Abstract
Thalamus abnormalities are common in neurological and psychiatric illnesses. Therefore, it is essential to understand the properties of the thalamus-related networks. The thalamic reticular nucleus (TRN) is a thin GABAergic layer interface strategically located between the thalamus and the neocortex. It is, at the very beginning of life, an essential neurodevelopmental guide for the accurate build up of reciprocal anatomical glutamatergic connections between the thalamus and neocortex. It is more than the mediator of selective attention. It appears as a combinatorial matrix because it holds and can combine multiple functional modalities. TRN cells work like integrators, thanks to their extraordinary intrinsic electrophysiological properties, under the contextual and leading influence of corticothalamic inputs. The TRN and thalamus principally form 2-neuron open-loop circuits (no reciprocal connection). The major functioning principle of such GABAergic-glutamatergic circuits is lateral inhibition, which is a gold standard device to set up, via differential amplifications, coherent structured thalamocortical activity patterns. Thereby, it selects relevant streams of information and deletes distractors during action, resting states, and information integration, including during consciousness, cognition, emotion, and thought. Disruption of thalamic lateral inhibition may contribute to a lack of coordination in activity between brain regions, as observed in psychiatric disorders like schizophrenia.
Keywords: brain rhythms, cerebral cortex, neurodevelopment, neurophysiology, pathophysiology, thalamic reticular nucleus
A great challenge for modern neurobiologists is to understand neural mechanisms that underlie the disintegration of complex psychic processes, like consciousness and thought. Hence, it is essential to develop and to study animal and network models to understand global brain operations and their dysfunction. Models are also useful tools to understand the natural functioning of brain networks and to accelerate the development of translational research from bench to bedside. Conceptualizing pathophysiological mechanisms from models provides a fundamental prelude for the preclinical development of therapeutic concepts. Advances in basic neurosciences disseminate translational concepts and tools, which are necessary for building synergies with neurological and psychiatric research programmes.
Dysfunction of Thalamus-Related Networks in Schizophrenia
Schizophrenia is caused and/or aggravated by genetic, neurodevelopmental, and environmental factors.1 Its pathophysiological mechanisms are multidimensional because it is characterized by multiple disorders, affecting higher-order brain operations, like consciousness, thought, attention, cognition, emotion, perception, and sensorimotor integration. These are likely to be due to hypoactivity of prefrontal cortex in schizophrenics, which is thought to be caused by dysfunction of related networks.2–4 Cerebral dysconnections may have various and diverse neurochemical, molecular, genetic, structural, and/or functional causes. What actually are cerebral dysconnections? Here, we need to highlight the anatomofunctional role of the gamma amino butyric acid (GABA)ergic thalamic reticular nucleus (TRN) in thalamocortical (TC), corticothalamic (CT), and corticocortical (CC) networks. Moreover, resting states and higher-order brain operations go on, thanks to spatiotemporal interactions between the neocortex and the thalamus, this latter structure being central in the constellation of interactive large-scale CC systems.5 The thalamus also innervates the striatum, amygdala, and hippocampus (figure 1A). Postmortem and high-resolution anatomofunctional studies lend credence to the hypothesis of dysfunctional CT/TC and basal ganglia networks in schizophrenic patients.6,7 Ferrarelli and Tononi8 provide strong experimental and clinical arguments in favor of the hypothesis of dysfunctional thalamic networks, involving especially the TRN in schizophrenia. The TRN is a modulator of TC activities, a mediator of selective attention, and an initiator of rhythmic TC activities.9 The TRN is often thought of as an attentional searchlight because TRN lesions induce different forms of behavioral neglect.10 Attention deficits are core features in schizophrenia (Ferrarelli and Tononi8).
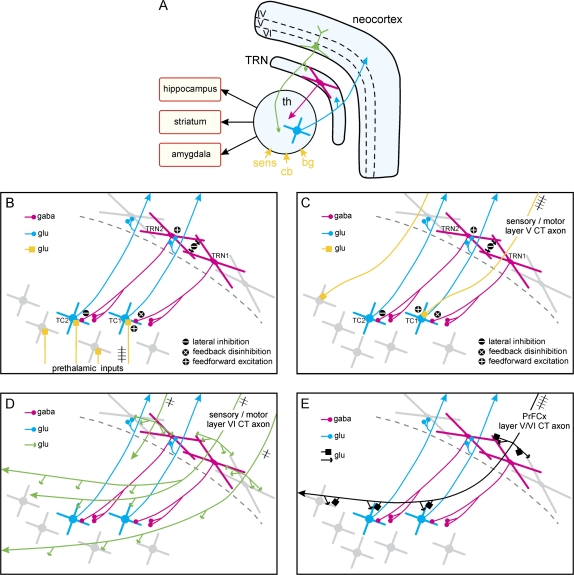
Fig. 1.
Thalamic Lateral Inhibition And Corticothalamic (CT) Influence. (A) The 3 principal elements of the thalamocortical (TC)/CT systems. The CT (of layer VI, in green) and TC (blue) axons are glutamatergic and cross the thalamic reticular nucleus (TRN), where they give off axon collaterals. The principal prethalamic inputs (deep yellow) originate from sensory receptors (sens), the cerebellum (cb), and basal ganglia (bg). Parts of the thalamus innervate the hippocampus, striatum, and amygdala. (B–E) The 4 drawings illustrate likely synaptic interactions between single TC, CT neurons, which are glutamatergic, and TRN neurons, which are GABAergic (in red). TC and TRN neurons form open-loop circuits. Some of the terminal axonal boutons of 1 TC neuron contact 1 TRN cell; similarly, some terminal boutons of 1 TRN cell make synaptic contacts with 1 TC neuron. The oversimplified drawings do not include thalamic interneurons and do not respect the anatomical scale in dimension and in number. (B) In a first-order (eg, specific sensory or motor) thalamic nucleus, activation (feedforward excitation) starts with neuron TC1, for instance, following an afferent discharge on a specific prethalamic, sensory or motor, glutamatergic axonal input (yellow). TC1 cell, via its axon collaterals, excites TRN2 cell, which then inhibits TC2 cell (lateral inhibition). TRN2 neuron, via dendrodendritic GABAergic synapses, inhibits TRN1 cell, which then disinhibits TC1 neuron (feedback disinhibition). (C) The same scenario might occur in higher-order (nonspecific, associative, cognitive) thalamic nuclei with layer V CT axons (yellow), which are thought to be drivers like prethalamic sensory inputs. In this scenario, the active CT input is indicated by the train of action potentials. (D) Layer VI axons (green), in contrast to layer V CT axons, innervate both the TRN and the thalamus; in addition, they reciprocally innervate larger territories in first-order and higher-order thalamic nuclei. CT axons are thought to supply contextual information to the TC-TRN circuits. (E) The prefrontal cortex (PrFCx) contains layer V and VI CT neurons (black), which innervate TRN and TC neurons in sensory, motor, and limbic systems. Because prefrontal CT axons have a dual mode of terminals (small and large), they may be seen as differential drivers. The functional scenario may roughly be a combination of those suggested in (C) and (D). For clarity, the prethalamic inputs shown in (B) (yellow) are not shown in (C), (D), and (E). The functional role of the distinct CT pathways and how they work together remain a grand mystery.
The TRN, an Essential Developmental Guidance Cue in Building Up Accurate TC And CT Connections
The not widely recognized, very essential developmental role of the TRN may occur during the embryonic development.11–13 At this stage (13/15th and 6/7th gestational day and month in rodent and human, respectively), the TRN is large relative to the thalamus because of the presence, in the adjacent internal capsule, of a neuronal population, which forms the perireticular nucleus. TRN axons start to innervate the thalamus at the embryonic stage earlier than CT and retinal axons. The early TRN and periRN appear to play a guidance role for naturally sprouting CT and TC axons. These axons cross the TRN, whereas corticospinal and corticobulbar axons avoid or may pass directly through the periRN to reach their respective anatomical targets. At mature stages, the TRN and particularly the periRN are reduced in size. The periRN disappears when the topographical organization of TC and CT axons is established.
The TRN, a Combinatorial Matrix With Elementary Integrators
The multiple areas of the neocortex communicate between each other directly and indirectly through subcortical nuclei, including through transthalamic pathways.14 Moreover, the thalamus is reciprocally connected with both the neocortex and the TRN (figure 1A). On the other hand, the TRN does not innervate the neocortex. The thalamus relays to the neocortex sensory information generated by the external world and mental information stored in cortex-related networks. The thalamus-TRN circuits work in tandem with the neocortex to generate physiological and pathological brain rhythms during natural and pathological behavior, respectively.9 The TRN is an essential multifunction interface innervated by matching topographical maps of CT and TC glutamatergic axonal projections. Most of its constituent elements are GABAergic cells, which express, among others, the calcium-binding parvalbumin and dopaminergic receptors.15 They are an important extrinsic source of thalamic GABA receptor–mediated inhibitions; furthermore, they can communicate with each other through dendrodendritic chemical and electrical synapses.
The TRN may be more than a searchlight. Indeed, it is a key structure involved in multiple conscious integration processes, including sensorimotor information processing, attention, and cognition.9 It contains virtually all functional modalities and occupies a strategic position between the neocortex and the thalamus (figure 1A). TRN cells have all the necessary wiring and intrinsic properties to combine more than 1 functional modality/submodality, endowing them with the power to control the spatial and temporal binding involved not only during global brain operations but also during resting states.
TRN cells operate as integrators with a wide range of complexity levels. Indeed, they are endowed with powerful electro-responsive, pacemaking, oscillatory, and resonant properties.9 This set of properties is accounted for in part by the presence of high-density dendritic calcium currents.16 The TRN is capable of an extraordinary functional plasticity that is dependent on the state of the neocortex. For instance, when its excitability increases, TRN neurons exhibit unfailing and efficient rhythmic burst firing,17 thereby modulating GABA receptor–mediated TC activities. Field potential oscillations at γ frequencies (30–80 Hz) in the TC and CT systems support the dynamics of global brain operations. The γ clock-like properties of the TRN18 may play a crucial role in mental operation-related spatiotemporal binding in these systems especially during focused arousal. Accordingly, the TRN has anatomofunctional properties designed to ensure coherence of spatiotemporal dynamics of interstructure communications, “horizontally” within the thalamus and “vertically” between the thalamus and the neocortex, with significant impact in CC communications.9 The TRN is thus endowed with structural, chemical, electrophysiological, and networking properties, which can be combined in multiple fashions. Thereby, the TRN can bind multiple functional modalities. It thus appears as a combinatorial matrix involved in setting up coherent structured TC activity patterns during resting states and conscious and unconscious global brain operations.
Any TRN cell is a potential elementary searchlight because it can exhibit a firing pattern independently of its immediate neighbors.9 Because any TRN neuron is integrated within a complex set of connections, it can belong to more than 1 neuronal circuit. Every TRN cell is therefore a potential individual searchlight, which may be able to connect to an appropriate network, as for example during focused attention. The theory that the TRN operates like a combinatorial matrix is also supported by the singular bundles of interwoven dendrites, which may conceal synaptic (chemical and electrical) and nonsynaptic mechanisms of cell-cell communication.
TRN Cells, Differential Amplifiers via Lateral Inhibition
How do TRN and TC neurons work together, for instance during the relay of sensorimotor information from the thalamus to the neocortex? It is true to say that the thalamus and the TRN are reciprocally connected (figure 1A). But the reciprocity principle does not apply at the single-cell level (figure 1B). Indeed, using the single-cell juxtacellular labeling technique, Pinault and Deschênes19 demonstrated that the glutamatergic TC and GABAergic TRN neurons form open- rather than closed-loop connections. The singularity of such 2-neuron open-loop circuits is that only 1 of these 2 neurons innervates the other (no reciprocal connection; figure 1B).
The major functioning principle of such 2-neuron GABAergic-glutamatergic circuits is lateral inhibition around the TC neuron that innervates the GABAergic cell. The principle of a lateral inhibition mechanism implies the generation of thalamic hot spots of synchronized activities, whose constituent TC cells convey these amplified bits of information to the related neocortical areas. In short, TRN cells modulate the activity of TC neurons through GABA receptor–mediated inhibitions and disinhibitions (counterpart of lateral inhibition). Thus, TRN cells modulate TC activities through lateral inhibition, an efficient tool to amplify relevant information that should be conveyed to neocortical areas and to attenuate or delete nonrelevant or distractive information. In addition, TRN cells can produce indirect excitation, ie, a postinhibitory rebound transient discharge (resulting from a low-threshold Ca2+ potential topped by a high-frequency burst of action potentials, the elementary event of rhythmic activity) in their target TC cells and/or discharge of varying duration in nontarget TC (disinhibition). Thereby, TRN cells are differential amplifiers that play a leading role in setting up and modulating TC maps and streams of information to the neocortex and also in the generation of physiological and pathological synchronized TC-CT oscillations. The TRN can thus be a modulator of cortical excitability and plasticity.
Here, we have deliberately attempted to give a simple concept of the principal role played by the TC and TRN neurons. We should not rule out that TRN cells may also to a certain degree be involved in recurrent inhibition.19 We should also be aware that the thalamus contains GABAergic interneurons, which are intrinsic sources of thalamic inhibitions and which are greater in number in felines and primates than in rodents.14,20 It also receives additional GABAergic inputs from other external sources, including the basal ganglia, the zona incerta, and the pretectum.21 Accordingly, this should somehow complicate the mechanics of the TC-TRN–mediated lateral inhibition.
The Contextual And Leading Influence of The Neocortex
The 2-neuron TC-TRN circuit is under the great influence of cortical inputs.22 At least 2 types of CT innervations are conceptually considered: driver and modulator.14 Layer V neurons can be regarded as drivers like prethalamic afferents (figure 1B,C). Layer V CT axons innervate the so-called higher-order (roughly equivalent to association, cognitive, and nonspecific) thalamic nuclei. Their axon terminations are large and clustered so as to innervate small populations of TC neurons on their proximal dendrites. Thereby, they generate large excitatory postsynaptic potentials through activation of ionotropic glutamate receptors. Furthermore, layer V CT neurons, again like prethalamic afferents, do not innervate the TRN, contrasting with layer VI CT neurons.23 Layer V CT neurons are likely to play an important role in large-scale CC communication.
Layer VI CT neurons, thought to be modulators, exceed in number by 1 order of magnitude TC neurons. In addition, layer VI CT axons reciprocally innervate large territories in first-order (specific, sensory, and motor) and higher-order thalamic nuclei (figure 1D). They have small and dispersed terminals ending on distal TC dendrites, and they generate small excitatory postsynaptic potentials through activation of ionotropic and metabotropic glutamate receptors. Layer VI CT neurons can provide the context to TC-TRN circuits, thereby subserving the generation of coherent patterns of rhythmic activities in large TC assemblies during brain operations. Assuming that the TRN is the searchlight operator, experimental data suggest that it is most likely led by layer VI CT inputs.17,24,25
Remarkably, the prefrontal cortex contains layers V and VI CT neurons that project to higher-order thalamic nuclei, including the mediodorsal nucleus, and to the sensorimotor and prefrontal sectors of the TRN26 (also see Ferrarelli and Tononi8). Prefrontal axonal inputs to the TRN may subserve the combinatorial matrix role of the TRN during conscious states, functional integration, and selective attention. Prefrontal CT neurons have a dual mode of axon terminals, small, and large, apparently the substrate of an intricate role (figure 1E). It is tempting to put forward the corollary prediction that prefrontal CT axons can play a differential driver role because, for a given axonal discharge, large axonal boutons are expected to generate a greater synaptic strength than the smaller ones, at least in sensorimotor TC-TRN circuits. On one hand, prefrontal cortico-TRN-thalamic circuits may be involved in attentional regulation, selecting relevant sensory stimuli and suppressing distractors. On the other hand, prefrontal cortical projections to the anterior TRN may be implicated in regulating CC communication and may critically contribute to the deficits in CC networks in schizophrenia.
Conclusion And Perspectives
In this essay, we have put important structural and functional properties of the TRN at the forefront. It is a thin GABAergic layer interface strategically located between the thalamus and the neocortex. It is endowed with increasing responsibilities since the very beginning of life. It is connected with multiple circuits involved in sensorimotor information processing, in cognition and in emotion. We consider the TRN as a combinatorial matrix that plays a central role in information integration and in the generation of conscious experience. It is the mediator of attention under the notable contextual and leading influence of the neocortex and should be the object of attention in future studies aiming to understand the pathophysiology of disorders of consciousness and cognition observed in psychiatry and neurology. Indeed, the TRN appears as a fascinating tool for studying the neurodevelopmental hypothesis of schizophrenia. It is worth saying that more than 80% of the neurons that make the CT, TC, and CC circuits are glutamatergic. In addition, the TRN displays N-methyl d-aspartate receptor-related activities. Therefore, the TRN is also an attractive model to study the glutamatergic and GABAergic hypotheses of schizophrenia and to understand the accompanying pathological brain rhythms and deficits in perception- and cognition-related synchrony. Moreover, Ferrarelli and colleagues, using high-density electroencephalographic recordings in schizophrenic patients, demonstrated that sleep spindles are reduced in number, amplitude, and duration.27,28 It is worth to emphasize that this reduction was due to the disease rather than to the treatment. This is a very important piece of the puzzling neurobiological mechanisms underlying schizophrenia because reduction in sleep spindles might be the signature of a weakening of TRN-mediated rhythmic GABAergic activity.
Functional interactions between TC and TRN neurons are under the influence of neuromodulators (acetylcholine, noradrenalin, serotonin, histamine, and dopamine) from the forebrain and brainstem and of massive cortical glutamatergic inputs. Disorders in neuromodulatory transmission might affect attentional and cognitive mechanisms at least in TC-TRN circuits. More specifically, neurochemical-induced dysfunction of the TRN would result in an impairment of the signal-to-noise ratio, subserving the emergence of hallucinations,29 may be through disruption of thalamic lateral inhibitions.
Funding
The National Institute of Health and Biomedical Research (Inserm); The University of Strasbourg; The fondation pour la recherche médicale; Neurex.
Acknowledgments
I thank Ray Guillery for discussion and critical reading of the manuscript. I also acknowledge my colleagues and all scientists who contributed to the expansion of our knowledge on the thalamus and especially those who have not been quoted here. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Friston KJ. Dysfunctional connectivity in schizophrenia. World Psychiatry. 2002;1:66–71. [PMC free article] [PubMed] [Google Scholar]
- 3.Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Tononi G, Edelman GM. Schizophrenia and the mechanisms of conscious integration. Brain Res Rev. 2000;31:391–400. doi: 10.1016/s0165-0173(99)00056-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhang N, Zhu XH, Zhang Y, Chen W. An fMRI study of neural interaction in large-scale cortico-thalamic visual network. Neuroimage. 2008;42:1110–1117. doi: 10.1016/j.neuroimage.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csernansky JG, Cronenwett WJ. Neural networks in schizophrenia. Am J Psychiatry. 2008;165:937–939. doi: 10.1176/appi.ajp.2008.08050700. [DOI] [PubMed] [Google Scholar]
- 7.Clinton SM, Meador-Woodruff JH. Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, and in vivo imaging abnormalities. Schizophr Res. 2004;69:237–253. doi: 10.1016/j.schres.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Ferrarelli F, Tononi G. The thalamic reticular nucleus schizophrenia. Schizophr Bull. doi: 10.1093/schbul/sbq142. December 3, 2010; doi:10.1093/schbul/sbq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Rev. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitrofanis J, Guillery RW. New views of the thalamic reticular nucleus in the adult and the developing brain. Trends Neurosci. 1993;16:240–245. doi: 10.1016/0166-2236(93)90163-g. [DOI] [PubMed] [Google Scholar]
- 12.Ulfig N, Nickel J, Bohl J. Transient features of the thalamic reticular nucleus in the human foetal brain. Eur J Neurosci. 1998;10:3773–3784. doi: 10.1046/j.1460-9568.1998.00390.x. [DOI] [PubMed] [Google Scholar]
- 13.Deng J, Elberger AJ. Corticothalamic and thalamocortical pathfinding in the mouse: dependence on intermediate targets and guidance axis. Anat Embryol (Berl) 2003;207:177–192. doi: 10.1007/s00429-003-0338-1. [DOI] [PubMed] [Google Scholar]
- 14.Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 15.Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- 16.Destexhe A, Contreras D, Steriade M, Sejnowski TJ, Huguenard JR. In vivo, in vitro, and computational analysis of dendritic calcium currents in thalamic reticular neurons. J Neurosci. 1996;16:169–185. doi: 10.1523/JNEUROSCI.16-01-00169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinault D. Cellular interactions in the rat somatosensory thalamocortical system during normal and epileptic 5-9 Hz oscillations. J Physiol. 2003;552:881–905. doi: 10.1113/jphysiol.2003.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinault D, Deschênes M. Voltage-dependent 40-Hz oscillations in rat reticular thalamic neurons in vivo. Neuroscience. 1992;51:245–258. doi: 10.1016/0306-4522(92)90312-p. [DOI] [PubMed] [Google Scholar]
- 19.Pinault D, Deschênes M. Anatomical evidence for a mechanism of lateral inhibition in the rat thalamus. Eur J Neurosci. 1998;10:3462–3469. doi: 10.1046/j.1460-9568.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones EG. Thalamic organization and function after Cajal. Prog Brain Res. 2002;136:333–357. doi: 10.1016/s0079-6123(02)36029-1. [DOI] [PubMed] [Google Scholar]
- 21.Bokor H, Frere SG, Eyre MD, et al. Selective GABAergic control of higher-order thalamic relays. Neuron. 2005;45:929–940. doi: 10.1016/j.neuron.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 22.Andolina IM, Jones HE, Wang W, Sillito AM. Corticothalamic feedback enhances stimulus response precision in the visual system. Proc Natl Acad Sci U S A. 2007;104:1685–1690. doi: 10.1073/pnas.0609318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deschênes M, Bourassa J, Pinault D. Corticothalamic projections from layer V cells in rat are collaterals of long-range corticofugal axons. Brain Res. 1994;664:215–219. doi: 10.1016/0006-8993(94)91974-7. [DOI] [PubMed] [Google Scholar]
- 24.Golshani P, Liu XB, Jones EG. Differences in quantal amplitude reflect GluR4- subunit number at corticothalamic synapses on two populations of thalamic neurons. Proc Natl Acad Sci U S A. 2001;98:4172–4177. doi: 10.1073/pnas.061013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montero VM. Attentional activation of the visual thalamic reticular nucleus depends on ‘top-down’ inputs from the primary visual cortex via corticogeniculate pathways. Brain Res. 2000;864:95–104. doi: 10.1016/s0006-8993(00)02182-x. [DOI] [PubMed] [Google Scholar]
- 26.Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrarelli F, Huber R, Peterson MJ, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 28.Ferrarelli F, Peterson MJ, Sarasso S, et al. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167:1339–1348. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behrendt RP. Dysregulation of thalamic sensory “transmission” in schizophrenia: neurochemical vulnerability to hallucinations. J Psychopharmacol. 2006;20:356–372. doi: 10.1177/0269881105057696. [DOI] [PubMed] [Google Scholar]