted bodyweight increase) and higher airway pressures (odds ratio: 2.32 per 1 cm H2O increase) during single lung ventilation were independently associated with postoperative ALI [95]. Kim et al. found that a lower predicted postoperative forced expiratory volume in 1 s (FEV1) and a greater perfusion fraction of resected lung independently predicted postoperative ARDS [96]. Incidence of ARDS was 17% when the perfusion fraction of resected lung was above 35% compared with 3.3% when it was not and was independent of the predicted postoperative FEV1.
Kuzniar et al. reviewed 84 cases of talc pleurodesis. Postprocedure ALI was uncommon (6%) but severe hypoxemia (defined by a requirement of an increase in FiO2 of 0.15 on two successive measurements within 6 h) was common (30%). The presence of preprocedure peripheral edema, any preprocedure oxygen requirement or receipt of chemotherapy in the previous 14 days were independent predictors for postprocedure ALI or severe hypoxemia [97].
Other subgroups
In the subset of ALI secondary to the acute chest syndrome, younger age, homozygous SS disease, lower levels of fetal hemoglobin, higher hemoglobin and leukocyte levels, avascular bone necrosis and a prior history of acute chest syndrome have all been associated with the development of ALI [98].
Lee et al. found ARDS occurred in 14 out of 124 cases (11%) of endoscopic sclerotherapy for bleeding esophageal varicies [99]. Sepsis, low baseline albumin, use of balloon tamponade and more than one sclerotherapy session (but not type or volume of sclerosant used) independently predicted ARDS.
Utilizing automated surveillance to enhance early detection
The need to improve recognition of ALI and to identify patients with ALI earlier in their clinical course has prompted attempts to use automated identification with real-time surveillance of the electronic medical record. Herasevich et al. at the Mayo clinic in Rochester (MN, USA) performed an automated continuous surveillance of 3795 ICU patients using their ‘ALI sniffer’ (PaO2/FiO2 <300; Boolean query of radiographic reports for ‘bilateral’ and ‘infiltrate’ or ‘edema’) and identified 325 patients with ALI with a sensitivity and specificity of 95 and 89%, respectively [100]. Physician recognition of ALI was present at the time of ‘sniffer’ identification in only 27% of patients and there was an associated use of larger tidal volumes (9.2 vs 8.0 ml/kg predicted bodyweight) in unrecognized cases of ALI. Azzam et al. demonstrated that automated electronic screening of ICU trauma patients without congestive heart failure identified patients with ALI with 87% sensitivity and 89% specificity before or within 24 h of identification by two blinded physician reviewers [101]. Given the evidence of rapid progression to respiratory failure and ALI following initial injury [29,40,78] and the widespread adoption of electronic medical records, refined automated surveillance systems have the potential to play a role in identification and treatment of patients with developing lung injury.
Imaging of early ALI
The degree of consolidation on a chest radiograph has been shown to correlate with the extravascular lung water (assessed by weight corrected for predicted bodyweight of explanted lungs) [102]. However, the chest radiograph is not a reliable predictor of progression to ALI. Our research group compared the value of the radiographic component of the Lung Injury Score (LIS) to a modified LIS (scoring each quadrant as 0 for no opacity, 0.5 for <50% opacified or interstitial edema, and 1 for >50% opacified) for predicting progression to ALI in patients admitted to the hospital with bilateral radiographic opacities not due to isolated left atrial hypertension [103]. Agreement between two independent chest radiologists was poor (kappa 0.37 and 0.25 for the LIS and modified LIS, respectively) and scores were not associated with progression to ALI. A qualitative classification system found bilateral consolidation beyond the bases (compared with limited bibasilar opacities or interstitial edema) was associated with progression to ALI on univariate analysis, but not on multivariate analysis including the level of supplemental oxygen requirement [40].
Computed tomography (CT) scoring of the degree of consolidation and fibroproliferative change (traction bronchiectasis and bronchiolectasis) [104], diffuse versus patchy or lobar attenuation [105], and percentage of recruitable lung [106] are associated with greater mortality [104–106], worse lung compliance [105], and fewer ventilator-free days and more pneumothoraces in patients with ALI [104]. However, CT has not been well evaluated for predicting progression to ALI. A review of CT scans in trauma patients reported that CT scanning was more sensitive than plain radiographs for identifying factures and contusions, but fractures and contusions visible on plain films were more predictive of progression to respiratory failure [107].
Ultrasound is an inexpensive and noninvasive technology that allows rapid and accurate bedside assessment of the lung in ALI. Ultrasound can accurately distinguish normal lung (lung sliding with horizontal A lines), pulmonary edema (anterior B lines with lung sliding), pneumothorax (absent lung sliding with anterior A lines and a positive lung point), lung consolidation and pleural effusions [108]. A tight correlation exists between CT and ultrasound, but not chest radiograph, assessment of lung re-aeration after antibiotic treatment of ventilator-associated pneumonia [109], suggesting ultrasound may be a useful technology to evaluate PEEP-induced alveolar recruitment in ALI.
In a small series of eight patients with blunt trauma and pulmonary contusion, fluorodeoxyglucose positron emission tomography (FDG-PET) identified increased diffuse signal in the lung of patients who developed ARDS compared with those who did not [110]. FDG-PET scanning identified increased uptake, presumably due to neutrophil sequestration, in areas of normally aerated lung in patients with ALI, suggesting that it may be a sensitive and early marker of lung injury prior to evidence of radiographic consolidation [111,112]. However, the clinical utility of labor-intensive, advanced imaging in ALI is unclear.
Expert commentary
Other than lung-protective ventilation, clinical trials in ALI have failed to demonstrate improvements in survival. Studying heterogeneous patient populations with potential different prognoses and pathophysiologies as well as delaying initiation of therapy until after respiratory failure requiring mechanical ventilation (inclusion requirement of most clinical trials) may limit our ability to identify effective treatments. Identifying better biologic markers of lung injury and genomic and proteinomic signals of alterations in cellular pathways contributing to lung injury may allow more specific targeting and earlier initiation of treatment.
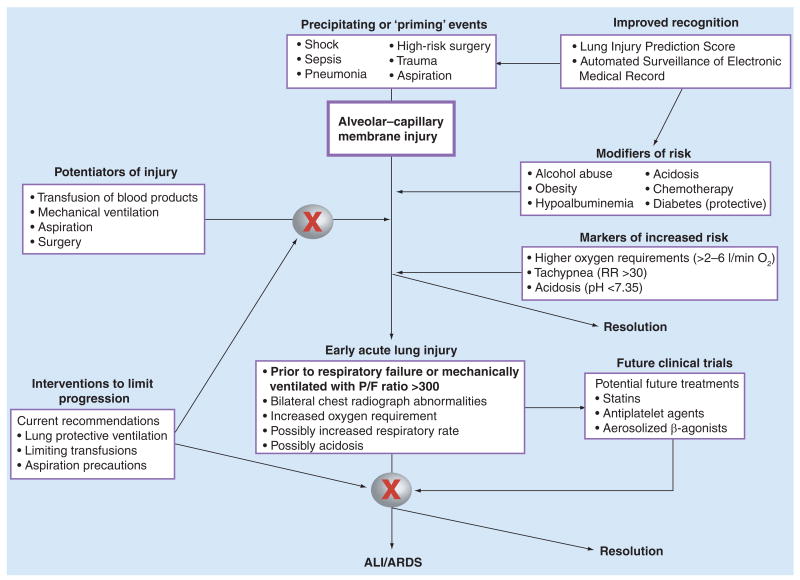
However, these techniques are not currently ready for clinical practice. Better characterization of important clinical risk factors for developing ALI and of patients with early lung injury who are at high risk for progression to respiratory failure, may allow early intervention to prevent or attenuate the progression of lung injury. However, direct application of current AECC criteria to spontaneous breathing patients outside the ICU may not identify a clinically relevant cohort without further validation of these criteria in these patient populations. The recently developed LIPS [41] and criteria for early ALI [40] offer an opportunity to accurately identify high-risk patients and initiate interventions prior to the onset of respiratory failure requiring mechanical ventilation. We suggest a paradigm of increased recognition of conditions (sepsis, trauma, high-risk surgery, and so on) that may either prime the lung for a secondary injury or induce early ALI with risk for progression to respiratory failure, as well as modifiers (such as transfusion of blood products and larger tidal volumes) that may perpetuate lung injury in susceptible patients (Figure 1). This conceptualization highlights potential targets, both pathways of lung injury and novel patient populations, for future clinical trials addressing prevention and early intervention to attenuate lung injury and progression to respiratory failure.
Figure 1. Approach to early identification and management of patients at risk for acute lung injury.
Improved recognition of at-risk patients (‘priming’ events) and risk modifiers (comorbid conditions) via automated surveillance and the Lung Injury Prediction Score, as well as potentiators of lung injury (transfusions of blood products, mechanical ventilation, aspiration, and so on), may allow early interventions to attenuate early injury and prevent the development of ALI. In addition, establishing validated clinical criteria for early but existing ALI in spontaneously breathing patients will provide novel cohorts for future clinical trials of treatments (possibly statins, antiplatelet agents or aerosolized β-agonists) prior to onset of respiratory failure. Initiating treatment earlier in the progression of lung injury may identify a therapeutic window and an opportunity to improve outcomes not attained when treatment is delayed until after the onset of respiratory failure and need for mechanical ventilation. ALI: Acute lung injury; ARDS: Acute respiratory distress syndrome.
To date, no disease-specific therapies other than lung-protective ventilation have proven to decrease mortality when initiated after progression to mechanical ventilation. However, clinical trials of additional pharmacologic therapies are ongoing and designing future clinical trials to initiate treatment earlier in the progression of lung injury may identify a therapeutic window and an opportunity to improve outcomes not attained when treatment is delayed until after onset of respiratory failure and need for mechanical ventilation. Currently, initiating interventions such as lower tidal volumes in mechanically ventilated patients and limiting blood and plasma transfusions in other high-risk patients may reduce rates of progression to ALI.
Five-year view
Improved understanding of clinical predictors of ALI and better characterization of the earliest phases of ALI will lead to novel empirically derived criteria such as the LIPS and early ALI identifying patients prior to the onset of acute respiratory failure requiring mechanical ventilation. These criteria will provide novel cohorts for future clinical trials that can target prevention of ALI and treatment of early ALI prior to progression to the need for mechanical ventilation. Similar to the paradigm of early goal-directed therapy of sepsis, clinical benefit may derive from earlier recognition and treatment.
In addition, the propagation of electronic medical records will allow refinement of automated surveillance systems that improve recognition of high-risk patients and patients with early lung injury and, along with increased protocol driven care, increase the use of earlier interventions to reduce rates of progression to ALI. Better characterization of risk factors across diverse subgroups of patients at risk for ALI may allow more targeted interventions in specific patient populations. Finally, improved recognition of the important role that transfusion of blood products plays in initiation and exacerbation of lung injury will lead to wider adoption of evidence-based guidelines to manage transfusion practices in critically ill patients.
Acknowledgments
Joseph E Levitt is funded by an NIH/NHLBI K23 grant (1K23HL091334-01A2) to study the early identification of acute lung injury. Michael A Matthay is the sponsor for Joseph E Levitt's K23 grant (1K23HL091334-01A2).
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR. Acute respiratory distress syndrome: a historical perspective. Am J Respir Crit Care Med. 2005;172(7):798–806. doi: 10.1164/rccm.200504-663OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milberg JA, Davis DR, Steinberg KP, et al. Improved survival of patients with acute respiratory distress syndrome (ARDS), 1983–1993. JAMA. 1995;273(4):306–309. [PubMed] [Google Scholar]
- 4.Bernard GR, Artigas A, Brigham KL, et al. Report of the American–European Consensus conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee. J Crit Care. 1994;9(1):72–81. doi: 10.1016/0883-9441(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 5.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33(4):319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anonymous. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 7.Slutsky AS. Improving outcomes in critically ill patients: the seduction of physiology. JAMA. 2009;302(18):2030–2032. doi: 10.1001/jama.2009.1653. [DOI] [PubMed] [Google Scholar]
- 8.Taccone P, Pesenti A, Latini R, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302(18):1977–1984. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 9.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 10.Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 11.Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 12.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 13.Calfee CS, Matthay MA. Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131(3):913–920. doi: 10.1378/chest.06-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levitt JE, Matthay MA. Treatment of acute lung injury: historical perspective and potential future therapies. Semin Respir Crit Care Med. 2006;27(4):426–437. doi: 10.1055/s-2006-948296. [DOI] [PubMed] [Google Scholar]
- 15.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz M, Keegan MT, Iscimen R, et al. Toward the prevention of acute lung injury: protocol-guided limitation of large tidal volume ventilation and inappropriate transfusion. Crit Care Med. 2007;35(7):1660–1666. doi: 10.1097/01.CCM.0000269037.66955.F0. [DOI] [PubMed] [Google Scholar]; • Important paper demonstrating reduced rates of acute lung injury (ALI) using protocols to limit transfusions and tidal volumes in at-risk patients.
- 17.Ciesla DJ, Moore EE, Jonson JL, et al. Decreased progression of postinjury lung dysfunction to the acute respiratory distress syndrome and multiple organ failure. Surgery. 2006;140(4):640–647. doi: 10.1016/j.surg.2006.06.015. discussion 647–648. [DOI] [PubMed] [Google Scholar]
- 18.Craig TR, Duffy MJ, Shyamsundar M, et al. Results of the HARP study: a randomized double blind Phase II trial of 80mg simvastatin in acute lung injury. Presented at: International Conference of the American Thoracic Society; New Orleans, LA, USA. 14–19 May 2010. [Google Scholar]
- 19.Kor DJ, Erlich J, Cartin-Ceba R, et al. Protective effects of anti-platelet agents in patients at risk for acute lung injury: a retrospective cohort study. Presented at: International Conference of the American Thoracic Society; New Orleans, LA, USA. 14–19 May 2010. [Google Scholar]; • Important abstract suggesting a protective effect of antiplatelet agents in reducing rates of ALI and a role for these agents in future clinical trials for treatment of at-risk patients prior to the onset of ALI.
- 20.Barnes KC. Genetic determinants and ethnic disparities in sepsis-associated acute lung injury. Proc Am Thorac Soc. 2005;2(3):195–201. doi: 10.1513/pats.200502-013AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam E, dos Santos CC. Advances in molecular acute lung injury/acute respiratory distress syndrome and ventilator-induced lung injury: the role of genomics, proteomics, bioinformatics and translational biology. Curr Opin Crit Care. 2008;14(1):3–10. doi: 10.1097/MCC.0b013e3282f42211. [DOI] [PubMed] [Google Scholar]
- 22.Levitt JE, Gould MK, Ware LB, et al. The pathogenetic and prognostic value of biologic markers in acute lung injury. J Intensive Care Med. 2009;24(3):151–167. doi: 10.1177/0885066609332603. [DOI] [PubMed] [Google Scholar]
- 23.Matthay MA, Zimmerman GA, Esmon C, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167(7):1027–1035. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 24.Meyer NJ, Garcia JG. Wading into the genomic pool to unravel acute lung injury genetics. Proc Am Thorac Soc. 2007;4(1):69–76. doi: 10.1513/pats.200609-157JG. [DOI] [PubMed] [Google Scholar]
- 25.Esteban A, Fernandez-Segoviano P, Frutos-Vivar F, et al. Comparison of clinical criteria for the acute respiratory distress syndrome with autopsy findings. Ann Intern Med. 2004;141(6):440–445. doi: 10.7326/0003-4819-141-6-200409210-00009. [DOI] [PubMed] [Google Scholar]
- 26.de Hemptinne Q, Remmelink M, Brimioulle S, et al. ARDS: a clinicopathological confrontation. Chest. 2009;135(4):944–949. doi: 10.1378/chest.08-1741. [DOI] [PubMed] [Google Scholar]
- 27.Rubenfeld GD, Cooper C, Carter G, et al. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med. 2004;32(6):1289–1293. doi: 10.1097/01.ccm.0000127266.39560.96. [DOI] [PubMed] [Google Scholar]
- 28.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson ND, Frutos-Vivar F, Esteban A, et al. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Crit Care. 2007;11(5):R96. doi: 10.1186/cc6113. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reports the results of an observational prospective study of 815 (mostly) ward patients admitted with at least one risk factor for ALI.
- 30.Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171(9):995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 31.Freishtat RJ, Mojgani B, Mathison DJ, et al. Toward early identification of acute lung injury in the emergency department. J Investig Med. 2007;55(8):423–429. doi: 10.2310/6650.2007.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quartin AA, Campos MA, Maldonado DA, et al. Acute lung injury outside of the ICU: incidence in respiratory isolation on a general ward. Chest. 2009;135(2):261–268. doi: 10.1378/chest.08-0280. [DOI] [PubMed] [Google Scholar]
- 33.Waldau T, Larsen VH, Bonde J. Evaluation of five oxygen delivery devices in spontaneously breathing subjects by oxygraphy. Anaesthesia. 1998;53(3):256–263. doi: 10.1046/j.1365-2044.1998.00318.x. [DOI] [PubMed] [Google Scholar]
- 34.Fowler AA, Hamman RF, Good JT, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983;98(5 Pt 1):593–597. doi: 10.7326/0003-4819-98-5-593. [DOI] [PubMed] [Google Scholar]
- 35.Hudson LD, Milberg JA, Anardi D, et al. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151(2 Pt 1):293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 36.Pepe PE, Potkin RT, Reus DH, et al. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144(1):124–130. doi: 10.1016/0002-9610(82)90612-2. [DOI] [PubMed] [Google Scholar]
- 37.Gong MN, Thompson BT, Williams P, et al. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33(6):1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 38.Iscimen R, Cartin-Ceba R, Yilmaz M, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36(5):1518–1522. doi: 10.1097/CCM.0b013e31816fc2c0. [DOI] [PubMed] [Google Scholar]
- 39.Mangialardi RJ, Martin GS, Bernard GR, et al. Hypoproteinemia predicts acute respiratory distress syndrome development, weight gain, and death in patients with sepsis. Ibuprofen in Sepsis Study Group. Crit Care Med. 2000;28(9):3137–3145. doi: 10.1097/00003246-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Levitt JE, Bedi H, Calfee CS, et al. Identification of early acute lung injury at initial evaluation in an acute care setting prior to the onset of respiratory failure. Chest. 2009;135(4):936–943. doi: 10.1378/chest.08-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The first paper to attempt to establish empiric clinical criteria for patients with early but existing acute lung injury prior to the onset of respiratory failure.
- 41.Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2010 doi: 10.1164/rccm.201004-0549OC. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Landmark observational multicenter study of more than 5000 patients admitted with at least one previously published risk factor for ALI and provides validation of the lung injury prediction score.
- 42.Trillo-Alvarez C, Cartin-Ceba R, Kor DJ, et al. Acute lung injury prediction score: derivation and validation in a population based sample. Eur Respir J. 2010 doi: 10.1183/09031936.00036810. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32(9):1817–1824. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]; • This paper suggests a protective effect of lower tidal volumes in reducing rates of ALI in mechanically ventilated patients without ALI at time of intubation.
- 44.Jia X, Malhotra A, Saeed M, et al. Risk factors for ARDS in patients receiving mechanical ventilation for > 48 h. Chest. 2008;133(4):853–861. doi: 10.1378/chest.07-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14(1):R1. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Suggests a protective effect of lower tidal volumes in reducing rates of ALI in mechanically ventilated patients without ALI at time of intubation.
- 46.Manzano F, Fernandez-Mondejar E, Colmenero M, et al. Positive-end expiratory pressure reduces incidence of ventilator-associated pneumonia in nonhypoxemic patients. Crit Care Med. 2008;36(8):2225–2231. doi: 10.1097/CCM.0b013e31817b8a92. [DOI] [PubMed] [Google Scholar]
- 47.van Kaam AH, Lachmann RA, Herting E, et al. Reducing atelectasis attenuates bacterial growth and translocation in experimental pneumonia. Am J Respir Crit Care Med. 2004;169(9):1046–1053. doi: 10.1164/rccm.200312-1779OC. [DOI] [PubMed] [Google Scholar]
- 48.Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344(7):481–487. doi: 10.1056/NEJM200102153440703. [DOI] [PubMed] [Google Scholar]
- 49.Agarwal R, Reddy C, Aggarwal AN, et al. Is there a role for noninvasive ventilation in acute respiratory distress syndrome? A meta-analysis. Respir Med. 2006;100(12):2235–2238. doi: 10.1016/j.rmed.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Agarwal R, Handa A, Aggarwal AN, et al. Outcomes of noninvasive ventilation in acute hypoxemic respiratory failure in a respiratory intensive care unit in north India. Respir Care. 2009;54(12):1679–1687. [PubMed] [Google Scholar]
- 51.Yoshida Y, Takeda S, Akada S, et al. Factors predicting successful noninvasive ventilation in acute lung injury. J Anesth. 2008;22(3):201–206. doi: 10.1007/s00540-008-0637-z. [DOI] [PubMed] [Google Scholar]
- 52.Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18–25. doi: 10.1097/01.CCM.0000251821.44259.F3. [DOI] [PubMed] [Google Scholar]
- 53.Rana S, Jenad H, Gay PC, et al. Failure of non-invasive ventilation in patients with acute lung injury: observational cohort study. Crit Care. 2006;10(3):R79. doi: 10.1186/cc4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaiwat O, Lang JD, Vavilala MS, et al. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology. 2009;110(2):351–360. doi: 10.1097/ALN.0b013e3181948a97. [DOI] [PubMed] [Google Scholar]
- 55.Croce MA, Tolley EA, Claridge JA, et al. Transfusions result in pulmonary morbidity and death after a moderate degree of injury. J Trauma. 2005;59(1):19–23. doi: 10.1097/01.ta.0000171459.21450.dc. discussion 23–14. [DOI] [PubMed] [Google Scholar]
- 56.Gajic O, Gropper MA, Hubmayr RD. Pulmonary edema after transfusion: how to differentiate transfusion-associated circulatory overload from transfusion-related acute lung injury. Crit Care Med. 2006;34(5 Suppl):S109–S113. doi: 10.1097/01.CCM.0000214311.56231.23. [DOI] [PubMed] [Google Scholar]
- 57.Gajic O, Rana R, Mendez JL, et al. Acute lung injury after blood transfusion in mechanically ventilated patients. Transfusion. 2004;44(10):1468–1474. doi: 10.1111/j.1537-2995.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- 58.Netzer G, Shah CV, Iwashyna TJ, et al. Association of RBC transfusion with mortality in patients with acute lung injury. Chest. 2007;132(4):1116–1123. doi: 10.1378/chest.07-0145. [DOI] [PubMed] [Google Scholar]
- 59.Shorr AF, Duh MS, Kelly KM, et al. Red blood cell transfusion and ventilator-associated pneumonia: a potential link? Crit Care Med. 2004;32(3):666–674. doi: 10.1097/01.ccm.0000114810.30477.c3. [DOI] [PubMed] [Google Scholar]
- 60.Gajic O, Rana R, Winters JL, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case–control study. Am J Respir Crit Care Med. 2007;176(9):886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This landmark paper is the first to establish the incidence of transfusion-related ALI in critically ill patients.
- 61.Gajic O, Dzik WH, Toy P. Fresh frozen plasma and platelet transfusion for nonbleeding patients in the intensive care unit: benefit or harm? Crit Care Med. 2006;34(5 Suppl):S170–S173. doi: 10.1097/01.CCM.0000214288.88308.26. [DOI] [PubMed] [Google Scholar]
- 62.Khan H, Belsher J, Yilmaz M, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007;131(5):1308–1314. doi: 10.1378/chest.06-3048. [DOI] [PubMed] [Google Scholar]
- 63.Rana R, Fernandez-Perez ER, Khan SA, et al. Transfusion-related acute lung injury and pulmonary edema in critically ill patients: a retrospective study. Transfusion. 2006;46(9):1478–1483. doi: 10.1111/j.1537-2995.2006.00930.x. [DOI] [PubMed] [Google Scholar]
- 64.Gajic O, Yilmaz M, Iscimen R, et al. Transfusion from male-only versus female donors in critically ill recipients of high plasma volume components. Crit Care Med. 2007;35(7):1645–1648. doi: 10.1097/01.CCM.0000269036.16398.0D. [DOI] [PubMed] [Google Scholar]
- 65.Koch C, Li L, Figueroa P, et al. Transfusion and pulmonary morbidity after cardiac surgery. Ann Thorac Surg. 2009;88(5):1410–1418. doi: 10.1016/j.athoracsur.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 66.Watson GA, Sperry JL, Rosengart MR, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67(2):221–227. doi: 10.1097/TA.0b013e3181ad5957. discussion 228–230. [DOI] [PubMed] [Google Scholar]
- 67.Church GD, Matthay MA, Liu K, et al. Blood product transfusions and clinical outcomes in pediatric patients with acute lung injury. Pediatr Crit Care Med. 2009;10(3):297–302. doi: 10.1097/PCC.0b013e3181988952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silliman CC. The two-event model of transfusion-related acute lung injury. Crit Care Med. 2006;34(5 Suppl):S124–S131. doi: 10.1097/01.CCM.0000214292.62276.8E. [DOI] [PubMed] [Google Scholar]
- 69.Looney MR, Nguyen JX, Hu Y, et al. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119(11):3450–3461. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Looney MR, Su X, Van Ziffle JA, et al. Neutrophils and their Fcγ receptors are essential in a mouse model of transfusion-related acute lung injury. J Clin Invest. 2006;116(6):1615–1623. doi: 10.1172/JCI27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI) Br J Haematol. 2007;136(6):788–799. doi: 10.1111/j.1365-2141.2007.06492.x. [DOI] [PubMed] [Google Scholar]
- 72.Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44(12):1774–1789. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 73.Toy P, Popovsky MA, Abraham E, et al. Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33(4):721–726. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 74.Popovsky MA, Abel MD, Moore SB. Transfusion-related acute lung injury associated with passive transfer of antileukocyte antibodies. Am Rev Respir Dis. 1983;128(1):185–189. doi: 10.1164/arrd.1983.128.1.185. [DOI] [PubMed] [Google Scholar]
- 75.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25(6):573–577. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 76.Marik PE, Corwin HL. Acute lung injury following blood transfusion: expanding the definition. Crit Care Med. 2008;36(11):3080–3084. doi: 10.1097/CCM.0b013e31818c3801. [DOI] [PubMed] [Google Scholar]
- 77.Benson AB, Austin GL, Berg M, et al. Transfusion-related acute lung injury in ICU patients admitted with gastrointestinal bleeding. Intensive Care Med. 2010;36(10):1710–1717. doi: 10.1007/s00134-010-1954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pepe PE, Hudson LD, Carrico CJ. Early application of positive end-expiratory pressure in patients at risk for the adult respiratory-distress syndrome. N Engl J Med. 1984;311(5):281–286. doi: 10.1056/NEJM198408023110502. [DOI] [PubMed] [Google Scholar]
- 79.Sadis C, Dubois MJ, Melot C, et al. Are multiple blood transfusions really a cause of acute respiratory distress syndrome? Eur J Anaesthesiol. 2007;24(4):355–361. doi: 10.1017/S0265021506001608. [DOI] [PubMed] [Google Scholar]
- 80.Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440–452. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 81.Rixen D, Siegel JH. Metabolic correlates of oxygen debt predict posttrauma early acute respiratory distress syndrome and the related cytokine response. J Trauma. 2000;49(3):392–403. doi: 10.1097/00005373-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Navarrete-Navarro P, Rivera-Fernandez R, Rincon-Ferrari MD, et al. Early markers of acute respiratory distress syndrome development in severe trauma patients. J Crit Care. 2006;21(3):253–258. doi: 10.1016/j.jcrc.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 83.Miller PR, Croce MA, Kilgo PD, et al. Acute respiratory distress syndrome in blunt trauma: identification of independent risk factors. Am Surg. 2002;68(10):845–850. discussion 850–841. [PubMed] [Google Scholar]
- 84.Eberhard LW, Morabito DJ, Matthay MA, et al. Initial severity of metabolic acidosis predicts the development of acute lung injury in severely traumatized patients. Crit Care Med. 2000;28(1):125–131. doi: 10.1097/00003246-200001000-00021. [DOI] [PubMed] [Google Scholar]
- 85.Rainer TH, Lam PK, Wong EM, et al. Derivation of a prediction rule for post-traumatic acute lung injury. Resuscitation. 1999;42(3):187–196. doi: 10.1016/s0300-9572(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 86.Pallister I, Dent C, Wise CC, et al. Early post-traumatic acute respiratory distress syndrome and albumin excretion rate: a prospective evaluation of a ‘point-of care’ predictive test. Injury. 2001;32(3):177–181. doi: 10.1016/s0020-1383(00)00149-2. discussion 183. [DOI] [PubMed] [Google Scholar]
- 87.Mascia L, Zavala E, Bosma K, et al. High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med. 2007;35(8):1815–1820. doi: 10.1097/01.CCM.0000275269.77467.DF. [DOI] [PubMed] [Google Scholar]
- 88.Kahn JM, Caldwell EC, Deem S, et al. Acute lung injury in patients with subarachnoid hemorrhage: incidence, risk factors, and outcome. Crit Care Med. 2006;34(1):196–202. doi: 10.1097/01.ccm.0000194540.44020.8e. [DOI] [PubMed] [Google Scholar]
- 89.Matthay MA, Jayr C. Acute respiratory distress syndrome after surgery: can the risk be decreased? Anesth Analg. 2010;111(2):268–269. doi: 10.1213/ANE.0b013e3181e75ced. [DOI] [PubMed] [Google Scholar]
- 90.Hughes CG, Weavind L, Banerjee A, et al. Intraoperative risk factors for acute respiratory distress syndrome in critically ill patients. Anesth Analg. 2010 doi: 10.1213/ANE.0b013e3181d8a16a. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 91.Weingarten TN, Whalen FX, Warner DO, et al. Comparison of two ventilatory strategies in elderly patients undergoing major abdominal surgery. Br J Anaesth. 2010;104(1):16–22. doi: 10.1093/bja/aep319. [DOI] [PubMed] [Google Scholar]
- 92.Blum JM, Fetterman DM, Park PK, et al. A description of intraoperative ventilator management and ventilation strategies in hypoxic patients. Anesth Analg. 2010;110(6):1616–1622. doi: 10.1213/ANE.0b013e3181da82e1. [DOI] [PubMed] [Google Scholar]
- 93.Lee PC, Helsmoortel CM, Cohn SM, et al. Are low tidal volumes safe? Chest. 1990;97(2):430–434. doi: 10.1378/chest.97.2.430. [DOI] [PubMed] [Google Scholar]
- 94.Clark SC. Lung injury after cardiopulmonary bypass. Perfusion. 2006;21(4):225–228. doi: 10.1191/0267659106pf872oa. [DOI] [PubMed] [Google Scholar]
- 95.Jeon K, Yoon JW, Suh GY, et al. Risk factors for post-pneumonectomy acute lung injury/acute respiratory distress syndrome in primary lung cancer patients. Anaesth Intensive Care. 2009;37(1):14–19. doi: 10.1177/0310057X0903700110. [DOI] [PubMed] [Google Scholar]
- 96.Kim JB, Lee SW, Park SI, et al. Risk factor analysis for postoperative acute respiratory distress syndrome and early mortality after pneumonectomy: the predictive value of preoperative lung perfusion distribution. J Thorac Cardiovasc Surg. 2010;140:26–31. doi: 10.1016/j.jtcvs.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 97.Kuzniar TJ, Blum MG, Kasibowska-Kuzniar K, et al. Predictors of acute lung injury and severe hypoxemia in patients undergoing operative talc pleurodesis. Ann Thorac Surg. 2006;82(6):1976–1981. doi: 10.1016/j.athoracsur.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 98.Siddiqui AK, Ahmed S. Pulmonary manifestations of sickle cell disease. Postgrad Med J. 2003;79(933):384–390. doi: 10.1136/pmj.79.933.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee H, Hawker FH, Selby W, et al. Intensive care treatment of patients with bleeding esophageal varices: results, predictors of mortality, and predictors of the adult respiratory distress syndrome. Crit Care Med. 1992;20(11):1555–1563. doi: 10.1097/00003246-199211000-00013. [DOI] [PubMed] [Google Scholar]
- 100.Herasevich V, Yilmaz M, Khan H, et al. Validation of an electronic surveillance system for acute lung injury. Intensive Care Med. 2009;35(6):1018–1023. doi: 10.1007/s00134-009-1460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Azzam HC, Khalsa SS, Urbani R, et al. Validation study of an automated electronic acute lung injury screening tool. J Am Med Inform Assoc. 2009;16(4):503–508. doi: 10.1197/jamia.M3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neyrinck AP, O'Neal H, Lee JW, et al. Quantification of pulmonary edema on chest radiograph compared with direct measurements of extravascular lung water in humans. Presented at: International Conference of the American Thoracic Society; San Diego, CA, USA. 15–20 May 2009. [Google Scholar]
- 103.Levitt JE, Weinacker A. A prospective evaluation of early lung injury prior to the onset of respiratory failure and mechanical ventilation. Presented at: International Conference of the American Thoracic Society; San Francisco, CA, USA. 18–23 May 2007. [Google Scholar]
- 104.Ichikado K, Suga M, Muranaka H, et al. Prediction of prognosis for acute respiratory distress syndrome with thin-section CT: validation in 44 cases. Radiology. 2006;238(1):321–329. doi: 10.1148/radiol.2373041515. [DOI] [PubMed] [Google Scholar]
- 105.Rouby JJ, Puybasset L, Cluzel P, et al. Regional distribution of gas and tissue in acute respiratory distress syndrome. II Physiological correlations and definition of an ARDS Severity Score CT Scan ARDS Study Group. Intensive Care Med. 2000;26(8):1046–1056. doi: 10.1007/s001340051317. [DOI] [PubMed] [Google Scholar]
- 106.Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354(17):1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 107.Livingston DH, Shogan B, John P, et al. CT diagnosis of rib fractures and the prediction of acute respiratory failure. J Trauma. 2008;64(4):905–911. doi: 10.1097/TA.0b013e3181668ad7. [DOI] [PubMed] [Google Scholar]
- 108.Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134(1):117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bouhemad B, Liu ZH, Arbelot C, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med. 2010;38(1):84–92. doi: 10.1097/CCM.0b013e3181b08cdb. [DOI] [PubMed] [Google Scholar]
- 110.Rodrigues RS, Miller PR, Bozza FA, et al. FDG-PET in patients at risk for acute respiratory distress syndrome: a preliminary report. Intensive Care Med. 2008;34(12):2273–2278. doi: 10.1007/s00134-008-1220-7. [DOI] [PubMed] [Google Scholar]
- 111.Bellani G, Messa C, Guerra L, et al. Lungs of patients with acute respiratory distress syndrome show diffuse inflammation in normally aerated regions: a [18F]-fluoro-2-deoxy-D-glucose PET/CT study. Crit Care Med. 2009;37(7):2216–2222. doi: 10.1097/CCM.0b013e3181aab31f. [DOI] [PubMed] [Google Scholar]
- 112.Chen DL, Schuster DP. Positron emission tomography with [18F] fluorodeoxyglucose to evaluate neutrophil kinetics during acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L834–L840. doi: 10.1152/ajplung.00339.2003. [DOI] [PubMed]
Websites
- 201.Drug Study of Albuterol to Treat Acute Lung Injury (ALTA) http://clinicaltrials.gov/ct2/show/NCT00434993.
- 202.Statins for Acutely Injured Lungs From Sepsis (SAILS) doi: 10.1007/s00134-018-5378-3. http://clinicaltrials.gov/ct2/show/NCT00979121. [DOI] [PMC free article] [PubMed]