Abstract
Background: The thalamic reticular nucleus (TRN) is a shell-shaped gamma amino butyric acid (GABA)ergic nucleus, which is uniquely placed between the thalamus and the cortex, because it receives excitatory afferents from both cortical and thalamic neurons and sends inhibitory projections to all nuclei of the dorsal thalamus. Method: A review of the evidence suggesting that the TRN is implicated in the neurobiology of schizophrenia. Results: TRN-thalamus circuits are implicated in bottom-up as well as top-down processing. TRN projections to nonspecific nuclei of the dorsal thalamus mediate top-down processes, including attentional modulation, which are initiated by cortical afferents to the TRN. TRN-thalamus circuits are also involved in bottom-up activities, including sensory gating and the transfer to the cortex of sleep spindles. Intriguingly, deficits in attention and sensory gating have been consistently found in schizophrenics, including first-break and chronic patients. Furthermore, high-density electroencephalographic studies have revealed a marked reduction in sleep spindles in schizophrenics. Conclusion: On the basis of our current knowledge on the molecular and anatomo-functional properties of the TRN, we suggest that this thalamic GABAergic nucleus may be involved in the neurobiology of schizophrenia.
Keywords: GABA, sleep spindles, auditory sensory gating, attention regulation
Introduction
The thalamic reticular nucleus (TRN) is a shell-shaped, gamma amino butyric acid (GABA)ergic nucleus, which envelops most of the thalamus.1 The TRN is strategically located between the thalamus and the cortex and represents an ideal hub for corticothalamic communications because it receives excitatory projections from thalamocortical and corticothalamic neurons and sends inhibitory efferents to all nuclei of the dorsal thalamus. Electrophysiological studies have shown that the TRN is involved in sensory gating2 and attentional modulation.3–6 Recent findings have revealed diffuse prefrontal projections to frontal as well as sensory TRN sectors, which may regulate the ability to perform tasks in an environment with competing sensory inputs.7 Finally, the TRN is responsible for the generation of sleep spindles.8,9.
In this review, we will first briefly describe the anatomical, neurochemical, and electrophysiological properties of the TRN. We will then present the evidence showing the critical role of the TRN in generating sleep spindles as well as in processing sensory information during waking. Next, we will review the studies reporting TRN abnormalities in animal models of schizophrenia. Moreover, we will present the results of sleep high-density electroencephalographic (hd-EEG) studies that established marked spindle deficits in schizophrenics. Finally, we will discuss how these findings may contribute to the understanding of certain aspects of the phenomenology and the neurobiology of schizophrenia.
Anatomical, Neurochemical, And Electrophysiological Properties of The TRN
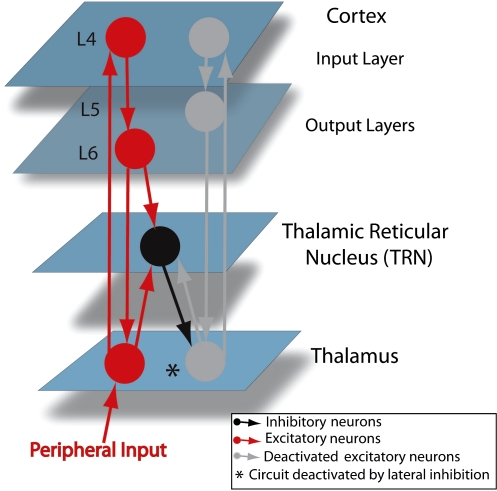
The TRN is a thin thalamic nucleus that receive projections from both thalamocortical and corticothalamic neurons10 (figure 1), which are responsible for its reticulated structure.11
Fig. 1.
Connectivity Between The Cortex, The Thalamic Reticular Nucleus (TRN), and The Thalamus. The asterisk indicates a cortex-TRN-thalamus circuit thought to be involved in lateral inhibition (see text).
Other projections to the TRN are from the brainstem and the basal forebrain.3 Brainstem inputs are cholinergic and monoaminergic12,13; basal forebrain afferents are GABAergic.14 While acetylcholine hyperpolarizes and decreases discharge activity in TRN neurons,15 noradrenalin and serotonin depolarize TRN cells.16 Dopamine receptors (D4) have also been established on TRN cells. However, the origins of these projections (substantia nigra) and the effects of dopamine on TRN neurons are still controversial.17,18
The TRN is entirely composed of GABAergic cells.19 Besides GABA, calcium-binding proteins such as calbindin, calretinin, and parvalbumin are expressed.20 These proteins act as Ca+ buffers, regulating intracellular availability of Ca+ and therefore modulating membrane potential, firing pattern, and synaptic transmission of TRN neurons.21
Increasing evidence suggests that the TRN, initially regarded as a nucleus with nonspecific diffuse projections within the thalamocortical system, has a topographic organization consistent with those of the cortex and the thalamus.3 Anatomical and electrophysiological studies of the cat, rabbit, and rat brain have contributed to identify 7 sectors: 5 sensory (visual, auditory, gustatory, visceral, and somatosensory), 1 motor, and 1 limbic.22–26 Each sector is involved in a specific modality, receives afferents from corresponding cortical and thalamic neurons, and projects back to the thalamic nuclei by which it is innervated. These thalamic nuclei can be classified as “first-order” and “higher-order” nuclei.27 First-order thalamic nuclei receive afferents from ascending sensory inputs, and relay information from the periphery to the cortex. Higher-order thalamic nuclei receive afferents from cortical axons of layer V neurons and are thought to be involved in corticocortical communication.28
The TRN projects to 2 thalamic targets: thalamocortical cells and local interneurons.29 TRN efferents to thalamocortical neurons, which represent ∼90% of reticular projections, exert an inhibitory control on thalamic transmission to the cortex.29 This inhibitory control is local and topographically organized when driven by thalamoreticular or corticothalamic afferents. When activated by afferents from neuromodulatory systems and the brainstem reticular formation, the TRN can exert a global, nonspecific inhibitory control on thalamocortical transmission, which regulates the level of vigilance as well as the overall attentive state.1 About 10% of TRN projections are to local thalamic interneurons, which in turn send inhibitory afferents to thalamocortical cells.29 It has been suggested recently that these TRN-thalamus circuits might be implicated in granting cortical access to peripheral inputs.9 For example, prefrontal pathways may facilitate cortical access to salient stimuli via TRN-local interneurons-thalamocortical circuits, while efficiently suppressing irrelevant inputs via TRN-thalamic cells connections.30
TRN cells can fire in single-spike or burst-spike. In vitro and in vivo studies have shown that trains of single-spike occur at depolarized membrane potential values (greater than or equal to −60 μV), while burst-spikes are observed in hyperpolarized cells.9,31,32
Single-spike is the predominant firing activity during active waking and rapid-eye movement (REM) sleep, while burst-spike occurs mostly during non-rapid eye movement (NREM) sleep.33 These spike-bursts play a pivotal role in generating and synchronizing sleep spindles.34
Spindles are waxing and waning fast oscillations that characterize NREM sleep, especially stage N2. Cortically recorded spindles are first generated in the TRN, as a sequence of rhythmic spike-bursts triggered by cortical depolarizing inputs. These spike-bursts produce inhibitory postsynaptic potential followed by rhythmic rebound in thalamic relay cells, which are then transferred to cortical neurons and cause them to oscillate at 12–16 Hz.35 The TRN is critical for spindles to occur, as indicated by the absence of sleep spindles in the isolated cortex,9,36 by the abolition of spindles following disconnection of TRN from the thalamocortical system,37 and by the presence of spindles in isolated reticular neurons.38
Spindle oscillations within reticular neurons are produced by low-threshold Ca+ currents, which generate periodic burst-spikes in the spindle frequency.39,40 However, the mechanism underlying synchronization across TRN cells is still largely unknown. Two mechanisms have been proposed: GABAergic synapses between TRN neurons or intrareticular electrical coupling mediated by gap junctions.
In support of the electrical coupling mechanism, recent studies in the rat TRN have demonstrated that 1/3 of reticular neurons are endowed with electrical coupling, that this coupling favors synchronous oscillations up to 15 Hz, and that reduced electrical coupling significantly dampens synchronization between adjacent TRN neurons.41,42. Furthermore, in vitro experiments in knocked out mice with no GABAergic receptors on TRN cells found increased epileptiform activity in these animals, thus suggesting that GABA receptors may reduce proepileptogenic synchronization between reticular neurons.43 These receptors, however, may still contribute to spindle synchronization. Indeed, recent electrophysiological and modeling studies have shown that, at hyperpolarized membrane potential levels (greater than or equal to −70 μV), GABA receptors give rise to depolarizing currents in TRN neurons, which generate persistent spindle oscillations in these cells.44,45
During wakefulness, the TRN, which sends both parallel and divergent inhibitory projections to thalamocortical neurons,46–50 may be implicated in processes regulated within the thalamocortical network, including lateral inhibition.51 Lateral inhibition refers to the ability of neurons activated by a stimulus (eg, visual, somatosensory) to inhibit neighbor cells, thus increasing the contrast and sharpness of brain responses to this stimulus. Figure 1 depicts how the TRN may implement lateral inhibition within a corticothalamic circuit. Notably, by employing single-cell and multiunit juxtacellular labeling techniques, Pinault and Deschenes49 provided the first anatomical demonstration of lateral inhibition in the rodent thalamus. Furthermore, a recent study in rats combining photic stimulation with dual recording in reticular and sensory thalamic neurons has shown that TRN neurons send local topographically organized projections to the ventral posterior lateral or medial thalamic nuclei and that these projections are ideally suited to mediate local lateral inhibition between thalamic neurons.52
Reticular neurons may also participate in cortically regulated functions and especially in attentional regulation. Recent findings have revealed that prefrontal areas, which are critically involved in the control of attention, send widespread projections to the TRN.6 These projections to frontal as well as sensory TRN sectors may regulate the ability to perform tasks in an environment with competing sensory inputs.7 Furthermore, prefrontal projections to the TRN largely overlap with afferents from sensory association cortices.53 Thus, TRN may be the site where anterior and posterior cortical areas converge to enhance cognitively/emotionally relevant stimuli and to suppress irrelevant inputs.
TRN And Schizophrenia: From Molecular to Patient Findings
Evidence for a role of TRN deficits in schizophrenia is fairly recent, but it comes from both animal and human studies. Electrophysiological and pharmacological studies in rodents have shown that dysfunctions of the TRN result in deficits of auditory gating and attentional shift,2,54 2 core features of schizophrenia known to be defective in medication-naive, first-break, as well as chronic schizophrenic patients.55–57 Furthermore, pharmacological studies in the rat TRN have demonstrated metabolic and histopathological changes caused by N-Methyl-D-aspartic acid (NMDA) receptor antagonists, including phencyclidine (PCP) and ketamine, which can produce psychotic symptoms in healthy individuals.58–62 A genetic study in the developing mouse brain has revealed that TRN neurons express high levels of a gene associated with schizophrenia, DISC-1 (disrupted in schizophrenia).63 Finally, 2 recent hd-EEG sleep studies in humans have shown that patients with schizophrenia had marked deficits in sleep spindles, which are generated by the TRN, compared with healthy and psychiatric controls. These findings are summarized in table 1, along with the information on the type of study and the species on which the study was conducted. These findings are presented more in detail below.
Table 1.
TRN Findings Related to The Neurobiology of Schizophrenia
| Type of Study | Species | Main Findings |
| Genetic | Rodents (mice) | TRN neurons had high expression of a gene associated with schizophrenia, disrupted in schizophrenia, DISC-1, in the developing mouse brain, when the connections within the thalamocortical system take shape63 |
| Pharmacological | Rodents (rats) | PCP impaired rats’ extradimensional attentional shifting, which is known to be defective in schizophrenics, caused a decrease in the expression of Zif-268 and parvalbumin in the infralimbic cortex and the TRN54 |
| Pharmacological | Rodents (rats) | PCP administration, which can induce psychosis in healthy humans, resulted in metabolic hypofunction within the prefrontal cortex, the auditory system, and TRN of rats, which was reversed by the coadministration of typical (haloperidol) or atypical (clozapine) antipsychotics with PCP58,59 |
| Pharmacological | Rodents (rats) | NMDA antagonists, including PCP, ketamine, and MK801, induced lesions in posterior cingulate and retrosplenial cortices of rats following injections in the anterior thalamus. These lesions were prevented by administering haloperidol and clozapine60–62 |
| Electrophysiological | Rodents (rats) | Auditory gating, which is defective in schizophrenics, was established in TRN neurons with single-cell recordings in anesthetized rats. Amphetamine, a dopamine agonist which can induce psychosis, disrupted the TRN-mediated auditory gating, while haloperidol reversed this deficit2 |
| Electrophysiological | Humans | Two sleep hd-EEG studies reported a marked deficits in sleep spindles, EEG oscillations generated by the TRN, in schizophrenics compared with healthy and psychiatric controls71,72 |
Note: EEG, electroencephalogram; hd-EEG, high-density electroencephalogram; NMDA, N-Methyl-D-aspartic acid; PCP, phencyclidine; TRN, thalamic reticular nucleus.
Auditory gating refers to a reduced responsiveness (habituation) to repetitive stimulation and is measured as the ratio of the responses to 2 consecutive stimuli (S2/S1). An increased S2/S1, which indicates reduced auditory gating, has been consistently reported by electrophysiological studies in schizophrenics,64–66 and in a recent functional Magnetic Resonance Imaging (fMRI) study, in which schizophrenics showed an increased hemodynamic response in the thalamus during an auditory gating paradigm, it was suggested that the TRN may be responsible for such gating deficit.67 An involvement of the TRN in sensory gating is also supported by a recent study employing single-unit recordings in anesthetized rodents, which demonstrated gated responses in TRN neurons, as reflected by a reduced number of spikes to the second of 2 paired tones played a second apart.2 In this study, the authors also tested the effects of amphetamine, a dopamine agonist that can induce psychosis, and of haloperidol, a dopamine antagonist prescribed as an antipsychotic medication, on TRN auditory gating and found that amphetamine disrupted it, while haloperidol reversed such deficit.2
Attentional deficits are commonly present in schizophrenics.57 In a study aimed at reproducing these deficits in an animal model of schizophrenia, PCP was injected in rats to test for attentional shift impairments within and across stimulus dimensions (ie, spatial location and level of illumination). Attentional shift refers to the ability to move the focus of attention from one object to another, and it is a top-down mechanism regulated by the frontal cortex in combination with thalamic nuclei.68 PCP selectively impaired rats’ ability to shift attention across stimulus dimensions or extradimensional shifting. These attentional impairments were associated with reduced expression of Zif-268 and parvalbumin, 2 markers of local neuronal activity, in the infralimbic cortex and in the TRN.54
Other pharmacological studies investigating the effects of PCP on the rat brain found induced metabolic hypofunction within the prefrontal cortex, the auditory system, and the TRN, which were reversed after coadministration of typical (haloperidol) or atypical (clozapine) antipsychotics with PCP.58,59 PCP could also induce excitotoxic lesions in the posterior cingulate and retrosplenial cortices of rats following injections in the anterior thalamus. These lesions were likely due to reduced inhibitory control of the TRN on anterior thalamic nuclei, which in turn determined excessive activation (excitotoxicity) of corticolimbic areas. Of importance, these excitotoxic effects on the rat brain could be prevented by administering antipsychotic medications, such as haloperidol and clozapine.60–62
The DISC1 gene, located on chromosome 1q42, has been associated with schizophrenia by multiple human genetic studies.69 A recent study investigating the expression of DISC1 in the mouse brain found that it was expressed only in some brain structures, including hippocampus, part of the neocortex, hyphothalamus, stria terminalis, and TRN. Furthermore, in the mouse TRN, DISC1 was highly expressed during development, in a period when corticothalamic connections take shape. Other studies in rats have shown that the TRN and the perireticular nucleus, a narrow sheet of cells surrounding the TRN, are significantly larger relative to their adult size during development, when corticothalamic and thalamocortical pathways are first formed, thus suggesting that these thalamic nuclei may be critically implicated in this process.70
Based on these results, which, however, need to be replicated in species with a more complex corticothalamic circuitry, it was suggested that the TRN may play a critical role in the ontogenesis of connections within the thalamocortical system and that deficits in the TRN may be implicated in the neurodevelopmental vulnerability to schizophrenia.63
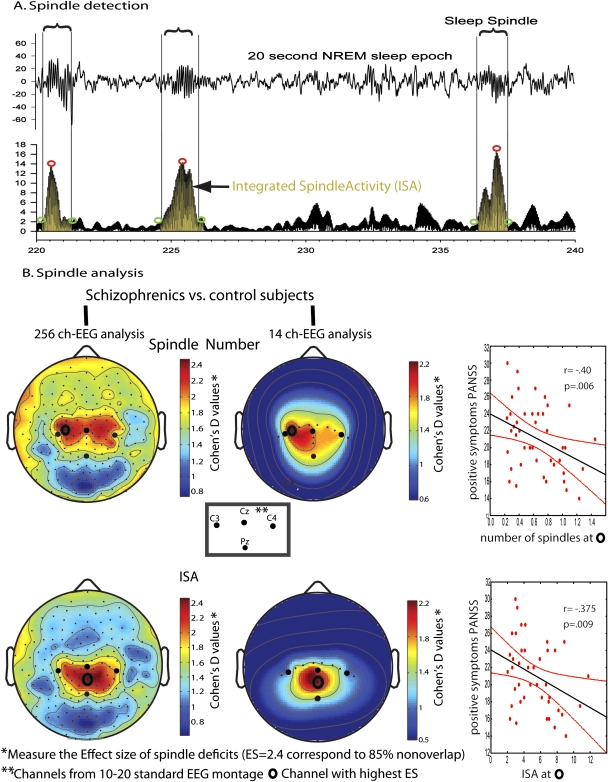
Consistent with these findings from animal studies, 2 sleep hd-EEG studies in humans have recently provided suggestive evidence for TRN defects in schizophrenia patients.71,72 An initial study found that 18 schizophrenics had marked deficits in TRN-generated NREM sleep spindles.9 Schizophrenics showed significant reductions in spindle duration, amplitude, number, and integrated spindle activity (ISA), calculated by integrating spindle amplitude over time, compared with 17 healthy controls and 15 depressed patients in the first NREM episode. A follow-up study extended these findings to 49 schizophrenics in whole-night recordings and established that nonschizophrenic patients taking antipsychotics had no spindle activity reduction, thus indicating that spindle deficits were not simply due to medications. Deficits in spindle number and ISA had an effect size (ES) ≥2.21, corresponding to ≥85% separation between schizophrenics and control subjects (figure 2), and similar ES were observed employing a 14-channel, low-density montage that included C1 and CPz, the channels with highest ES for the hd-EEG analysis. Additionally, in schizophrenics, both spindle number (at C1) and ISA (at CPz) were inversely correlated to the positive symptoms of the Positive and Negative Symptoms Scales. Given the magnitude of spindle deficits and their correlation with clinical symptoms in schizophrenics, these findings suggest that reduced TRN function may be implicated in the neurobiology of schizophrenia and may contribute to its clinical features.
Fig. 2.
Spindles Deficits in Schizophrenics Have High Effect Size (ES); Can Be Detected With A Few Electroencephalographic (EEG) Channels; and Correlate With Clinical Symptoms. (A) The top trace shows 20 s of non-rapid eye movement (NREM) sleep, with vertical lines enclosing sleep spindles. The bottom trace represents the rectified EEG signal filtered in the spindle range (12–16 Hz). Circles indicate the beginning and end of each spindle, as well as its maximal amplitude, while the highlighted area reflects spindle activity integrated over time (ISA). (B) Left panel: high-density (185 channels) topography of the ES of spindle deficits in schizophrenics. Spindle deficits had high ES, corresponding to 85% separation between schizophrenics and control subjects. Similar ES were obtained with a 14-channel EEG montage (middle panel), and they were correlated with the clinical symptoms of schizophrenics (right panel).
At this stage we can only speculate about the contribution of TRN deficits to the clinical symptoms of schizophrenia, which include hallucinations, delusions, as well as attentional, learning, and memory deficits. Auditory hallucinations are thought to reflect a reduced ability to distinguish externally generated stimuli, which are relayed by thalamocortical sensory pathways, from internally generated inputs, which are processed by corticothalamic circuits.73 Intriguingly, a study using juxtacellular recordings and labeling techniques has recently shown that the rat auditory TRN receives tonotopically organized afferents from both the cortex and the thalamus.74 A defect in the TRN may therefore account for the simultaneous impairment of thalamocortical and corticothalamic auditory circuits in schizophrenia. Furthermore, a reduced inhibitory control of the TRN on other thalamic nuclei would increase the response of thalamocortical neurons to internally cortically generated inputs, which in turn would facilitate hallucinatory experiences. A reduced activity of TRN neurons, as indicated by decreased cholinergic binding, has been reported in a postmortem study in schizophrenics and patients with Lewy bodies dementia, a disorder characterized by visual hallucinations.75
Together with hallucinations, patients with schizophrenia commonly experience delusions. Delusional thoughts tend to arise in a state of hypervigilance, characterized by an increased neuronal activity and an enhanced response to incoming inputs. It is therefore possible that a reduced inhibitory control of the TRN on thalamocortical activity may result in hyperactivation of the cortex, which, in turn, may produce psychotic delusional symptoms.
Schizophrenics also report cognitive deficits and especially attentional impairments.76 The TRN has long been viewed as critical for attention regulation, as indicated in the searchlight hypothesis.68 This hypothesis suggests explicitly that the TRN is involved in rapidly moving the center of attention between external inputs, based on a decision made by the frontal cortex. Recent electrophysiological studies in primates have provided experimental evidence for the involvement of the TRN in the control of attention and have shown how TRN dysfunctions may result in attentional deficits.77,78
Other cognitive processes found to be impaired in schizophrenia patients are learning and memory.76 While many factors, including changes in the level of attention, decreased motivation, and presence of active symptoms may affect these cognitive processes, it is intriguing that such processes are critically regulated during sleep by sleep-specific rhythms, including sleep spindles.79 Higher spindle activity is associated with better performances in verbal memory, visuospatial memory, as well as declarative learning tasks.79 A defective TRN function, which initiates spindle oscillations, may therefore interfere with the ability to learn as well as with memory consolidation processes and may account for some of the cognitive impairments found in schizophrenia.
Future Directions
Noninvasive direct measurements of activity in the human TRN are difficult. However, both pharmacological and nonpharmacological strategies can be utilized to investigate TRN activity deficits in schizophrenia patients.
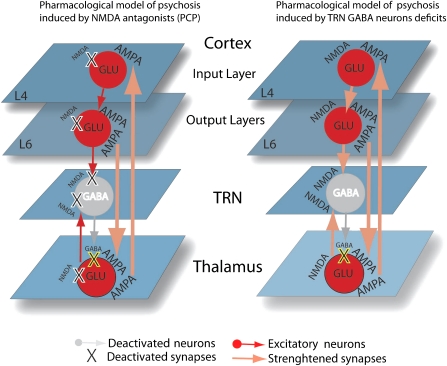
A pharmacological approach would involve testing whether compounds that target the GABAergic system might improve spindle deficits in schizophrenics and whether spindle improvements may predict amelioration in the clinical symptoms of these patients. It has been shown recently that Gabapentin, a compound that selectively increases GABA activity, improved clinical symptoms in a treatment-resistant schizophrenia patient.80 Furthermore, it has been suggested that a reduced GABA release by TRN neurons is a key mechanism underlying the psychosis induced by NMDA receptor antagonists.61 This pharmacological model of psychosis proposes that a reduction in GABA release from TRN to other thalamic nuclei, due to inactivation of NMDA receptors on TRN neurons, would increase the firing rate of thalamic relay neurons to the cortex. Hyperactivated cortical neurons would then determine psychotic symptoms, while corticothalamic excitatory feedbacks would sustain this effect (figure 3, left panel). Intriguingly, a decreased activity of TRN neurons could cause a reduction in GABA release similar to that determined by NMDA receptor antagonists (figure 3, right panel).
Fig. 3.
Pharmacological Models of Psychosis Induced by NMDA Antagonists (eg, PCP) or by Deficits of TRN Gamma Amino Butyric Acid (GABA)ergic Neurons. Left panel: PCP blocks (X) NMDA receptors in the Cortex, TRN, and Thalamus. The overall effect is a reduction in TRN GABA release, which results in an increase in the activity of cortex-thalamus and thalamus-cortex α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) excitatory synapses. Right panel: deficits in TRN GABAergic neurons (X) can induce a similar increase in the activity of cortex-thalamus and thalamus-cortex connections, which is mediated by both AMPA and NMDA synapses.
A complementary nonpharmacological approach to ascertain TRN deficits in schizophrenia involves employing a combination of electrophysiological/neuroimaging techniques: transcranial magnetic stimulation (TMS) with hd-EEG and TMS/fMRI. TMS/hd-EEG and TMS/fMRI allow implementing a “perturb and measure” approach in which neural responses to single pulses of TMS delivered to the cortex can be measured as proxies for TRN responses. Corticothalamic afferents are 10-fold more numerous than thalamocortical projections.10 Furthermore, the number of glutamatergic corticothalamic synapses onto TRN neurons is 3.7 times higher than corticothalamic synapses onto thalamocortical neurons, and the amplitude of excitatory postsynaptic currents (EPSCs) is about 2.5 times larger in TRN than in thalamocortical neurons.81 Thus, cortical stimulation is a priori the most effective way of testing the activity of the TRN, measured at the thalamus by fMRI and at scalp electrodes directly under the TMS coil by EEG. It is also plausible that the same putative TRN dysfunction underlying sleep spindles will result in abnormalities in responses to single pulses of TMS in schizophrenics. Specifically, if TRN activity is reduced, the TMS-evoked blood oxygen level–dependent response in the thalamus should be of lower magnitude in schizophrenic patients vs healthy control subjects, and the size of this decrease might correlate with the magnitude of spindle deficits. Similarly, early components of the EEG response to TMS, which are largely contributed by a thalamic rebound from the TMS-evoked cortical activation,82 should be reduced in schizophrenics in a manner that correlates with spindle deficits. By using TMS/hd-EEG, we recently found reduced TMS-evoked early gamma oscillations in the frontal cortex of schizophrenics compared with healthy controls.83 Altogether, results from these experiments may provide further evidence of TRN deficits in schizophrenia and may help establishing the contribution of the TRN to the neurobiology of this disorder.
Funding
This work was supported by the schizophrenia program of the Health-Emotions Research Institute and a National Institutes of Health-National Institute of Mental Health Conte Center (1P20MH077967-01A1 to G.T.) and by a European Union Marie Curie International Reintegration Grant (IRG-No208779 to F.F.).
Acknowledgments
Dr Tononi has declared the following conflicts of interest statement: Philips Healthcare/Respironics—Currently the David P. White Chair in Sleep Medicine at the University of Wisconsin-Madison. The chair is endowed by Philips Respironics; Sponsors of Slow Wave Induction grant (05/01/2009-04/30/2010); Consulting fee of <$20,000; Symposium Speaker of <$500; Invited Speaker, 2008, June 8: “Advancing Sleep Science Through Interdisciplinary Research,” Sleep Research Society, Baltimore, Maryland.
References
- 1.Guillery RW, Harting JK. Structure and connections of the thalamic reticular nucleus: advancing views over half a century. J Comp Neurol. 2003;463:360–371. doi: 10.1002/cne.10738. [DOI] [PubMed] [Google Scholar]
- 2.Krause M, Hoffmann WE, Hajos M. Auditory sensory gating in hippocampus and reticular thalamic neurons in anesthetized rats. Biol Psychiatry. 2003;53:244–253. doi: 10.1016/s0006-3223(02)01463-4. [DOI] [PubMed] [Google Scholar]
- 3.Guillery RW, Feig SL, Lozsadi DA. Paying attention to the thalamic reticular nucleus. Trends Neurosci. 1998;21:28–32. doi: 10.1016/s0166-2236(97)01157-0. [DOI] [PubMed] [Google Scholar]
- 4.Mayo JP. Intrathalamic mechanisms of visual attention. J Neurophysiol. 2009;101:1123–1125. doi: 10.1152/jn.91369.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAlonan K, Brown VJ, Bowman EM. Thalamic reticular nucleus activation reflects attentional gating during classical conditioning. J Neurosci. 2000;20:8897–8901. doi: 10.1523/JNEUROSCI.20-23-08897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zikopoulos B, Barbas H. Circuits for multisensory integration and attentional modulation through the prefrontal cortex and the thalamic reticular nucleus in primates. Rev Neurosci. 2007;18:417–438. doi: 10.1515/revneuro.2007.18.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazhenov M, Timofeev I, Steriade M, Sejnowski T. Spiking-bursting activity in the thalamic reticular nucleus initiates sequences of spindle oscillations in thalamic networks. J Neurophysiol. 2000;84:1076–1087. doi: 10.1152/jn.2000.84.2.1076. [DOI] [PubMed] [Google Scholar]
- 9.Fuentealba P, Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Jones EG, editor. The Thalamus. 2nd ed. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- 11.Crabtree JW. Intrathalamic sensory connections mediated by the thalamic reticular nucleus. Cell Mol Life Sci. 1999;56:683–700. doi: 10.1007/s000180050462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol. 1987;262:105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- 13.Morrison JH, Foote SL. Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J Comp Neurol. 1986;243:117–138. doi: 10.1002/cne.902430110. [DOI] [PubMed] [Google Scholar]
- 14.Asanuma C. GABAergic and pallidal terminals in the thalamic reticular nucleus of squirrel monkeys. Exp Brain Res. 1994;101:439–451. doi: 10.1007/BF00227337. [DOI] [PubMed] [Google Scholar]
- 15.Sillito AM, Kemp JA, Berardi N. The cholinergic influence on the function of the cat dorsal lateral geniculate nucleus (dLGN) Brain Res. 1983;280:299–307. doi: 10.1016/0006-8993(83)90059-8. [DOI] [PubMed] [Google Scholar]
- 16.McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex. J Clin Neurophysiol. 1992;9:212–223. doi: 10.1097/00004691-199204010-00004. [DOI] [PubMed] [Google Scholar]
- 17.Floran B, Floran L, Erlij D, Aceves J. Activation of dopamine D4 receptors modulates [3H]GABA release in slices of the rat thalamic reticular nucleus. Neuropharmacology. 2004;46:497–503. doi: 10.1016/j.neuropharm.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- 19.Houser CR, Vaughn JE, Barber RP, Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980;200:341–354. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- 20.FitzGibbon T, Solomon SG, Goodchild AK. Distribution of calbindin, parvalbumin, and calretinin immunoreactivity in the reticular thalamic nucleus of the marmoset: evidence for a medial leaflet of incertal neurons. Exp Neurol. 2000;164:371–383. doi: 10.1006/exnr.2000.7436. [DOI] [PubMed] [Google Scholar]
- 21.Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15:303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- 22.Coleman KA, Mitrofanis J. Organization of the visual reticular thalamic nucleus of the rat. Eur J Neurosci. 1996;8:388–404. doi: 10.1111/j.1460-9568.1996.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 23.Hayama T, Hashimoto K, Ogawa H. Anatomical location of a taste-related region in the thalamic reticular nucleus in rats. Neurosci Res. 1994;18:291–299. doi: 10.1016/0168-0102(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 24.Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Shosaku A, Kayama Y, Sumitomo I, Sugitani M, Iwama K. Analysis of recurrent inhibitory circuit in rat thalamus: neurophysiology of the thalamic reticular nucleus. Prog Neurobiol. 1989;32:77–102. doi: 10.1016/0301-0082(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 26.Stehberg J, Acuna-Goycolea C, Ceric F, Torrealba F. The visceral sector of the thalamic reticular nucleus in the rat. Neuroscience. 2001;106:745–755. doi: 10.1016/s0306-4522(01)00316-5. [DOI] [PubMed] [Google Scholar]
- 27.Sherman SM. Thalamic relay functions. Prog Brain Res. 2001;134:51–69. doi: 10.1016/s0079-6123(01)34005-0. [DOI] [PubMed] [Google Scholar]
- 28.Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 29.Steriade M. Alertness, Quiet Sleep, Dreaming. Vol 9. New York, NY: Plenum; 1991. [Google Scholar]
- 30.Barbas H, Zikopoulos B. The prefrontal cortex and flexible behavior. Neuroscientist. 2007;13:532–545. doi: 10.1177/1073858407301369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bal T, McCormick DA. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. J Physiol. 1993;468:669–691. doi: 10.1113/jphysiol.1993.sp019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 33.McCormick DA, Feeser HR. Functional implications of burst firing and single spike activity in lateral geniculate relay neurons. Neuroscience. 1990;39:103–113. doi: 10.1016/0306-4522(90)90225-s. [DOI] [PubMed] [Google Scholar]
- 34.Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996;274:771–774. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- 35.Steriade M. Sleep oscillations and their blockage by activating systems. J Psychiatry Neurosci. 1994;19:354–358. [PMC free article] [PubMed] [Google Scholar]
- 36.Contreras D, Steriade M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J Physiol. 1996;490:159–179. doi: 10.1113/jphysiol.1996.sp021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pare D, Steriade M, Deschenes M, Oakson G. Physiological characteristics of anterior thalamic nuclei, a group devoid of inputs from reticular thalamic nucleus. J Neurophysiol. 1987;57:1669–1685. doi: 10.1152/jn.1987.57.6.1669. [DOI] [PubMed] [Google Scholar]
- 38.Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57:260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- 39.Contreras D, Curro Dossi R, Steriade M. Bursting and tonic discharges in two classes of reticular thalamic neurons. J Neurophysiol. 1992;68:973–977. doi: 10.1152/jn.1992.68.3.973. [DOI] [PubMed] [Google Scholar]
- 40.Huguenard JR, Chung JM, Prince DA. Excitability changes in thalamic and neocortical neurons after injury. Epilepsy Res Suppl. 1996;12:129–135. [PubMed] [Google Scholar]
- 41.Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- 42.Landisman CE, Long MA, Beierlein M, Deans MR, Paul DL, Connors BW. Electrical synapses in the thalamic reticular nucleus. J Neurosci. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- 44.Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Self-sustained rhythmic activity in the thalamic reticular nucleus mediated by depolarizing GABAA receptor potentials. Nat Neurosci. 1999;2:168–174. doi: 10.1038/5729. [DOI] [PubMed] [Google Scholar]
- 45.Golomb D, Wang XJ, Rinzel J. Synchronization properties of spindle oscillations in a thalamic reticular nucleus model. J Neurophysiol. 1994;72:1109–1126. doi: 10.1152/jn.1994.72.3.1109. [DOI] [PubMed] [Google Scholar]
- 46.Battaglia G, Lizier C, Colacitti C, Princivalle A, Spreafico R. A reticuloreticular commissural pathway in the rat thalamus. J Comp Neurol. 1994;347:127–138. doi: 10.1002/cne.903470110. [DOI] [PubMed] [Google Scholar]
- 47.Chen S, Raos V, Bentivoglio M. Connections of the thalamic reticular nucleus with the contralateral thalamus in the rat. Neurosci Lett. 1992;147:85–88. doi: 10.1016/0304-3940(92)90780-b. [DOI] [PubMed] [Google Scholar]
- 48.Pinault D, Bourassa J, Deschenes M. Thalamic reticular input to the rat visual thalamus: a single fiber study using biocytin as an anterograde tracer. Brain Res. 1995;670:147–152. doi: 10.1016/0006-8993(94)01303-y. [DOI] [PubMed] [Google Scholar]
- 49.Pinault D, Deschenes M. Anatomical evidence for a mechanism of lateral inhibition in the rat thalamus. Eur J Neurosci. 1998;10:3462–3469. doi: 10.1046/j.1460-9568.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- 50.Scheibel ME, Scheibel AB. Specialized organizational patterns within the nucleus reticularis thalami of the cat. Exp Neurol. 1972;34:316–322. doi: 10.1016/0014-4886(72)90177-x. [DOI] [PubMed] [Google Scholar]
- 51.Deschenes M, Timofeeva E, Lavallee P, Dufresne C. The vibrissal system as a model of thalamic operations. Prog Brain Res. 2005;149:31–40. doi: 10.1016/S0079-6123(05)49003-2. [DOI] [PubMed] [Google Scholar]
- 52.Lam YW, Sherman SM. Different topography of the reticulothalmic inputs to first- and higher-order somatosensory thalamic relays revealed using photostimulation. J Neurophysiol. 2007;98:2903–2909. doi: 10.1152/jn.00782.2007. [DOI] [PubMed] [Google Scholar]
- 53.Zikopoulos B, Barbas H. Parallel driving and modulatory pathways link the prefrontal cortex and thalamus. PLoS One. 2007;2:e848. doi: 10.1371/journal.pone.0000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Egerton A, Reid L, McKerchar CE, Morris BJ, Pratt JA. Impairment in perceptual attentional set-shifting following PCP administration: a rodent model of set-shifting deficits in schizophrenia. Psychopharmacology (Berl) 2005;179:77–84. doi: 10.1007/s00213-004-2109-y. [DOI] [PubMed] [Google Scholar]
- 55.Adler LE, Olincy A, Waldo M, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- 56.Freedman R, Olincy A, Ross RG, et al. The genetics of sensory gating deficits in schizophrenia. Curr Psychiatry Rep. 2003;5:155–161. doi: 10.1007/s11920-003-0032-2. [DOI] [PubMed] [Google Scholar]
- 57.Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cochran SM, Fujimura M, Morris BJ, Pratt JA. Acute and delayed effects of phencyclidine upon mRNA levels of markers of glutamatergic and GABAergic neurotransmitter function in the rat brain. Synapse. 2002;46:206–214. doi: 10.1002/syn.10126. [DOI] [PubMed] [Google Scholar]
- 59.Cochran SM, Kennedy M, McKerchar CE, Steward LJ, Pratt JA, Morris BJ. Induction of metabolic hypofunction and neurochemical deficits after chronic intermittent exposure to phencyclidine: differential modulation by antipsychotic drugs. Neuropsychopharmacology. 2003;28:265–275. doi: 10.1038/sj.npp.1300031. [DOI] [PubMed] [Google Scholar]
- 60.Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA. NMDA antagonist neurotoxicity: mechanism and prevention. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- 61.Sharp FR, Tomitaka M, Bernaudin M, Tomitaka S. Psychosis: pathological activation of limbic thalamocortical circuits by psychomimetics and schizophrenia? Trends Neurosci. 2001;24:330–334. doi: 10.1016/s0166-2236(00)01817-8. [DOI] [PubMed] [Google Scholar]
- 62.Tomitaka S, Tomitaka M, Tolliver BK, Sharp FR. Bilateral blockade of NMDA receptors in anterior thalamus by dizocilpine (MK-801) injures pyramidal neurons in rat retrosplenial cortex. Eur J Neurosci. 2000;12:1420–1430. doi: 10.1046/j.1460-9568.2000.00018.x. [DOI] [PubMed] [Google Scholar]
- 63.Austin CP, Ky B, Ma L, Morris JA, Shughrue PJ. Expression of Disrupted-In-Schizophrenia-1, a schizophrenia-associated gene, is prominent in the mouse hippocampus throughout brain development. Neuroscience. 2004;124:3–10. doi: 10.1016/j.neuroscience.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 64.Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- 65.Cadenhead KS, Light GA, Geyer MA, Braff DL. Sensory gating deficits assessed by the P50 event-related potential in subjects with schizotypal personality disorder. Am J Psychiatry. 2000;157:55–59. doi: 10.1176/ajp.157.1.55. [DOI] [PubMed] [Google Scholar]
- 66.Light GA, Braff DL. Human and animal studies of schizophrenia-related gating deficits. Curr Psychiatry Rep. 1999;1:31–40. doi: 10.1007/s11920-999-0008-y. [DOI] [PubMed] [Google Scholar]
- 67.Tregellas JR, Davalos DB, Rojas DC, et al. Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res. 2007;92:262–272. doi: 10.1016/j.schres.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brandon NJ, Millar JK, Korth C, Sive H, Singh KK, Sawa A. Understanding the role of DISC1 in psychiatric disease and during normal development. J Neurosci. 2009;29:12768–12775. doi: 10.1523/JNEUROSCI.3355-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitrofanis J, Guillery RW. New views of the thalamic reticular nucleus in the adult and the developing brain. Trends Neurosci. 1993;16:240–245. doi: 10.1016/0166-2236(93)90163-g. [DOI] [PubMed] [Google Scholar]
- 71.Ferrarelli F, Huber R, Peterson MJ, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 72.Ferrarelli F, Peterson MJ, Sarasso S, et al. Whole night deficits in slow and fast spindles point to thalamic dysfunction in schizophrenia. Am J Psychiatry. 2010;167:1339–1348. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hugdahl K. “Hearing voices”: auditory hallucinations as failure of top-down control of bottom-up perceptual processes. Scand J Psychol. 2009;50:553–560. doi: 10.1111/j.1467-9450.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- 74.Kimura A, Imbe H, Donishi T. Axonal projections of auditory cells with short and long response latencies in the medial geniculate nucleus: distinct topographies in the connection with the thalamic reticular nucleus. Eur J Neurosci. 2009;30:783–799. doi: 10.1111/j.1460-9568.2009.06880.x. [DOI] [PubMed] [Google Scholar]
- 75.Court J, Spurden D, Lloyd S, et al. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. J Neurochem. 1999;73:1590–1597. doi: 10.1046/j.1471-4159.1999.0731590.x. [DOI] [PubMed] [Google Scholar]
- 76.Gur RE, Nimgaonkar VL, Almasy L, et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164:813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- 77.McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. J Neurosci. 2006;26:4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–394. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fogel SM, Nader R, Cote KA, Smith CT. Sleep spindles and learning potential. Behav Neurosci. 2007;121(1):1–10. doi: 10.1037/0735-7044.121.1.1. [DOI] [PubMed] [Google Scholar]
- 80.Demily C, Franck N. Gabapentin for ultra resistant schizophrenia with aggressive behavior. Schizophr Res. 2008;100:349–350. doi: 10.1016/j.schres.2007.12.482. [DOI] [PubMed] [Google Scholar]
- 81.Golshani P, Liu XB, Jones EG. Differences in quantal amplitude reflect GluR4- subunit number at corticothalamic synapses on two populations of thalamic neurons. Proc Natl Acad Sci U S A. 2001;98:4172–4177. doi: 10.1073/pnas.061013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Der Werf YD, Sadikot AF, Strafella AP, Paus T. The neural response to transcranial magnetic stimulation of the human motor cortex. II. Thalamocortical contributions. Exp Brain Res. 2006;175:246–255. doi: 10.1007/s00221-006-0548-x. [DOI] [PubMed] [Google Scholar]
- 83.Ferrarelli F, Massimini M, Peterson MJ, et al. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. Am J Psychiatry. 2008;165:996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]