Abstract
The mitosomes of Giardia intestinalis are thought to be mitochondria highly-reduced in response to the oxygen-poor niche. We performed a quantitative proteomic assessment of Giardia mitosomes to increase understanding of the function and evolutionary origin of these enigmatic organelles. Mitosome-enriched fractions were obtained from cell homogenate using Optiprep gradient centrifugation. To distinguish mitosomal proteins from contamination, we used a quantitative shot-gun strategy based on isobaric tagging of peptides with iTRAQ and tandem mass spectrometry. Altogether, 638 proteins were identified in mitosome-enriched fractions. Of these, 139 proteins had iTRAQ ratio similar to that of the six known mitosomal markers. Proteins were selected for expression in Giardia to verify their cellular localizations and the mitosomal localization of 20 proteins was confirmed. These proteins include nine components of the FeS cluster assembly machinery, a novel diflavo-protein with NADPH reductase activity, a novel VAMP-associated protein, and a key component of the outer membrane protein translocase. None of the novel mitosomal proteins was predicted by previous genome analyses. The small proteome of the Giardia mitosome reflects the reduction in mitochondrial metabolism, which is limited to the FeS cluster assembly pathway, and a simplicity in the protein import pathway required for organelle biogenesis.
Introduction
Mitochondria are eukaryotic organelles that are thought to have evolved from an alpha-proteobacterial endosymbiont about two billion years ago. The loss of bacterial autonomy and transition of the endosymbiont to a “protomitochondrion” were associated with a reduction in the number of genes in the endosymbiont genome; these genes were either transferred to the nuclear genome or lost. While the genome of the extant alpha-proteobacterium Rickettsia prowazekii contains 834 protein-coding genes [1], the largest number of genes (67 protein-coding genes) in a mitochondrial genome is found in Reclinomonas americana [2], with only three protein-coding genes present in the Plasmodium falciparum mitochondrial genome [3]. Paradoxically, the reduction of the mitochondrial genome did not lead to a reduction of the organellar proteome [4]. The acquisition of a mechanism for mitochondrial import at the earliest stage of the endosymbiont-to-protomitochondrion transition allowed the recruitment of the proteins of endosymbiotic origin that were now encoded in the nucleus, and the import of proteins of other origins [5]. Contemporary mitochondrial proteomes contain hundreds of proteins, up to 1100 proteins in the mouse [6].
Mitosomes are the most highly reduced forms of mitochondria, having completely lost their genomes and dramatically reduced their proteomes. Mitosomes have also lost many of the typical mitochondrial functions, such as respiration, the citric acid cycle, and ATP synthesis. Biosynthesis of FeS clusters is the only mitochondrial function seen to be retained by at least some mitosomes [7]. Mitosomes have become established independently in diverse groups of unicellular eukaryotes (protists); many of them once considered to be amitochondrial because they lack organelles with the expected mitochondrial morphology [8].
Organisms with mitosomes live under oxygen-limiting conditions, like the human intestinal parasites Giardia intestinalis [9] and Entamoeba histolytica [10], or are intracellular parasites like the microsporidians Encephalitozoon cuniculi and Trachipleistophora hominis [11], [12] and the apicomplexan Cryptosporidium parvum [13]. Mitosomes are tiny ovoid organelles enclosed by two membranes. Unlike mitochondria, the inner membrane of mitosomes does not form cristae. The morphology of the mitosome is reminiscent of the hydrogenosome, another form of mitochondrion that is present in some anaerobic protists, such as Trichomonas vaginalis. Unlike mitosomes, however, hydrogenosomes are metabolically active organelles that produce ATP by substrate level phosphorylation [14].
The limited knowledge of mitosomal proteomes has been gained mainly from analyses of genome sequences and localization studies of a few model mitosomal proteins [9], [11], [15]–[21]. The only published proteomics study that focused on mitosomes was that recently reported for the amoeba E. histolytica, identifying a unique sulfate activation pathway [22]. To increase our understanding of the function and origin of these enigmatic organelles, we established a large-scale proteomic approach to analyze the mitosomes of Giardia intestinalis. This organism was selected because Giardia intestinalis is a common human intestinal pathogen, its genome sequence has been published [23], [24], and it is considered to be among the most basal eukaryotes [25]. Moreover, previous analysis of the G. intestinalis genome provided little new information pertaining to the putative mitosomal proteome [24], so there are substantial gaps in our knowledge of the structure and function of this essential organelle. Here, we quantitatively analyzed the presence of isobarically-tagged proteins in mitosome enriched fractions. This technique allowed us to discriminate the mitosomal proteins from those of contaminating cellular structures. Combined with an exhaustive bioinformatics analysis, this strategy identified 139 putative mitosomal proteins; 20 of which were experimentally confirmed to be localized in mitosomes. Our results revealed that the proteome of the G. intestinalis mitosome is selectively reduced and houses a single metabolic pathway for FeS cluster assembly, a novel diflavin protein with NADPH reductase activity, a minimal protein import machinery and proteins that may be important for the interaction of mitosomes with other cellular compartments.
Results and Discussion
Identification of putative mitosomal proteins by isobaric tagging
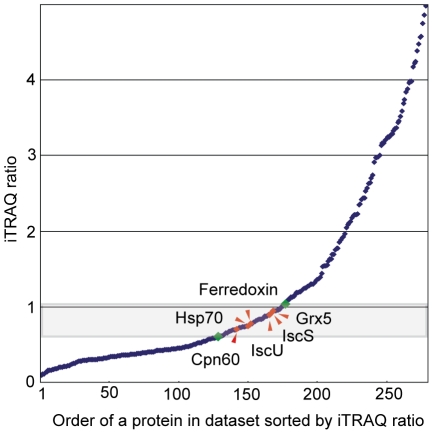
Mitosome-enriched fractions were separated from a Giardia homogenate by preparative centrifugation using a discontinuous Optiprep (iodixanol) gradient [26]. This method produced five dense organellar fractions (Fig. 1A). The mitosomal marker protein IscU was particularly enriched in fraction #4 and to a lesser extent in fraction #3 (Fig. 1B). Electron microscopy confirmed the presence of mitosomes in both fractions; however, co-fractionating vesicles of similar densities were also found (data not shown).To discriminate between putative mitosomal proteins and those of contaminating cellular structures, we compared the relative distribution of each protein in fractions #3 and #4. Because the mitosomal proteins necessarily co-fractionate (i.e. being contained within mitosomes) during gradient centrifugation, each of the bona fide mitosomal protein should display similar distribution ratios [27]. To this end, the proteins of fractions #3 and #4 were digested in parallel with trypsin and each peptide population was labeled with a distinct iTRAQ reagent and then combined. The isobaric mass characteristics of the iTRAQ reagents means the differentially-labeled peptides from fractions #3 and #4 form a single peak in the MS scan for protein identification. MS/MS analysis of the iTRAQ-labelled peptides liberates the isotope-encoded reporter ions, the ratio of which can reflects the distribution of the protein across the two fractions. In our analysis, the pooled peptides were analyzed by tandem mass spectrometry after subsequent separation with isoelectric focusing and nano-liquid chromatography (nano-LC MS/MS). The iTRAQ ratio was then calculated for each protein, and the proteins were sorted according to the relative distributions in the fractions (Fig. 2).
Figure 1. Isolation of mitosome-rich fractions.
(A) Trophozoites were disrupted and centrifuged to remove unbroken cells, nuclei and cytoskeletal residue. The high-speed pellet was resuspended in sucrose buffer, layered onto an Optiprep density gradient, and centrifuged overnight. Five distinct fractions were obtained. (B) Fractions were collected and analyzed by SDS-PAGE and Western blot. The mitosomal marker GiIscU was detected in fractions #3 and #4 using a polyclonal rabbit antibody. (C–D) Electron microscopy of subcellular fractions. Fraction #3 (C) contains numerous vesicles of variable sizes, while fraction #4 (D) contains vesicles of more homogeneous sizes. Arrows indicate mitosomes.
Figure 2. iTRAQ ratios define protein subcellular localization.
Proteins in fractions #3 and #4 isolated on the Optiprep gradient were labeled with the iTRAQ-114 and iTRAQ-115 reagents, respectively, analyzed by LC MS/MS, and sorted according to the iTRAQ ratios. Mitosomal marker proteins (red diamonds) fall into a narrow range of iTRAQ ratios. Green diamondsdindicate the zone of proteins considered as mitosomal candidates (mitosomal distribution, MiD).
Validating the methodology, mitosomal markers (IscS, IscU, [2Fe2S] ferredoxin, Cpn60, Hsp70 and glutaredoxin 5) [28] clustered together with similar iTRAQ ratios (Fig. 2). Proteins with ratios between the lowest and highest values for the markers were considered to be candidate mitosomal proteins. We also extended this window on both sides by half of the distance between the limiting markers and included all proteins in this extended window (Fig. 2). In total, we identified 638 proteins (Table S1), with 139 of these proteins meeting the defined criteria for mitosomal proteins (Tables 1– 7). Each of the 139 mitosomal candidates was assigned to a probable function based on current annotations in the GiardiaDB, PSI BLAST searches in the NCBI nr database, and motif and domain searches in the Pfam database. Three additional bioinformatics tools were used to predict cellular localization (PsortII, TargetP 1.1 and SignalP 3.0), and two web-based programs were used to predict alpha-helical transmembrane region segments (TMHMM and Memsat3) (Tables S2–S4, summary is given in Tables 1– 7). The candidate proteins were clustered into 13 groups according to their predicted functions (Tables 1– 7, Fig. 3). The proteomic data confirmed the validity of 250 hypothetical genes predicted from the complete genome sequence of Giardia [24]; 40 of these formed the largest group of candidate mitosomal proteins.
Table 1. Putative mitosomal proteins classified by predicted function: Iron-sulfur cluster assembly, chaperones, redox mechanism and protein translocation and processing.
| Accession number | Annotation | Identification | Localization | Structure | |||||||
| MASCOT | Coverage | MiD | SignalP | Target P | Psort II | Exp Ver. | MEMSAT3 | SGP | TMHMM | ||
| score | peptides | % mito | TM No. | TM No. | |||||||
| Iron-sulfur cluster assembly | |||||||||||
| GL50803_14519 | IscS, cysteine desulfurase | 296 | 4 | Y | N | O | M | 0 | # | 0 | |
| GL50803_15196 | IscU | 243 | 5 | Y | N | M | 17% | M | 0 | # | 0 |
| EAA38809 | Nfu | 60 | 2 | Y | N | M | 39% | M | 1 | 0 | |
| GL50803_14821 | IscA | 198 | 3 | Y | N | O | 35% | M | 0 | # | 0 |
| GL50803_2013 | Glutaredoxin 5 | 249 | 3 | Y | N | O | 13% | M | 0 | # | 0 |
| Molecular chaperones | |||||||||||
| GL50803_14581 | mitochondrial type HSP70 | 404 | 7 | Y | N | O | 13% | M | 0 | # | 0 |
| GL50803_1376 | GrpE | 29 | 1 | Y | N | M | 39% | M | 0 | # | 0 |
| GL50803_17030 | DnaJ protein, Jac1 | * | * | * | N | O | 35% | M | 0 | # | 0 |
| GL50803_9751 | DnaJ protein, Type III | 34 | 1 | Y | N | O | 13% | M | 1 | 1 | |
| GL50803_103891 | Cpn60 | 336 | 6 | Y | N | O | M | 0 | # | 0 | |
| GL50803_29500 | Cpn10 | 68 | 1 | Y | Y | O | 9% | M | 0 | # | 0 |
| Redox mechanism | |||||||||||
| GL50803_27266 | [2Fe-2S] ferredoxin | 182 | 2 | Y | N | M | 48% | M | 0 | # | 0 |
| GL50803_91252 | GiOR-1, oxidoreductase | 40 | 1 | N | N | O | 13% | M | 0 | # | 0 |
| GL50803_15897 | GiOR-2, oxidoreductase | * | * | * | N | O | 21% | O | 0 | # | 0 |
| GL50803_9827 | Thioredoxin reductase | * | * | * | N | M | 13% | O | 0 | # | 0 |
| GL50803_9719 | NADH oxidase | 271 | 5 | Y | N | O | 9% | ** | 0 | # | 0 |
| GL50803_16076 | Peroxiredoxin 1 | 293 | 5 | Y | N | O | 9% | 0 | # | 0 | |
| Protein translocation and processing | |||||||||||
| GL50803_17161 | Tom40 | 208 | 2 | Y | N | O | 13% | M | 0 | # | 0 |
| XP_002364144 | Pam18 | 68 | 1 | Y | N | M | 30% | M | 0 | # | 0 |
| GL50803_19230 | Pam16 | 35 | 1 | Y | N | O | 13% | M | 0 | # | 0 |
| GL50803_9478 | GPP, processing peptidase | 30 | 1 | Y | N | O | 4% | M | 0 | # | 0 |
Mascot score, Mascot total ion score for the identified protein. Coverage, number of unique peptides per identified protein. MiD, mitosomal distribution. Proteins are marked “Y” if their distributions in fractions #3 and #4 of the Optiprep gradient (measured by the iTRAQ ratio) were within the range between Cpn10 and IscU and the window that extended in both directions by half of the distance between these markers. Proteins with ratios outside of this range are indicated with “N”. TargetP and PsortII were used to predict the subcellular location of Giardia proteins. S, secretory; N, non-secretory; M, mitochondrial; O, other. Exp. ver., experimental verification of protein localization using the pONDRA expression vector. The recombinant tagged proteins were localized by fluorescence microscopy. M, mitosome; ER, endoplasmic reticulum; O, other; ? inconclusive. MEMSAT3 and TMHMM were used to predict transmembrane domains. SGP, predicted soluble proteins are marked with number sign (#). Asterisk (*) is used where no data were available. (**) transformed Giardia did not express the recombinant tagged protein.
Table 2. Putative mitosomal proteins classified by predicted function: transporters and proteins known to operate in endoplasmic reticulum and tramsport vesicles.
| Accession number | Annotation | Identification | Localization | Structure | |||||||
| MASCOT | Coverage | MiD | SignalP | Target P | Psort II | Exp Ver. | MEMSAT3 | SGP | TMHMM | ||
| score | peptides | % mito | TM No. | TM No. | |||||||
| Transporters | |||||||||||
| GL50803_114777 | major facilitator superfamily mfs_1 | 658 | 8 | N | N | M | 4% | ER | 10 | 12 | |
| GL50803_17296 | major facilitator superfamily mfs_1 | 32 | 1 | Y | N | M | ** | 7 | 10 | ||
| GL50803_17342 | major facilitator superfamily mfs_1 | 151 | 3 | Y | N | M | ** | 10 | 12 | ||
| GL50803_87446 | ABC transporter, A family, putative | 554 | 7 | Y | N | O | ** | 4 | 7 | ||
| GL50803_3470 | ABC transporter, A family, putative | 95 | 2 | Y | N | M | ** | 6 | 7 | ||
| GL50803_17165 | ABC transporter, A family, putative | 113 | 2 | Y | N | O | 4% | 8 | 7 | ||
| GL50803_21411 | ABC transporter, A family, putative | 429 | 10 | Y | N | S | 0 | 14 | |||
| ER, vesicle transport | |||||||||||
| GL50803_5744 | Sec61-alpha | 175 | 3 | Y | N | M | 22% | ER | 10 | 9 | |
| GL50803_16906 | Phosphatidate cytidylyltransferase | 48 | 2 | Y | N | M | 9% | ER | 7 | 8 | |
| GL50803_14200 | Molybdenum cofactor sulfurase | 56 | 1 | Y | N | O | 22% | ER | 2 | 1 | |
| GL50803_14670 | Protein disulfide isomerase PDI3 | 69 | 1 | Y | Y | S | 22% | 1 | 0 | ||
| GL50803_8064 | Protein disulfide isomerase PDI5 | 58 | 1 | Y | Y | S | 13% | ER | 1 | 1 | |
| GL50803_17121 | ER Hsp70 (Bip) | 1626 | 24 | Y | Y | S | 11% | ER | 0 | # | 1 |
| GL50803_15204 | Endosomal cargo receptor 3 | 95 | 2 | Y | Y | S | 1 | 1 | |||
| GL50803_14469 | R-SNARE 3 | 45 | 1 | Y | N | O | 1 | 2 | |||
| GL50803_8559 | Vacuolar ATP synthase 16 kDa proteolipid subunit | 90 | 1 | Y | N | O | 11% | 4 | 4 | ||
| GL50803_7532 | Vacuolar ATP synthase catalytic subunit A | 146 | 2 | Y | N | O | 17% | 1 | 0 | ||
| GL50803_13000 | Vacuolar ATP synthase subunit d | 342 | 5 | Y | N | O | 13% | 1 | 0 | ||
| GL50803_23833 | Vacuolar protein sorting 35 | 26 | 1 | Y | N | O | 11% | 1 | 0 | ||
| GL50803_18470 | Vacuolar proton-ATPase subunit, putative | 608 | 8 | Y | N | O | 4% | 6 | 6 | ||
| GL50803_96670 | Potassium-transporting ATPase alpha chain 1 | 473 | 9 | Y | N | O | 4% | 10 | 8 | ||
Mascot score, Mascot total ion score for the identified protein. Coverage, number of unique peptides per identified protein. MiD, mitosomal distribution. Proteins are marked “Y” if their distributions in fractions #3 and #4 of the Optiprep gradient (measured by the iTRAQ ratio) were within the range between Cpn10 and IscU and the window that extended in both directions by half of the distance between these markers. Proteins with ratios outside of this range are indicated with “N”. TargetP and PsortII were used to predict the subcellular location of Giardia proteins. S, secretory; N, non-secretory; M, mitochondrial; O, other. Exp. ver., experimental verification of protein localization using the pONDRA expression vector. The recombinant tagged proteins were localized by fluorescence microscopy. M, mitosome; ER, endoplasmic reticulum; O, other; ? inconclusive. MEMSAT3 and TMHMM were used to predict transmembrane domains. SGP, predicted soluble proteins are marked with number sign (#). Asterisk (*) is used where no data were available. (**) transformed Giardia did not express the recombinant tagged protein.
Table 3. Putative mitosomal proteins classified by predicted function: protein modification, cztosceletal and motor proteins.
| Accession number | Annotation | Identification | Localization | Structure | ||||||
| MASCOT | Coverage | MiD | SignalP | Target P | Psort II | MEMSAT3 | SGP | TMHMM | ||
| score | peptides | % mito | TM No. | TM No. | ||||||
| Protein modification | ||||||||||
| GL50803_8587 | Kinase, AGC NDR | 22 | 1 | Y | N | O | 4% | 0 | # | 0 |
| GL50803_14223 | Kinase, NEK | 124 | 2 | Y | N | O | 13% | 0 | # | 0 |
| GL50803_16824 | Kinase, NEK | 87 | 2 | Y | N | O | 0 | # | 0 | |
| GL50803_17510 | Kinase, NEK | 25 | 1 | Y | N | O | 17% | 0 | # | 0 |
| GL50803_5375 | Kinase, NEK | 46 | 1 | Y | N | O | 17% | 0 | # | 0 |
| GL50803_11775 | Kinase, NEK-frag | 50 | 2 | Y | N | O | 17% | 0 | # | 0 |
| GL50803_7183 | Kinase, NEK-frag | 22 | 1 | Y | N | O | 13% | 0 | # | 0 |
| GL50803_8805 | Kinase, SCY1 | 159 | 2 | Y | N | O | 11% | 1 | 0 | |
| GL50803_7110 | Ubiquitin | 360 | 5 | Y | N | O | 17% | 0 | # | 0 |
| Cytoskeletal and motor proteins | ||||||||||
| GL50803_11654 | Alpha-1 giardin | 934 | 17 | Y | N | O | 13% | 1 | 0 | |
| GL50803_7796 | Alpha-2 giardin | 478 | 8 | Y | N | O | 17% | 1 | 0 | |
| GL50803_5649 | Alpha-10 giardin | 294 | 5 | Y | N | O | 9% | 1 | 0 | |
| GL50803_15097 | Alpha-14 giardin | 643 | 9 | Y | N | O | 4% | 0 | # | 0 |
| GL50803_112079 | Alpha-tubulin | 394 | 7 | Y | N | O | 0 | # | 0 | |
| GL50803_136020 | Beta tubulin | 841 | 13 | Y | N | O | 0 | # | 0 | |
| GL50803_42285 | Ciliary dynein heavy chain 11 | 23 | 1 | Y | N | 1 | 0 | |||
| GL50803_93736 | Dynein heavy chain | 29 | 1 | Y | N | 13% | 0 | 0 | ||
| GL50803_16993 | FtsJ cell division protein, putative | 24 | 1 | Y | N | O | 17% | 0 | # | 0 |
| GL50803_102101 | Kinesin-3 | 85 | 1 | Y | N | O | 26% | 0 | # | 0 |
| GL50803_21444 | Spindle pole protein, putative | 63 | 2 | Y | N | O | 22% | 0 | # | 0 |
| GL50803_8589 | Suppressor of actin 1 | 81 | 2 | Y | N | O | 11% | 3 | 2 | |
Mascot score, Mascot total ion score for the identified protein. Coverage, number of unique peptides per identified protein. MiD, mitosomal distribution. Proteins are marked “Y” if their distributions in fractions #3 and #4 of the Optiprep gradient (measured by the iTRAQ ratio) were within the range between Cpn10 and IscU and the window that extended in both directions by half of the distance between these markers. Proteins with ratios outside of this range are indicated with “N”. TargetP and PsortII were used to predict the subcellular location of Giardia proteins. S, secretory; N, non-secretory; M, mitochondrial; O, other. Exp. ver., experimental verification of protein localization using the pONDRA expression vector. The recombinant tagged proteins were localized by fluorescence microscopy. M, mitosome; ER, endoplasmic reticulum; O, other; ? inconclusive. MEMSAT3 and TMHMM were used to predict transmembrane domains. SGP, predicted soluble proteins are marked with number sign (#). Asterisk (*) is used where no data were available. (**) transformed Giardia did not express the recombinant tagged protein.
Table 4. Putative mitosomal proteins classified by predicted function: various metabolic processes, lipid metabolism.
| Accession number | Annotation | Identification | Localization | Structure | |||||||
| MASCOT | Coverage | MiD | SignalP | Target P | Psort II | Exp Ver. | MEMSAT3 | SGP | TMHMM | ||
| score | peptides | % mito | TM No. | TM No. | |||||||
| Various metabolic processes | |||||||||||
| GL50803_7203 | Guanylate kinase | * | * | * | N | M | 65% | O | 0 | # | 0 |
| GL50803_3287 | Acetyl-CoA acetyltransferase | * | * | * | N | M | 22% | O | 0 | # | 0 |
| GL50803_8163 | Manganese-dependent inorganic pyrophosphatase, putative | 25 | 1 | Y | N | O | 22% | 0 | # | 0 | |
| GL50803_6497 | Metal-dependent hydrolase | 30 | 1 | Y | N | O | 13% | 1 | 0 | ||
| GL50803_10311 | Ornithine carbamoyltransferase | 665 | 8 | Y | N | O | 9% | 1 | 0 | ||
| GL50803_14993 | Pyrophosphate-fructose 6-phosphate 1-phosphotransferase alpha subunit | 56 | 1 | Y | N | M | 35% | 1 | 0 | ||
| GL50803_15380 | CDC8 Thymidylate kinase | * | * | * | N | O | 35% | O | 0 | # | 0 |
| Lipid metabolism | |||||||||||
| GL50803_9062 | Long chain fatty acid CoA ligase 5 | 279 | 3 | Y | N | O | 22% | ** | 0 | # | 0 |
| GL50803_21118 | Long chain fatty acid CoA ligase 5 | 25 | 1 | Y | N | O | 26% | 0 | # | 0 | |
| GL50803_113892 | Long chain fatty acid CoA ligase, putative | 224 | 4 | Y | N | O | 26% | 0 | # | 0 | |
| GL50803_7259 | CDP-diacylglycerol-glycerol-3-phosphate 3-phosphatidyltransferase | 43 | 1 | N | N | M | 22% | 6 | 2 | ||
Mascot score, Mascot total ion score for the identified protein. Coverage, number of unique peptides per identified protein. MiD, mitosomal distribution. Proteins are marked “Y” if their distributions in fractions #3 and #4 of the Optiprep gradient (measured by the iTRAQ ratio) were within the range between Cpn10 and IscU and the window that extended in both directions by half of the distance between these markers. Proteins with ratios outside of this range are indicated with “N”. TargetP and PsortII were used to predict the subcellular location of Giardia proteins. S, secretory; N, non-secretory; M, mitochondrial; O, other. Exp. ver., experimental verification of protein localization using the pONDRA expression vector. The recombinant tagged proteins were localized by fluorescence microscopy. M, mitosome; ER, endoplasmic reticulum; O, other; ? inconclusive. MEMSAT3 and TMHMM were used to predict transmembrane domains. SGP, predicted soluble proteins are marked with number sign (#). Asterisk (*) is used where no data were available. (**) transformed Giardia did not express the recombinant tagged protein.
Table 5. Putative mitosomal proteins classified by predicted function: miscellaneous.
| Accession number | Annotation | Identification | Localization | Structure | |||||||
| MASCOT | Coverage | MiD | SignalP | Target P | Psort II | Exp Ver. | MEMSAT3 | SGP | TMHMM | ||
| score | peptides | % mito | TM No. | TM No. | |||||||
| Miscellaneous | |||||||||||
| GL50803_11953 | Coatomer alpha subunit (WD40) | 31 | 1 | Y | N | O | 0 | # | 0 | ||
| GL50803_88765 | Cytosolic HSP70 | 22 | 1 | Y | N | O | 4% | 1 | 0 | ||
| GL50803_112312 | Elongation factor 1-alpha | 424 | 10 | Y | N | O | 4% | 1 | 0 | ||
| GL50803_12102 | Elongation factor 1-gamma | 158 | 3 | Y | N | M | 13% | 1 | 0 | ||
| GL50803_28379 | Multidrug resistance-associated protein 1 | 210 | 4 | Y | N | O | 0 | 10 | |||
| GL50803_16313 | Pescadillo (ribosome biogenesis) | 52 | 1 | Y | N | M | 17% | ** | 0 | # | 0 |
| GL50803_15380 | CDC8 Thymidylate kinase | * | * | * | N | O | 35% | O | 0 | # | 0 |
| GL50803_16354 | Protein 21.1 | 25 | 1 | Y | N | O | 4% | 0 | # | 0 | |
| GL50803_17288 | Protein 21.1 | 54 | 2 | Y | N | O | 4% | 0 | 0 | ||
| GL50803_23492 | Protein 21.1 | 130 | 1 | Y | N | O | 30% | 1 | 0 | ||
| GL50803_86855 | Protein 21.1 | 22 | 1 | Y | N | O | 9% | 0 | # | 0 | |
| GL50803_88245 | Protein 21.1 | 23 | 1 | Y | N | O | 17% | 0 | # | 0 | |
| GL50803_21662 | Coiled-coil protein | 31 | 1 | N | N | M | ** | 0 | # | 0 | |
| GL50803_16152 | Coiled-coil protein | 57 | 2 | Y | N | O | 0 | # | 0 | ||
| GL50803_8564 | Coiled-coil protein | 74 | 3 | Y | N | O | 0 | 0 | |||
| GL50803_9515 | Coiled-coil protein | 61 | 2 | Y | N | O | 0 | # | 0 | ||
| GL50803_40244 | P24, putative | 53 | 1 | Y | N | O | 13% | 1 | 1 | ||
| GL50803_6430 | 14-3-3 protein | 78 | 2 | Y | N | O | 13% | 1 | 0 | ||
| GL50803_8903 | Copine I | 190 | 4 | Y | N | O | 44% | O | 0 | # | 0 |
| GL50803_14225 | CXC-rich protein | 494 | 8 | Y | Y | S | 0 | 1 | |||
| GL50803_17476 | CXC-rich protein | 255 | 7 | Y | Y | S | 4% | 0 | 1 | ||
| GL50803_113656 | Cysteine protease | 73 | 2 | Y | Y | S | 1 | 1 | |||
| GL50803_103454 | High cysteine membrane protein Group 1 | 1038 | 14 | Y | Y | S | 1 | 1 | |||
| GL50803_17328 | High cysteine membrane protein Group 2 | 113 | 3 | Y | Y | S | 0 | 1 | |||
| GL50803_91099 | High cysteine membrane protein Group 2 | 65 | 1 | Y | Y | S | 13% | 0 | # | 1 | |
| GL50803_114042 | High cysteine membrane protein Group 4 | 330 | 5 | Y | Y | S | 1 | 1 | |||
| GL50803_11359 | Ribosomal protein S4 | 31 | 1 | Y | N | O | 17% | 1 | 0 | ||
| GL50803_17411 | TCP-1 chaperonin subunit gamma | 24 | 1 | Y | N | O | 1 | 0 | |||
Mascot score, Mascot total ion score for the identified protein. Coverage, number of unique peptides per identified protein. MiD, mitosomal distribution. Proteins are marked “Y” if their distributions in fractions #3 and #4 of the Optiprep gradient (measured by the iTRAQ ratio) were within the range between Cpn10 and IscU and the window that extended in both directions by half of the distance between these markers. Proteins with ratios outside of this range are indicated with “N”. TargetP and PsortII were used to predict the subcellular location of Giardia proteins. S, secretory; N, non-secretory; M, mitochondrial; O, other. Exp. ver., experimental verification of protein localization using the pONDRA expression vector. The recombinant tagged proteins were localized by fluorescence microscopy. M, mitosome; ER, endoplasmic reticulum; O, other; ? inconclusive. MEMSAT3 and TMHMM were used to predict transmembrane domains. SGP, predicted soluble proteins are marked with number sign (#). Asterisk (*) is used where no data were available. (**) transformed Giardia did not express the recombinant tagged protein.
Table 6. Putative mitosomal proteins classified by predicted function: miscellaneous - continued; hypothetical proteins.
| Accession number | Annotation | Identification | Localization | Structure | |||||||
| MASCOT | Coverage | MiD | SignalP | Target P | Psort II | Exp Ver. | MEMSAT3 | SGP | TMHMM | ||
| score | peptides | % mito | TM No. | TM No. | |||||||
| GL50803_10330 | Tenascin precursor | 330 | 4 | Y | Y | S | 11% | 0 | # | 0 | |
| GL50803_16477 | Tenascin-37 | 178 | 4 | Y | Y | S | 17% | 1 | 0 | ||
| GL50803_16833 | Tenascin-like | 96 | 2 | Y | Y | S | 0 | # | 0 | ||
| GL50803_13561 | Translation elongation factor | 36 | 1 | Y | N | O | 13% | 1 | 0 | ||
| GL50803_15889 | UDP-N-acetylglucosamine-dolichyl-phosphateN-acetylglucosamine- phosphotransferase | 36 | 1 | Y | Y | S | 4% | 10 | 7 | ||
| GL50803_11521 | VSP | 198 | 3 | Y | Y | S | 1 | 1 | |||
| GL50803_137618 | VSP | 530 | 9 | Y | N | O | 4% | 2 | 1 | ||
| GL50803_11470 | VSP with INR | 220 | 3 | Y | N | O | 2 | 1 | |||
| GL50803_6733 | Zinc finger domain | 55 | 1 | Y | N | S | 22% | 4 | 4 | ||
| Hypothetical proteins | |||||||||||
| GL50803_12999 | Hypothetical protein | 414 | 5 | Y | Y | M | ? | 2 | 2 | ||
| GL50803_14939 | Hypothetical protein | 133 | 2 | Y | Y | M | 30% | M | 1 | 2 | |
| GL50803_15985 | Hypothetical protein (VAP, VAMP associated protein) | 35 | 1 | Y | N | M | 13% | M | 1 | 1 | |
| GL50803_16596 | Hypothetical protein | 177 | 3 | N | N | M | 30% | O | 0 | # | 0 |
| GL50803_4768 | Hypothetical protein | 21 | 1 | Y | N | M | 57% | O | 0 | # | 0 |
| GL50803_9296 | Hypothetical protein | 178 | 4 | Y | Y | M | 57% | M | 0 | # | 0 |
| GL50803_11237 | Hypothetical protein | 24 | 1 | Y | N | O | 9% | 1 | 0 | ||
| GL50803_11557 | Hypothetical protein | 41 | 1 | Y | N | O | 17% | 1 | 0 | ||
| GL50803_11866 | Hypothetical protein | 25 | 1 | Y | N | O | 22% | 0 | # | 0 | |
| GL50803_13288 | Hypothetical protein | 35 | 1 | Y | N | O | 9% | 1 | 0 | ||
| GL50803_13413 | Hypothetical protein | 95 | 2 | Y | N | O | 11% | 2 | 2 | ||
| GL50803_137685 | Hypothetical protein | 200 | 4 | Y | N | S | 13 | 9 | |||
| GL50803_137746 | Hypothetical protein | 25 | 1 | Y | N | O | 0 | # | 0 | ||
| GL50803_13922 | Hypothetical protein | 1121 | 14 | Y | Y | S | 1 | 1 | |||
| GL50803_14164 | Hypothetical protein | 23 | 1 | Y | N | O | 13% | 0 | # | 0 | |
| GL50803_14278 | Hypothetical protein | 31 | 1 | Y | N | O | 13% | 0 | # | 0 | |
| GL50803_14660 | Hypothetical protein | 105 | 2 | Y | N | O | 35% | 1 | 0 | ||
Mascot score, Mascot total ion score for the identified protein. Coverage, number of unique peptides per identified protein. MiD, mitosomal distribution. Proteins are marked “Y” if their distributions in fractions #3 and #4 of the Optiprep gradient (measured by the iTRAQ ratio) were within the range between Cpn10 and IscU and the window that extended in both directions by half of the distance between these markers. Proteins with ratios outside of this range are indicated with “N”. TargetP and PsortII were used to predict the subcellular location of Giardia proteins. S, secretory; N, non-secretory; M, mitochondrial; O, other. Exp. ver., experimental verification of protein localization using the pONDRA expression vector. The recombinant tagged proteins were localized by fluorescence microscopy. M, mitosome; ER, endoplasmic reticulum; O, other; ? inconclusive. MEMSAT3 and TMHMM were used to predict transmembrane domains. SGP, predicted soluble proteins are marked with number sign (#). Asterisk (*) is used where no data were available. (**) transformed Giardia did not express the recombinant tagged protein.
Table 7. Putative mitosomal proteins classified by predicted function: hypothetical proteins – continued.
| Accession number | Annotation | Identification | Localization | Structure | ||||||
| MASCOT | Coverage | MiD | SignalP | Target P | Psort II | MEMSAT3 | SGP | TMHMM | ||
| score | peptides | % mito | TM No. | TM No. | ||||||
| GL50803_14845 | Hypothetical protein | 69 | 2 | Y | N | O | 4% | 0 | # | 0 |
| GL50803_15084 | Hypothetical protein | 22 | 1 | Y | N | O | 0 | # | 0 | |
| GL50803_16424 | Hypothetical protein | 117 | 3 | Y | N | O | 26.1% | 0 | # | 0 |
| GL50803_16430 | Hypothetical protein | 32 | 1 | Y | N | O | 9% | 1 | 0 | |
| GL50803_16998 | Hypothetical protein | 24 | 1 | Y | N | O | 17% | 0 | # | 0 |
| GL50803_17236 | Hypothetical protein | 69 | 1 | Y | N | M | 10 | 10 | ||
| GL50803_1937 | Hypothetical protein | 75 | 2 | Y | N | S | 2 | 2 | ||
| GL50803_23389 | Hypothetical protein | 33 | 1 | Y | N | O | 4 | 6 | ||
| GL50803_28962 | Hypothetical protein | 39 | 1 | Y | Y | S | 4% | 1 | 1 | |
| GL50803_29327 | Hypothetical protein | 111 | 2 | Y | N | O | 17% | 1 | 0 | |
| GL50803_3021 | Hypothetical protein | 21 | 1 | Y | N | O | 13% | 0 | # | 0 |
| GL50803_32999 | Hypothetical protein | 98 | 3 | Y | N | O | 13% | 0 | # | 0 |
| GL50803_3491 | Hypothetical protein | 25 | 1 | Y | N | O | 30% | 1 | 0 | |
| GL50803_6617 | Hypothetical protein | 350 | 5 | Y | Y | S | 1 | 1 | ||
| GL50803_7188 | Hypothetical protein | 926 | 11 | Y | Y | S | 13% | 3 | 1 | |
| GL50803_7242 | Hypothetical protein | 69 | 1 | Y | N | O | 22% | 3 | 3 | |
| GL50803_7244 | Hypothetical protein | 144 | 3 | Y | N | O | 11% | 4 | 3 | |
| GL50803_94658 | Hypothetical protein | 27 | 1 | Y | N | O | 13% | 0 | # | 0 |
| GL50803_9503 | Hypothetical protein | 206 | 3 | Y | N | O | 9% | 0 | # | 0 |
| GL50803_9780 | Hypothetical protein | 333 | 5 | Y | Y | S | 11% | 0 | # | 0 |
| GL50803_9861 | Hypothetical protein | 137 | 2 | Y | N | O | 4% | 0 | # | 0 |
| GL50803_10016 | Hypothetical protein | 265 | 5 | Y | Y | S | 22% | 1 | 0 | |
| GL50803_111809 | Hypothetical protein | 34 | 1 | Y | N | O | 0 | # | 0 | |
Mascot score, Mascot total ion score for the identified protein. Coverage, number of unique peptides per identified protein. MiD, mitosomal distribution. Proteins are marked “Y” if their distributions in fractions #3 and #4 of the Optiprep gradient (measured by the iTRAQ ratio) were within the range between Cpn10 and IscU and the window that extended in both directions by half of the distance between these markers. Proteins with ratios outside of this range are indicated with “N”. TargetP and PsortII were used to predict the subcellular location of Giardia proteins. S, secretory; N, non-secretory; M, mitochondrial; O, other. Exp. ver., experimental verification of protein localization using the pONDRA expression vector. The recombinant tagged proteins were localized by fluorescence microscopy. M, mitosome; ER, endoplasmic reticulum; O, other; ? inconclusive. MEMSAT3 and TMHMM were used to predict transmembrane domains. SGP, predicted soluble proteins are marked with number sign (#). Asterisk (*) is used where no data were available. (**) transformed Giardia did not express the recombinant tagged protein.
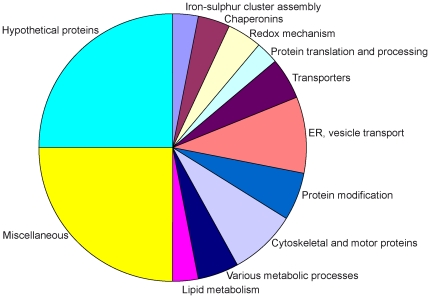
Figure 3. Classification of the identified proteins according to function.
Functions were assigned based upon GiardiaDB annotations, PSI-BLAST analysis and searches of the Pfam database (Tables 1, 2, 3, 4, 5, 6 and 7, Tables S2–S3).
Evolution-inspired orthology phylogenetic profiling
Previous phylogenetic analyses of known mitosomal proteins have generally confirmed their alpha-proteobacterial origin [28]–[30]. On this premise, we compared the genomes of G. intestinalis and Rickettsia typhi using the orthology phylogenetic profile tool at GiardiaDB (http://www.orthomcl.org/cgi-bin/OrthoMclWeb.cgi) to identify proteins of alpha-proteobacterial ancestry in the G. intestinalis genome. The phylogenetic profiling yielded 106 candidate genes that were analyzed with the topology prediction algorithms described above (Table S5). Based on these analyses, six additional proteins: acetyl CoA acetyl transferase, CDP-diacylglycerol-glycerol-3-phosphate 3-phosphatidyltransferase, guanylate kinase, J-protein HesB, thioredoxin reductase, and thymidylate kinase were added to the set of candidate mitosomal proteins identified by our proteomics approach (Tables 1– 7).
Experimental validation of protein subcellular localization
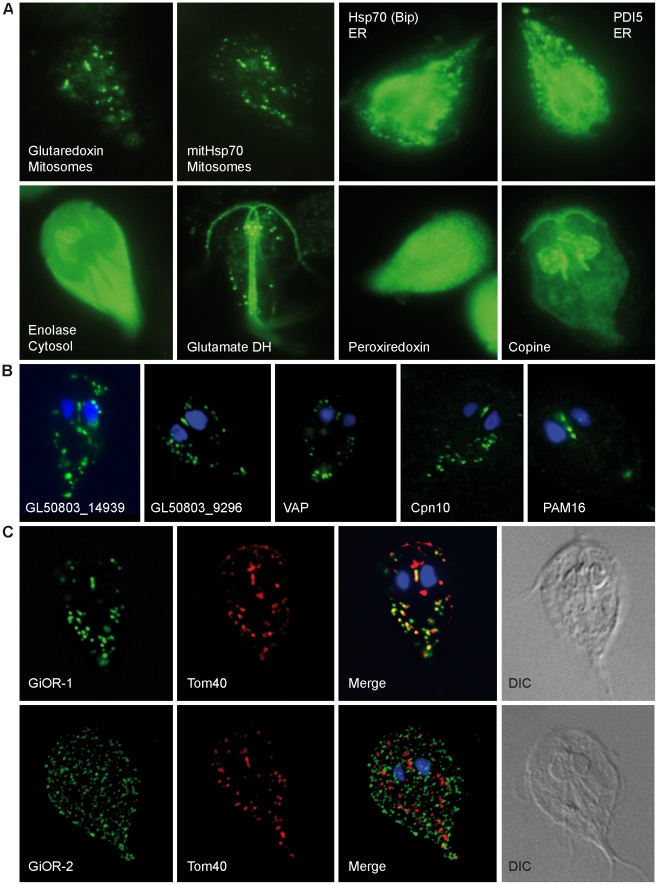
The cellular localization of the selected candidate proteins was observed by stable episomal expression in Giardia. To establish the morphology of subcellular localizations by this approach, we first observed the localization of five marker proteins: cytosolic enolase, two proteins from the endoplasmic reticulum (Hsp70 and protein disulfide isomerase 5), mitosomal Hsp70 and glutaredoxin (Fig. 4A). We added to these markers of known location, three proteins of untested location with iTRAQ ratios outside that of the mitosomal range: glutamate dehydrogenase, copine and peroxiredoxin. Glutamate dehydrogenase and copine were associated with cytoskeletal structures, while peroxiredoxin localizes to the endoplasmic reticulum network. This strategy was used to test the sub-cellular localization of 44 selected proteins. Of these 20 expressed fluorescent fusions that were found in the mitosomes (Tables 1– 7). By way of example, four of these: VAP, Pam16, Cpn10 and unknown proteins GL50803_9296 and GL50803_ 14939 are shown in Fig. 4B.
Figure 4. Sub-cellular localization of selected proteins in Giardia.
Transformed G. intestinalis cells with episomally-expressed HA-tagged proteins. (A) Marker proteins were stained using a mouse anti-HA antibody (green). Grx5, glutaredoxin 5; ER, endoplasmic reticulum; glutamate DH, glutamate dehydrogenase. (B) Predicted mitosomal proteins (GL50803_14939, GL50803_9296, VAP, Cpn10, Pam16) were stained using a mouse anti-HA antibody (green). (C) Cellular localization of tagged diflavin proteins GiOR-1 and GiOR-2 stained with mouse anti HA antibody (green). Tom40 was detected by polyclonal rabbit anti-Tom40 antibody (red).
Iron-sulfur cluster assembly
Proteins involved in FeS cluster assembly formed the most prominent functional group within the predicted mitosomal proteins. These included components required for the formation of transient FeS clusters on the molecular scaffold (IscS, IscU, Nfu) (Fig. S1) and components that have been proposed to transfer the transient FeS clusters to target apoproteins, including IscA (Fig. S2), the monothiol glutaredoxin 5, chaperone Hsp70 and its co-chaperones the J-protein HscB (Fig. S3) and nucleotide exchange factor GrpE (Fig. S4). The identification of the FeS cluster assembly machinery in the mitosomal proteome is consistent with the ability of the mitosome-enriched fraction to catalyze the formation of FeS clusters on a ferredoxin apoprotein [9]. However, when we compared the FeS cluster machinery of Giardia mitosomes to that of S. cerevisiae and Trypanosoma brucei mitochondria, we found that several mitochondrial components were absent from the mitosomes (Table 8).
Table 8. Comparison of iron-sulfur cluster assembly machineries in organisms with mitosomes (Giardia intestinalis, Cryptosporidium parvum, and Encephalitozoon cuniculi), hydrogenosomes (Trichomonas vaginalis), and mitochondria (Trypanosoma brucei, Saccharomyces cerevisiae).
| Name | G. intestinalis | C. parvum | E. cuniculi | T. vaginalis | T. brucei | S. cerevisiae |
| IscS (Nfs) | • | • | • | •• | • | • |
| Isd11 | ○ | ○ | • | •• | ••• | • |
| Nfu | • | ○ | ○ | ••• | ••• | • |
| IscU (Isu) | • | • | • | • | • | •• |
| IscA1(Isa1) | ○ | ○ | ○ | ○ | • | • |
| IscA2 (Isa2) | • | ○ | ○ | •••• | • | • |
| Iba57 | ○ | ○ | ○ | ○ | • | • |
| Ind | ○ | ○ | ○ | ••• | • | • |
| Grx5 | • | ○ | • | ○ | • | • |
| Ferredoxin (Yah1) | • | • | • | ••••••• | •• | • |
| FOR (Arh1) | ○ | • | • | ○ | • | • |
| Frataxin (Yfh1) | ○ | • | • | •• | • | • |
| HSP70 | • | • | • | ••• | •• | •♦♦ |
| Dna-J (Jac1) | • | • | • | •• | • | • |
| GrpE | • | • | • | •• | • | • |
| Atm1 | ○ | • | • | ○ | • | • |
| Erv1 | ○ | ○ | • | ○ | • | • |
Filled circles indicate the presence of protein exhibiting homology to the known component of mitochondrial iron-sulfur cluster assembly machinery identified by BLAST searches. Empty circles indicates absence of homologous protein. Mitochondria of S. cerevisiae possess three distinct Hsp70 of which Ssq1 is devoted for FeS cluster assembly (filled circle), while Ssc1, and Ecm10 have other fuctions (diamonds). Other eukaryotes possess multifunctional Hsp70. IscS, cysteine desulfurase; Isd11, IscS binding protein; Nfu, IscU, IscA, a scafold proteins; Iba57, IscA binding protein required for [4Fe4S] cluster assembly; Ind, P-loop NTPaseb required for assembly of respiratory complex I; Grx5, glutaredoxin 5; ferredoxin, [2Fe2S] ferredoxin that transport electrons; FOR, ferredoxin oxidoreductase; frataxin, iron binding protein; Hsp70, chaperone; DnaJ, GrpE, co-chaperones; Atm1, ABC half trasnporter; Erv1, sulfhydryl oxidase. Names of proteins used for S. cerevisiae orthologs are in brackets.
A striking deviation from other eukaryotes is the absence of frataxin in Giardia mitosomes. Frataxin is invariably present in eukaryotes that contain the ISC-type FeS cluster assembly machinery. The presence of frataxin in mitosomes was found in E. cuniculi [17], and genes encoding frataxin are present in the genomes of C. parvum and the diplomonad Spironucleus vortens, a close relative of Giardia. We failed to identify frataxin in the genomes of three G. intestinalis strains in the GiardiaDB, using either BLAST searches or the motif search tool.
Two IscA-like proteins, IscA1 (Isa1) and IscA2 (Isa2) are present in virtually all mitochondria [31] and are thought to act as scaffold proteins for transient FeS clusters [32]–[34] and/or serve as iron donors [35]. Interestingly, the Giardia mitosome contains only a single IscA-2 type protein (Fig. S2), while IscA-1 is absent. The same situation was found in hydrogenosomes of Trichomonas vaginalis (Table 8). No genes encoding IscA were found in the genomes of other organisms with mitosomes. The observed distributions of IscA therefore suggest that IscA-1 was lost in mitosomes and hydrogenosomes together with a specific set of mitochondrial FeS proteins, while IscA-2 was retained in Giardia mitosomes to function either in the maturation of specific FeS protein(s) or as an iron transporter [35].
The mitosomes did not contain Ind1 or Iba57. In mitochondria, these proteins are required for the formation of FeS clusters on specific substrates. Ind1 is a P-loop NTPase that is required for the maturation of FeS proteins of the multi-subunit respiratory complex I [36], [37]. Homologues of Ind1 are also present in the hydrogenosomes of T. vaginalis (Table 8), which contain a highly reduced form of complex I with only two FeS catalytic subunits [38]. The selective absence of Ind1 in the mitosomes of Giardia (Table 8) is thus consistent with the absence of complex I and highlights the specific role of Ind1 in the biogenesis of this respiratory complex. Iba57 forms a complex with the scaffold protein IscA (Isa1p and Isa2p in yeast), which plays a specific role in [4Fe4S] cluster assembly of aconitase-type proteins and the functional activation of mitochondrial radical-SAM FeS proteins [39]. As in the case of Ind1, the absence of Iba57 likely reflects the absence of the respective substrate proteins in mitosomes.
Pyridine nucleotide-driven electron transport in mitosomes
The formation of FeS clusters requires reducing equivalents, which are provided by a short electron chain consisting of the [2Fe2S] ferredoxin and ferredoxin:NADP+ reductase (FNR) [40]. The presence of this chain has been predicted in the mitosomes of C. parvum and E. cuniculi; however, [2Fe2S] ferredoxin, but not FNR, was found in Giardia mitosomes (Table 1). We identified a distinct protein with a possible redox activity named GiOR-1 (GL50803_91252), which is currently annotated in the GiardiaDB as an inducible nitric oxide synthase. This protein consists of a flavodoxin-like FMN-binding domain that is connected to a cytochrome p450 reductase-like domain, including a FAD binding pocket and an NADP(H) binding site (Fig. S5). These two domains are present in the C-termini of various oxidoreductases, such as cytochrome p450 reductase and nitric oxide synthase, and serve as electron donors (Fig. S5). GiOR-1 does not contain an N-terminal domain that determines the specific functions of known oxidoreductases.
The architecture of GiOR-1 resembles that of the recently identified protein Tah18 in Saccharomyces cerevisiae [41], [42]. Tah18 was shown to form a complex with Dre2 in the cytosol, where it participates in cytosolic FeS cluster assembly [43]. Under oxidative stress, the Dre2-Tag18 complex was destabilized, and Tah18 relocalized from the cytosol to the mitochondria. This behavior has been shown to be associated with apoptotic events. Searches for a Dre2 homologue in Giardia were unsuccessful. However, we identified a second paralogue of Tah18 named GiOR-2 (GL50803_15897, Fig. S5). The expression of tagged GiOR-1 and GiOR-2 in G. intestinalis confirmed that the GiOR-1 is localized to the mitosome, but GiOR-2 was found in numerous vesicles that did not correspond to mitosomes (Fig. 4C). To assess the oxidoreductase activity of GiOR-1, recombinant GiOR-1 was produced in Escherichia coli and isolated as a yellow protein, which is expected for diflavin oxidoreductases. GiOR-1 efficiently transferred electrons from NADPH to dichlorophenolindolphenol, whereas an about 30 fold lower activity was measured using NADH as the electron donor (Table 9). Low specific activities were observed also with methyl viologen and oxygen as electron acceptors (Table 9). No activity was observed when GiOR-1 was assayed with G. intestinalis mitosomal ferredoxin as a possible native electron acceptor. These results suggest that GiOR-1 does not act directly as a ferredoxin reductase in mitosomes, however, its ability to utilize NADPH as an electron donor indicates that pyridine nucleotides are involved in mitosomal electron transport.
Table 9. Activity of mitosomal diflavin oxidoreductase GiOR-1.
| Substrate | Specific activity [µg.min−1.mg−1] | Standard deviation |
| NADPH | 0 | |
| NADPH+DCIP | 9,053 | 0,111 |
| NADH + DCIP | 0,269 | 0,034 |
| NADPH + MV | 0,450 | 0,205 |
| NADPH + O2 | 0,144 | 0,042 |
| NADPH+ferredoxin | 0 |
Electron donors: NADPH, NADH.
Electron acceptors: DCIP, dichlorophenol-indolephenol; MV, methyl viologen; O2, aerobic conditions; ferredoxin, recombinant G. intestinalis [2Fe2S]ferredoxin.
Molecular chaperones in the mitosomal matrix: protein folding and assembly
A single mitosomal Hsp70, three J-protein co-chaperones and the nucleotide exchange factor GrpE were identified in the mitosomes. The J-proteins included HscB, an orthologue of yeast Jac1 (Fig. S3)that has a predicted role in FeS cluster biogenesis [44], and Pam18/Tim14, which is required for translocation of proteins across the mitochondrial inner membrane [45]. The third J-protein also contains an N-terminal DnaJ domain (type III family); however, its function cannot be inferred from domain structure or phylogenetic profiling. We also identified the chaperonins Cpn60 and Cpn10 (Fig. S6), that function in folding and assembly of newly-imported proteins [46], [47] (Table 1).
Protein import
We identified four components that are potentially involved in transporting proteins across the mitosomal membranes: a homologue of a mitochondrial Tom40, which would form a general import pore in the outer mitosomal membrane, and the three essential components of the PAM (presequence translocase-associated motor) complex: Pam18, Pam16 (Fig. S7) and mHsp70. Pam18 and Pam16 form an intimate complex that anchors a population of the matrix Hsp70 to the inner membrane and regulates its activity to drive protein translocation across the inner membrane [48]. Typically, it functions together with a TIM complex that forms the translocation pore for protein passage across the membrane. In representative organisms from all lineages of eukaryotes, the TIM complex is built from one or two proteins of the Tim17/22/23 family [49]. Surprisingly, we find no evidence for a member of this protein in our proteomics data, and sensitive hidden Markov model searches detected no related sequences in the Giardia genome (unpublished, see Methods). In eukaryotes, the Sec61 channel catalyzes protein transport across the endoplasmic reticulum [2], while a highly-related protein called SecY is the translocation channel in the inner membrane of bacteria, including the alpha-proteobacteria from which mitochondria are derived. Interestingly, Reclinomonas americana encodes a bacterial-type SecY protein translocation channel in its mitochondrial genome [50], and our proteomics analysis detected what appeared to be contamination of the mitosomal membranes with GiSecY/Sec61. We expressed a tagged version of this protein in Giardia but it localized to the endoplasmic reticulum, as expected for a cognate Sec61, rather than to the mitosomes. The nature of the mitosomal inner membrane protein translocation channel remains unknown, and yet must exist given that at least 17 of the proteins detected in the mitosomal proteome are likely to reside in the matrix.
We suggest that Tim23/17/22 protein(s) have been secondarily lost from Giardia, given that the these proteins appear to be derived from components of the ancestral endosymbiont [48] and are present in all other groups of eukaryotes including other members of the Excavata [51], particularly T. vaginalis (TrichDB, http://trichdb.org/trichdb; our unpublished data). Because there is evidence to suggest that T. vaginalis and G. intestinalis share a common ancestor [44], [52], the absence of a Tim23 homologue in Giardia likely reflects the overall simplification of the organelle than a primitive trait. Why has the TIM complex been replaced? In addition to a reliance on ATP hydrolysis mediated by the PAM motor, the TIM complex is powered by the membrane potential through its physical association with the respiratory complexes III and IV [53], [54]. Giardia mitosomes do not generate a large membrane potential, as shown by their inability to accumulate the routinely used mitochondrial probes that are sensitive to the membrane potential (e.g., mitotrackers, JC-1, our observations). Perhaps any membrane potential that is present, is insufficient to support the function of a TIM23 translocase.
Interaction of mitosomes with other cellular compartments
In the Giardia mitosomes, we identified a VAMP (vesicle-associated membrane protein)-associated protein, VAP (Table 6). VAPs are involved mainly in membrane trafficking and lipid metabolism. They provide membrane anchors for various lipid binding proteins on the surfaces of the endoplasmic reticulum and Golgi complex [53] and physically interact with SNARE proteins, with FFAT-motif containing lipid transport proteins and microtubules. Like other VAPs, the Giardia VAP protein contains an N-terminal domain that includes the VAP consensus sequence [55], a central coiled-coil domain and a C-terminal transmembrane domain with the putative dimerization motif GxxxG (Fig. S8). The presence of a VAP protein has not been reported in mitochondria or other mitosomes so far. In Giardia, GiVAP was found within the set of hypothetical proteins with distribution value corresponding to mitosomal proteins (Table 6) and its mitosomal localization was experimentally confirmed (Fig. 4B).
Hypothetical proteins
The set of mitosomal candidates contains 40 proteins annotated as hypothetical proteins. We selected six proteins with high mitochondrial score (Tables 6–7) for the verification of their sub-cellular localization. Three proteins were confirmed to reside in mitosomes (Table 6, Fig 4): (i) putative VAP (GL50803_15985) that is discussed above, (ii) hypothetical protein GL50803_14939 that contains two predicted transmembrane domains (residues 13–35 and 102–124), and (iii) a putative soluble globular protein GL50803_9296. The latter two proteins seem to be unique for giardia as no orthologues were identified in available databases. Two other hypothetical proteins (GL50803_16596 and GL50803_4768) were observed in the cytosol and in association with kinetosomes, respectively (Table 6, data not shown). The cellular localization of hypothetical protein GL50803_12999 remains inconclusive. Although the protein co-localized with IscU in some vesicles, it was not observed in typical rod-like structure between nuclei (data not shown).
Origin of mitosomes and perspectives
Mitosomes are thought to have evolved several times in different eukaryotic lineages through the reduction of ancestral mitochondria. For example, microsporidians are intracellular parasites allied with Fungi; whereas Fungi typically possess fully equipped mitochondria with large proteomes (>850 proteins) [11], [56], only twenty to thirty proteins have been identified from genome analysis of E. cuniculi as having similarity to bona fide mitochondrial proteins of Saccharomyces cerevisiae [15], [18], [21]. Apicomplexan parasites related to Plasmodium also include organisms with mitosomes, such as Cryptosporidium parvum and Cryptosporidium hominis. Based on genomic analyses, 37–54 proteins have been predicted to reside in these mitosomes [19], of which 18 were detected by mass spectrometry in whole C. parvum sporozoites [25].
An intriguing question concerns the nature of the mitochondrial progenitor from which mitosomes of G. intestinalis have evolved. Giardia is a member of the Excavate group, which has recently been re-considered to belong to the basal groups of eukaryotes based on its mechanism of cytochrome c and c1 biogenesis [25], [57]. These and other data have placed the root of eukaryotes between Excavata and Euglenozoa, a group of protists that includes trypanosomatids [58]. In this respect, there is an apparent simplicity in the protein import machinery of the Giardia mitosomes that deserves attention (Fig. 5). The proteomics analysis detected in mitosomes a protein recently shown to be Tom40, the protein translocation channel across the outer membrane [25], [59]. The current model for the evolution of the TOM complex posits that Tom40 was derived from a beta-barrel protein in the endosymbiont's outer membrane, perhaps of an usher or autotransporter type protein translocase [60]. Because two other proteins: Tom7 and Tom22, have been found in representative species of all other eukaryotic groups [58], the model further suggests that the first TOM complex was composed of Tom40, Tom22 and Tom7. Our proteomics finds no evidence of Tom7 or Tom22 in mitosomes, and sensitive hidden Markov model searches likewise fail to find any proteins encoded in the Giardia genome with similarity to Tom7 or Tom22 [25], [57]. Whether reflecting a secondary gene loss or the ancestral condition, GiTom40 would appear to be a selectively simple protein translocase. In addition to Tom40, mitochondria contain one other member of the mitochondrial porin family, the voltage-dependent anion channels (VDAC), which serve to exchange metabolites [61]. The absence of VDAC in Giardia mitosomes might reflect the disappearance of many of the metabolic pathways, and the concomitant decrease in metabolite flux across the outer membrane. It is likely that the Giardia Tom40, in addition to importing proteins, exchanges ions and small metabolites across the outer mitosomal membrane as has been demonstrated for the yeast Tom40 in mutants lacking VDAC [44], [62]–[64].
Figure 5. Schematic representation of protein import pathway in the mitosome of G. intestinalis.
Components identified in mitosome are highlighted by color. Components that are known to participate in the protein import into mitochondria of animals and fungi are shown in grey colour. OM, outer membrane; IMS, intermembrane space; IM, inner membrane; TOM, translocase of outer membrane; SAM, sorting and assembly machinery; TIM, translocase of inner membrane; PAM, presequence translocase-associated motor; VAP, VAMP (vesicle-associated membrane protein)-associated protein; VDAC, voltage-dependent anion channel; MPP, mitochondrial processing peptidase
Another surprising result, one that can only be explained by a secondary gene loss, is the absence of the outer membrane protein Sam50 in Giardia. Sam50 is a component of the SAM (sorting and assembly machinery) complex, which is required for the assembly of both Tom40 and VDAC [48], [65]. The apparent absence of Sam50 from the Giardia genome and from our proteomics data is unique among eukaryotes. A putative Sam50 homologue has been predicted in the genomes of all eukaryotes, including trypanosomatids [58], [65] and mitosome- and hydrogenosome-containing protists (C. parvum, E. cuniculi, E. histolytica and T. vaginalis) [66]. Numerous phylogenetic and functional analyses indicate that Sam50 was derived from the Omp85/BamA protein present in the outer membrane of the ancestral, alpha-proteobacterial endosymbiont and it must, therefore, have been present in the earliest mitochondria [44]. It is not clear how Giardia Tom40 is assembled within the outer membrane without the assistance of the SAM complex. It is known that even in yeast Tom40 mediates the import of new molecules of Tom40 into mitochondria [67] and it is tempting to speculate that the Giardia Tom40 is capable of mediating its own import and membrane insertion, given the highly simplified nature of the TOM complex in mitosomes.
Our proteomics data support the hypothesis that ISC assembly is an important and possibly the only biosynthetic function of Giardia mitosomes. Previous phylogenic analyses have indicated that the ISC assembly machinery was obtained from the alpha-proteobacterial endosymbiont; nearly complete ISC assembly machinery is present from trypanosomatids to higher eukaryotes. Therefore, the absence of certain components, such as IscA-1, Iba57, and Ind, in the mitosomal machines (Table 8) is apparently due to a secondary loss of specific target proteins. Noteworthy, we did not identify any proteins that would carry FeS clusters in Giardia mitosomes, except for components of the FeS cluster assembly machinery itself. It seems likely then that the main role of mitosomes could be to export preassembled FeS clusters, or other compounds that are essential for the biogenesis of FeS proteins, to other cellular compartments. In mitochondria, the export of these enigmatic compounds is dependent on the membrane ABC “half-transporter” Atm1 [68] and sulfhydryl oxidase Erv1 [69]. In the mitosome-enriched fraction, we identified four ABC half-transporters by mass spectrometry, and another candidate was predicted based on phyletic profiling of the G. intestinalis genome (Table 2). However, compared to other Atm1 homologues, these candidates lack the x-loop with the conserved arginine, which is essential for known Atm1 transporters (Fig. S9). No protein with homology to Erv1 was found by proteomics or by analysis of the Giardia genome.
Another remaining question pertains to the source of ATP that is required for the multiple processes identified in mitosomes including FeS cluster assembly and export, organelle division, protein import and protein folding. In E. histolytica, it has been shown that a mitochondrial carrier family (MCF) protein localizes to mitosomes and exchanges ATP and ADP across the inner membrane, effecting the import ATP into mitosomes [20]. E. cuniculi mitosomes contain a distinct bacterial nucleotide transporter that may fulfill the same function [23], [24]. However, our proteomic analysis did not revealed a candidate nucleotide transporter in the mitosomes of Giardia leaving open the question of ATP acquisition.
In conclusion, using iTRAQ-based mass spectrometry and bioinformatics we identified 139 candidate mitosomal proteins. Mitosomal localization was confirmed experimentally for 20 of 44 proteins tested, suggesting the complete mitosomal proteome of Giardia to be of the order of 50-100 proteins. Previous genome analyses failed to predict any of the novel mitosomal proteins identified here [70]; only by combining quantitative mass spectrometry and bioinformatics were these novel proteins identified. The small proteome of the G. intestinalis mitosome indicates a marked reduction in mitochondrial metabolic activity and reduced requirements for organelle biogenesis. These do not mirror the reductions seen in the mitosomal proteome of Cryptosporidium, supporting the view that lineage-specific reductions produce organelles with distinct metabolic pathways and specific “short-cut” pathways for biogenesis. Our findings provide new insight into aspects of mitochondrial evolution and the basis from which to begin reconstructing the details of precisely how these organelles are built and replicated to support Giardia growth and division.
Methods
Cell culture and fractionation
G. intestinalis strain WB (American Type Culture Collection) was grown in TYI-S-33 medium supplemented with 10% heat-inactivated bovine serum and 0.1% bovine bile [9]. Trophozoites were freeze-detached, washed in PBS and collected by centrifugation. Cells were then resuspended in hypotonic buffer (12 mM MOPS, pH 7.4) and incubated for 15 minutes. The cells were then pelleted at 680× g for 15 minutes, resuspended in the same buffer with DNase I (40 µg/mL) and homogenized by 10 passages through a 25G needle. After homogenization, the isotonicity was immediately restored with the addition of an equal volume of 500 mM sucrose in MOPS buffer. The homogenate was then treated with trypsin (200 µg/mL) for 10 minutes at 37°C to release the organelles from the cytoskeleton. Proteases inhibitors were then added (5 mg/mL of soybean trypsin inhibitor, leupeptin and TLCK), and the homogenate was diluted and centrifuged for 20 minutes at 2760× g to remove cellular debris. The collected supernatant was centrifuged using a Beckman rotor Ti 50 at 20,000 rpm for 30 minutes. After centrifugation, the pellet was collected and washed in SM buffer (250 mM sucrose and 12 mM MOPS, pH 7.4). Next, the pellet was resuspended in 0.5 mL of SM buffer and layered onto a discontinuous density OptiPrep (Axis-Shield PoC AS, Oslo, Norway) gradient, which consisted of 1 ml each of 15%, 20%, 25%, 30% and 50% OptiPrep diluted in 12 mM MOPS buffer. The gradient was centrifuged for 24 h in a Beckman SW 40 rotor at 120,000× g at 4°C. Fractions (1 mL each) were collected, washed and analyzed by immunoblot using a polyclonal rabbit anti-IscU antibody [71], [72].
Mass spectrometry analysis
Samples of two selected fractions (100 µg of total protein each) were precipitated with acetone at −20°C for 2 hours and then pelleted at 13,000× g for 15 min. The proteins were trypsin digested and labeled with sample-specific iTRAQ reagents according to the manufacturer's protocol (Applied Biosystems). Labeled samples were mixed and precipitated with acetone. The pellet was dissolved in 2 M urea in HPLC grade water, and the solution was subjected to IEF using 7 cm immobilized pH 3–10 gradient strips (Bio-Rad) for 20,000 VHrs. The strips were cut into 2-mm wide slices, and peptides were extracted using 50% ACN with 1% TFA. Extracted peptides were then separated using an Ultimate 3000 HPLC system (Dionex) coupled to a Probot micro-fraction collector (Dionex). The samples were loaded onto a PepMap 100 C18 RP column (3 µm particle size, 15 cm long, 75 µm internal diameter; Dionex) and separated with a gradient of 5% (v/v) ACN and 0.1% (v/v) TFA to 80% (v/v) ACN and 0.1% (v/v) TFA over 60 min at a flow rate of 300 nl/min. The eluate was mixed 1∶3 with matrix solution (20 mg/mL α-cyano-4-hydroxycinnamic acid in 80% ACN) prior to spotting onto a MALDI target. Spectra were acquired using a 4800 Plus MALDI TOF/TOF analyzer (Applied Biosystems/MDS Sciex) equipped with a Nd:YAG laser (355 nm, 200 Hz firing rate). All spots were measured in MS mode; up to 10 of the strongest precursors were selected for MS/MS analysis, which was performed using collision energy of 1 kV and operating pressure of the collision cell of 10−6 Torr. Peak lists from the MS/MS spectra were generated using GPS Explorer v. 3.6 (Applied Biosystems/MDS Sciex) subtraction of baseline enabled with peak width 50, smoothing with Savitsky-Golay algorithm of polynomial order of four and three points across peak, minimum signal to noise (S/N) 3, local noise window 250 m/z, cluster area S/N optimization enabled with S/N threshold 5. Spectra were searched with locally installed Mascot v. 2.1 (Matrix Science) against the GiardiaDB release 1.3 annotated protein database (4892 sequences, 2663813 residues) and GiardiaDB release 1.2 Open Reading Frame translations greater than 50 amino acids (85612 sequences, 9633221 residues). The database search criteria were as follows: trypsin; one missed cleavage site allowed; fixed modifications iTRAQ 4-plex on N-terminal- and lysine ε-amino group, methylthiolation of cysteine; variable modification methionine oxidation; peptide mass tolerance of 100 ppm; MS/MS tolerance of 0.2 Da; maximum peptide rank of 1, minimum ion score C.I. (peptide) of 95%.
Bioinformatics
Bioinformatics searches based on simple pair-wise alignment Psi-BLAST and hidden Markov models (HMMs) were applied to verify the automatic protein annotations and estimate their functions. Protein sequences (<1000 residues) were submitted (i) against a 90% redundancy reduced NCBI nr database for 8 iterations at an e-value cutoff of 10−3 and (ii) against Pfam 23.0 A+B database of families represented by multiple sequence alignments and hidden Markov models at an e-value of 0.044 (http://pfam.sanger.ac.uk/search). Where noted in the text, tailored HMM libraries were used to search for components of the protein import machinery [16].
Programs based on a combination of artificial neural networks (TargetP) and hidden Markov models (SignalP, both http://www.cbs.dtu.dk/services/) together with PsortII (http://psort.ims.u-tokyo.ac.jp/) were used to predict the subcellular localizations of the proteins. The secondary structures and topologies of alpha-helical integral membrane proteins were predicted using two bioinformatics tools: TMHMM, a program based on hidden Markov models (http://www.cbs.dtu.dk/services/), and Memsat3 (http://bioinf.cs.ucl.ac.uk/memsat/).
Transformation of G. intestinalis and immunofluorescence
Selected genes were amplified by PCR from genomic Giardia DNA and inserted into the pONDRA plasmid [73]. Table S6 contains a list of primers that were used for subcloning of genes into expression vector. Cells were transformed and selected as described previously [16]. G. intestinalis cells expressing the recombinant proteins fused to a hemagglutinin tag (HA) at the C-terminus were fixed and stained for immunofluorescence microscopy with a mouse monoclonal anti-HA antibody. A secondary AlexaFluor-488 (green) donkey anti-mouse antibody was used.
Preparation of recombinant proteins and enzyme assay
The coding region of GiOR-1 and [2Fe2S]ferredoxin was subcloned into pET42b and pET3a (Invitrogen), respectively and expressed in Escherichia coli BL21. The bacteria were induced with 0,5 mM IPTG (isopropyl-β-D-thiogalactopyranoside) and grown at 37°C in LB medium. For expression of GiOR-1, the LB medium was supplemented with 250 µM flavin adenine dinucleotide (FAD) and 250 µM flavin mononucleotide (FMN), whereas the LB medium supplemented with 400 µM ferric ammonium citrate was used for expression of ferredoxin. After induction, the cells were incubated overnight at 4°C. The harvested cells were homogenized, and soluble fraction was obtained by centrifugation at 250,000× g, 1 h, 4°C. The his-tagged GiOR-1was affinity purified under native conditions using a Ni-NTA column (Qiagen) according to manufacture's protocol. Ferredoxin was isolated by gel filtration chromatography using a BioLogic HR system (BioRad).
Enzyme activity of GiOR-1 was assayed spectrophotometrically at 25°C in anaerobic cuvettes under nitrogen atmosphere. The activity was monitored as a rate of NADPH or NADH (0,25 mM) oxidation in the presence of dichlorophenol-indolephenol (0,1 mM) or ferredoxin at 340 nm, or as a rate of methyl viologen (2 mM) reduction at 600 nm. NADPH oxidase activity was measured under aerobic conditions at 340 nm. The enzymatic activity was determined in phosphate buffer (100 mM KH2PO4/KOH, 150 mM NaCl, pH 7,4). Protein concentration was determined according to Lowry method.
Supporting Information
Sequence alignment of Giardia Nfu against ekaryotic and prokaryotic orthologues. Conserved thioredoxin-like CXXC motif is shown in green. Giardia, Giardia intestinalis EAA38809; Trichomonas, Trichomonas vaginalis, TVAG_146780; Trypanosoma, Trypanosoma brucei, XP_845796; Leishmania, Leishmania infantum, XP_001470367; Toxoplasma, Toxoplasma gondii, XP_002371042; Plasmodium, Plasmodium falciparum, CAX64255; Saccharomyces, Saccharomyces cerevisiae, NP_012884; Homo, Homo sapiens, AAI13695; Rickettsia, Rickettsia prowazekii, NP_221029; Stigmatella, Stigmatella aurantiaca, ZP_01463912.
(PDF)
Sequence alignment of Giardia IscA against eukaryotic and bacterial orthologs. The conserved cysteine residues are highlited in yellow. Organism names and accession numbers: Giardia, Giardia intestinalis GL50803_14821; Trichomonas, Trichomonas vaginalis TVAG_055320; Trypanosoma, Trypanosoma cruzi XP_806610; Saccharomyces, Saccharomyces cerevisiae Q12425; Homo, Homo sapiens NP_919255; Arabidopsis, Arabidopsis thaliana NP_179262; Chlamydomonas, Chlamydomonas reinhardtii XP_001697636; Rickettsia, Rickettsia conorii NP_360365; Escherichia, Escherichia coli CAQ32901; Mycobacterium, Mycobacterium leprae NP_301657.
(PDF)
Sequence alignment of Giardia Jac1 against eukaryotic and bacterial orthologs. The conserved HSP70 interactin site is highlited in green. Organism names and accession numbers: Giardia, Giardia intestinalis, GL50803_17030; Trichomonas, Trichomonas vaginalis, TVAG_422630; Trypanosoma, Trypanosoma brucei, XP_843770; Leishmania, Leishmania infantum, XP_001466207; Plasmodium, Plasmodium falciparum, CAX64223; Toxoplasma, Toxoplasma gondii, XP_002368309; Naegleria, Naegleria gruberi, EFC47366; Saccharomyces, Saccharomyces cerevisiae, NP_011497; Homo, Homo sapiens, AAN85282; Escherichia, Escherichia coli, YP_002408666.
(PDF)
Sequence alignment of Giardia Mge1 against eukaryotic (Mge1) and bacterial (GrpE) orthologs. The residues in yellow indicate a GrpE dimer interface. HSP70 binding sites are shown in green (Harrison CJ, Hayer-Hartl M, Di Liberto M, Hartl F, Kuriyan J, Crystal structure of the nucleotide exchange factor GrpE bound to the ATPase domain of the molecular chaperone DnaK, Science 1999, 276:431–435. Giardia intestinalis, GL50803_1376; Homo sapiens, NP_079472; Saccharomyces cerevisae, NP_014875; Escherichia coli, NP_417104; Arabidopsis thaliana, NP_567757; Trichomonas vaginalis, XP_001329309; Trypanosoma brucei, XP_845338; Dictyostelium discoideum, XP_638912; Bacillus subtilis, NP_390426; Halobacterium sp., NP_279548.
(PDF)
Sequence alignment of G. intestinalis mitosomal oxidoreductase OR-1 (GL50803_91252), against G. intestinalis non-mitosomal paralogue OR-2 (GL50803_15897) and structurally related proteins containing flavodoxin-like FMN-binding domain (conserved residua in blue), FAD binding pocket (residua involved in FAD binding in green) and NADP(H) binding site (residua involved in NADP(H) in red). Saccharomyces cerevisiae Tah18, DAA11472; Homo sapiens NDOR, NADPH dependent diflavin oxidoreductase, AAH15735; Rattus norvegicus NOS, nitric oxide synthase, AAC13747; Rattus norvegicus CPR, cytochrome P450 reductase, NP_113764; Escherichia coli SiR, sulfite reductase, YP_002330508; Homo sapiens MSR, methionine synthase reductase, NP_076915; Trichomonas vaginalis Hyd, hydrogenase, TVAG_136330; Leptospira interrogans FNR, ferredoxin reductase, YP_003372.
(PDF)
Conserved glycine which is present in all GroES and Cpn10 homologues is shown in green. Hsp60 binding site is shown in yellow (van der Giezen M, León-Avila G, Tovar J. (2005) Characterization of chaperonin 10 (Cpn10) from the intestinal human pathogen Entamoeba histolytica. Microbiology 151:3107-15). Giardia intestinalis GL50803_29500; Trichomonas vaginalis TVAG_191660; Saccharomyces cerevisiae NP_014663.1; Homo sapiens XP_001118014.1; Leishmania infantum XP_001470405.1; Plasmodium falciparum PFL0740c; Arabidopsis thaliana NP_563961.1; Dictyostelium discoideum XP_636819.1; Mycobacterium tuberculosis NP_217935.1; Escherichia coli NP_290775.1.
(PDF)
Sequence alignment of Giardia Pam16 against eukaryotic Pam 16 orthologues and giardial Pam 18 paralogue. Conserved leucin in an interacting hydrofobic pocket is shown in green (D'Silva PR, Schilke B, Hayashi M, Craig EA (2008) Interaction of the J-protein heterodimer Pam18/Pam16 of the mitochondrial import motor with the translocon of the inner membrane. Mol Biol Cell 19:424-32). The typical HPD motif (in blue) present in Pam18 is degenerated in Pam16, in yellow (Mokranjac D, Bourenkov G, Hell K, Neupert W, Groll M (2006) Structure and function of Tim14 and Tim16, the J and J-like components of the mitochondrial protein import motor. EMBO J 25:4675-85). Giardia intestinalis Pam 16 GL50803_19230; Trichomonas vaginalis TVAG_470110; Toxoplasma gondii XP_002367323.1; Saccharomyces cerevisiae NP_012431.1; Neurospora crassa XP_960477.1; Pediculus humanus XP_002428010.1; Schistosoma japonicum CAX74438.1; Homo sapiens NP_057153.8; Mus musculus NP_079847.1; Xenopus laevis NP_001084733.1; Giardia intestinalis Pam 18 XP_002364144.
(PDF)
Protein sequence alignment of VAP (VAMP-associated protein) homologues. Domain structure is depicted for each represented sequence according to HHPRED (http://toolkit.tuebingen.mpg.de/). Major sperm protein domain, yellow. Coiled-coil domain in green and dimerization motif GXXXG in red. The G. intestinalis VAP contains all protein characteristics as described for human homologue.
(PDF)
Sequence alignment of Giardia AbcB transporter against mitochondrial and bacterial orthologs. Giardia intestinalis AbcB, GL50803_17315; Saccharomyces cerevisiae Atm1, NP_014030; Saccharomyces cerevisae Mdl1, NP_013289; Homo sapiens AbcB7, NP_004290; Homo sapiens AbcB10, NP_036221; Arabidopsis thaliana Atm3, NP_200635; Naegleria gruberi Atm1,XP_002683195; Rhodobacter sphaeroides AbcB, YP_001168064; Halobacterium sp. AbcB, NP_279266. Walker A part of a conserved ATP-binding motif in yellow; Q-loop part of a conserved ATP-binding motif in green; ABC signature, a conserved sequence specific for ABC proteins in pink; Walker B part of a conserved ATP-binding motif in blue; D-loop part of a conserved ATP-binding motif in red; H-loop part of a conserved ATP-binding motif in purple; X-loop contains a conserved arginine in AbcB transporters (•), which is not present in Giardia sequence, in cyan (Dawson RJ, Locher KP (2006) Structure of a bacterial multidrug ABC transporter. Nature 443:180-185; Bernard DG, Cheng Y, Zhao Y, Balk J (2009) An allelic mutant series of ATM3 reveals its key role in the biogenesis of cytosolic iron-sulfur proteins in Arabidopsis. Plant Physiol 151: 590-602).
(PDF)
Complete list of proteins identified by LC MS/MS in mitosomal fractions labelled by iTRAQ reagents.
(PDF)
List of Giardia proteins within the mitosomal distribution range (MiD) identified by LC MS/MS.
(PDF)
Identification of protein families using PfamA+B databases.
(PDF)
Predictions of cellular localization.
(PDF)
Orthology phylogenetic profililng. Genomes of G. intestinalis and Rickettsia typhi were compared using orthology phylogenetic profile tool at GiardiaDB.
(PDF)
List of primers that were used for subcloning of genes into expression vector pONDRA to investigate subcellular localization of corresponding gene products in G. intestinalis.
(PDF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Czech Ministry of Education (MSM0021620858, LC07032) and the Czech Science Foundation grants 204/05/H023 (P.J.) and 204/06/0947 (J.T.) and the Australian Research Council (T.L.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Andersson SG, Zomorodipour A, Andersson JO, Sicheritz-Ponten T, Alsmark UC, et al. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 2.Lang BF, Burger G, O'Kelly CJ, Cedergren R, Golding GB, et al. An ancestral mitochondrial DNA resembling a eubacterial genome in miniature. Nature. 1997;387:493–497. doi: 10.1038/387493a0. [DOI] [PubMed] [Google Scholar]
- 3.Vaidya AB, Mather MW. Mitochondrial evolution and functions in malaria parasites. Annu Rev Microbiol. 2009;63:249–267. doi: 10.1146/annurev.micro.091208.073424. 10.1146/annurev.micro.091208.073424 [doi] [DOI] [PubMed] [Google Scholar]
- 4.Gabaldon T, Huynen MA. Shaping the mitochondrial proteome. Biochim Biophys Acta. 2004;1659:212–220. doi: 10.1016/j.bbabio.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Gabaldon T, Huynen MA. From endosymbiont to host-controlled organelle: the hijacking of mitochondrial protein synthesis and metabolism. PLoS Comput Biol. 2007;3:e219. doi: 10.1371/journal.pcbi.0030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tachezy J, Smid O. Mitosomes in parasitic protists. In: Tachezy J, editor. Hydrogenosomes and Mitosomes: Mitochondria of Anaerobic Eukaryotes. Berlin, Heidelberg: Springer-Verlag; 2008. pp. 201–230. [Google Scholar]
- 8.Cavalier-Smith T. The origin of eukaryotic and archaebacterial cells. Ann N Y Acad Sci. 1987;503:17–54:17–54. doi: 10.1111/j.1749-6632.1987.tb40596.x. [DOI] [PubMed] [Google Scholar]
- 9.Tovar J, Leon-Avila G, Sanchez LB, Sutak R, Tachezy J, et al. Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature. 2003;426:172–176. doi: 10.1038/nature01945. [DOI] [PubMed] [Google Scholar]
- 10.Tovar J, Fischer A, Clark CG. The mitosome, a novel organelle related to mitochondria in the amitochondrial parasite Entamoeba histolytica. Mol Microbiol. 1999;32:1013–1021. doi: 10.1046/j.1365-2958.1999.01414.x. [DOI] [PubMed] [Google Scholar]
- 11.Katinka MD, Duprat S, Cornillot E, Metenier G, Thomarat F, et al. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature. 2001;414:450–453. doi: 10.1038/35106579. [DOI] [PubMed] [Google Scholar]
- 12.Williams BAP, Hirt RP, Lucocq JM, Embley TM. A mitochondrial remnant in the microsporidian Trachipleistophora hominis. Nature. 2002;418:865–869. doi: 10.1038/nature00949. [DOI] [PubMed] [Google Scholar]
- 13.Riordan CE, Langreth SG, Sanchez LB, Kayser O, Keithly JS. Preliminary evidence for a mitochondrion in Cryptosporidium parvum: Phylogenetic and therapeutic implications. Journal of Eukaryotic Microbiology. 1999;46:52S–55S. [PubMed] [Google Scholar]
- 14.Hrdy I, Tachezy J, Müller M. Metabolism of trichomonad hydrogenosomes. In: Tachezy J, editor. Hydrogenosomes and Mitosomes:Mitochondria of Anaerobic Euakryotes. Berlin, Heidelberg: Springer-Verlag; 2008. pp. 114–145. [Google Scholar]
- 15.Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, et al. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304:441–445. doi: 10.1126/science.1094786. 10.1126/science.1094786 [doi];1094786 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Dolezal P, Smid O, Rada P, Zubacova Z, Bursac D, et al. Giardia mitosomes and trichomonad hydrogenosomes share a common mode of protein targeting. Proc Natl Acad Sci U S A. 2005;102:10924–10929. doi: 10.1073/pnas.0500349102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg AV, Molik S, Tsaousis AD, Neumann K, Kuhnke G, et al. Localization and functionality of microsporidian iron-sulphur cluster assembly proteins. Nature. 2008;452:624–628. doi: 10.1038/nature06606. [DOI] [PubMed] [Google Scholar]
- 18.Putignani L, Tait A, Smith HV, Horner D, Tovar J, et al. Characterization of a mitochondrion-like organelle in Cryptosporidium parvum. Parasitology. 2004;129:1–18. doi: 10.1017/s003118200400527x. [DOI] [PubMed] [Google Scholar]
- 19.Sanderson SJ, Xia D, Prieto H, Yates J, Heiges M, et al. Determining the protein repertoire of Cryptosporidium parvum sporozoites. Proteomics. 2008;8:1398–1414. doi: 10.1002/pmic.200700804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsaousis AD, Kunji ER, Goldberg AV, Lucocq JM, Hirt RP, et al. A novel route for ATP acquisition by the remnant mitochondria of Encephalitozoon cuniculi. Nature. 2008;453:553–556. doi: 10.1038/nature06903. nature06903 [pii];10.1038/nature06903 [doi] [DOI] [PubMed] [Google Scholar]
- 21.Xu P, Widmer G, Wang Y, Ozaki LS, Alves JM, et al. The genome of Cryptosporidium hominis. Nature. 2004;431:1107–1112. doi: 10.1038/nature02977. [DOI] [PubMed] [Google Scholar]
- 22.Mi-ichi F, Abu YM, Nakada-Tsukui K, Nozaki T. Mitosomes in Entamoeba histolytica contain a sulfate activation pathway. Proc Natl Acad Sci U S A. 2009;106:21731–21736. doi: 10.1073/pnas.0907106106. 0907106106 [pii];10.1073/pnas.0907106106 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franzen O, Jerlstrom-Hultqvist J, Castro E, Sherwood E, Ankarklev J, et al. Draft genome sequencing of Giardia intestinalis assemblage B isolate GS: is human giardiasis caused by two different species? PLoS Pathog. 2009;5:e1000560. doi: 10.1371/journal.ppat.1000560. 10.1371/journal.ppat.1000560 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison HG, McArthur AG, Gillin FD, Aley SB, Adam RD, et al. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science. 2007;317:1921–1926. doi: 10.1126/science.1143837. [DOI] [PubMed] [Google Scholar]
- 25.Cavalier-Smith T. Kingdoms Protozoa and Chromista and the eozoan root of the eukaryotic tree. Biol Lett. 2010;6:342–345. doi: 10.1098/rsbl.2009.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Billington D, Maltby PJ, Jackson AP, Graham JM. Dissection of hepatic receptor-mediated endocytic pathways using self-generated gradients of iodixanol (Optiprep). Anal Biochem. 1998;258:251–258. doi: 10.1006/abio.1998.2561. S0003-2697(98)92561-1 [pii];10.1006/abio.1998.2561 [doi] [DOI] [PubMed] [Google Scholar]
- 27.Dunkley TP, Watson R, Griffin JL, Dupree P, Lilley KS. Localization of organelle proteins by isotope tagging (LOPIT). Mol Cell Proteomics. 2004;3:1128–1134. doi: 10.1074/mcp.T400009-MCP200. 10.1074/mcp.T400009-MCP200 [doi];T400009-MCP200 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Smid O, Matuskova A, Harris SR, Kucera T, Novotny M, et al. Reductive evolution of the mitochondrial processing peptidases of the unicellular parasites Trichomonas vaginalis and Giardia intestinalis. PLoS Pathog. 2008;4:e1000243. doi: 10.1371/journal.ppat.1000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rada P, Smid O, Sutak R, Dolezal P, Pyrih J, et al. The monothiol single-domain glutaredoxin is conserved in the highly reduced mitochondria of Giardia intestinalis. Eukaryot Cell. 2009;8:1584–1591. doi: 10.1128/EC.00181-09. EC.00181-09 [pii];10.1128/EC.00181-09 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tachezy J, Sanchez LB, Müller M. Mitochondrial type iron-sulfur cluster assembly in the amitochondriate eukaryotes Trichomonas vaginalis and Giardia intestinalis, as indicated by the phylogeny of IscS. Molecular Biology and Evolution. 2001;18:1919–1928. doi: 10.1093/oxfordjournals.molbev.a003732. [DOI] [PubMed] [Google Scholar]
- 31.Vinella D, Brochier-Armanet C, Loiseau L, Talla E, Barras F. Iron-sulfur (Fe/S) protein biogenesis: phylogenomic and genetic studies of A-type carriers. PLoS Genet. 2009;5:e1000497. doi: 10.1371/journal.pgen.1000497. 10.1371/journal.pgen.1000497 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krebs C, Agar JN, Smith AD, Frazzon J, Dean DR, et al. IscA, an alternate scaffold for Fe-S cluster biosynthesis. Biochemistry. 2001;40:14069–14080. doi: 10.1021/bi015656z. bi015656z [pii] [DOI] [PubMed] [Google Scholar]
- 33.Pelzer W, Muhlenhoff U, Diekert K, Siegmund K, Kispal G, et al. Mitochondrial Isa2p plays a crucial role in the maturation of cellular iron-sulfur proteins. FEBS Lett. 2000;476:134–139. doi: 10.1016/s0014-5793(00)01711-7. S0014-5793(00)01711-7 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Song D, Tu Z, Lee FS. Human ISCA1 interacts with IOP1/NARFL and functions in both cytosolic and mitochondrial iron-sulfur protein biogenesis. J Biol Chem. 2009;284:35297–35307. doi: 10.1074/jbc.M109.040014. M109.040014 [pii];10.1074/jbc.M109.040014 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding H, Clark RJ, Ding B. IscA mediates iron delivery for assembly of iron-sulfur clusters in IscU under the limited accessible free iron conditions. J Biol Chem. 2004;279:37499–37504. doi: 10.1074/jbc.M404533200. 10.1074/jbc.M404533200 [doi];M404533200 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Bych K, Kerscher S, Netz DJ, Pierik AJ, Zwicker K, et al. The iron-sulphur protein Ind1 is required for effective complex I assembly. EMBO J. 2008;27:1736–1746. doi: 10.1038/emboj.2008.98. emboj200898 [pii];10.1038/emboj.2008.98 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheftel AD, Stehling O, Pierik AJ, Netz DJ, Kerscher S, et al. Human ind1, an iron-sulfur cluster assembly factor for respiratory complex I. Mol Cell Biol. 2009;29:6059–6073. doi: 10.1128/MCB.00817-09. MCB.00817-09 [pii];10.1128/MCB.00817-09 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hrdy I, Hirt RP, Dolezal P, Bardonova L, Foster PG, et al. Trichomonas hydrogenosomes contain the NADH dehydrogenase module of mitochondrial complex I. . Nature. 2004;432:618–622. doi: 10.1038/nature03149. [DOI] [PubMed] [Google Scholar]
- 39.Gelling C, Dawes IW, Richhardt N, Lill R, Mühlenhoff U. Mitochondrial Iba57p is required for Fe/S cluster formation on aconitase and activation of radical SAM enzymes. Mol Cell Biol. 2008;28:1851–1861. doi: 10.1128/MCB.01963-07. MCB.01963-07 [pii];10.1128/MCB.01963-07 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mühlenhoff U, Richhardt N, Gerber J, Lill R. Characterization of iron-sulfur protein assembly in isolated mitochondria. A requirement for ATP, NADH, and reduced iron. J Biol Chem. 2002;277:29810–29816. doi: 10.1074/jbc.M204675200. 10.1074/jbc.M204675200 [doi];M204675200 [pii] [DOI] [PubMed] [Google Scholar]
- 41.Vernis L, Facca C, Delagoutte E, Soler N, Chanet R, et al. A newly identified essential complex, Dre2-Tah18, controls mitochondria integrity and cell death after oxidative stress in yeast. PLoS One. 2009;4:e4376. doi: 10.1371/journal.pone.0004376. 10.1371/journal.pone.0004376 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Lyver ER, Nakamaru-Ogiso E, Yoon H, Amutha B, et al. Dre2, a conserved eukaryotic Fe/S cluster protein, functions in cytosolic Fe/S protein biogenesis. Mol Cell Biol. 2008;28:5569–5582. doi: 10.1128/MCB.00642-08. MCB.00642-08 [pii];10.1128/MCB.00642-08 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walsh P, Bursac D, Law YC, Cyr D, Lithgow T. The J-protein family: modulating protein assembly, disassembly and translocation. EMBO Rep. 2004;5:567–571. doi: 10.1038/sj.embor.7400172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N. Importing mitochondrial proteins: machineries and mechanisms. Cell. 2009;138:628–644. doi: 10.1016/j.cell.2009.08.005. S0092-8674(09)00967-2 [pii];10.1016/j.cell.2009.08.005 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horwich AL, Fenton WA, Chapman E, Farr GW. Two families of chaperonin: physiology and mechanism. Annu Rev Cell Dev Biol. 2007;23:115–145. doi: 10.1146/annurev.cellbio.23.090506.123555. 10.1146/annurev.cellbio.23.090506.123555 [doi] [DOI] [PubMed] [Google Scholar]
- 46.Elsner S, Simian D, Iosefson O, Marom M, Azem A. The Mitochondrial Protein Translocation Motor: Structural Conservation between the Human and Yeast Tim14/Pam18-Tim16/Pam16 co-Chaperones. Int J Mol Sci. 2009;10:2041–2053. doi: 10.3390/ijms10052041. 10.3390/ijms10052041 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mokranjac D, Bourenkov G, Hell K, Neupert W, Groll M. Structure and function of Tim14 and Tim16, the J and J-like components of the mitochondrial protein import motor. EMBO J. 2006;25:4675–4685. doi: 10.1038/sj.emboj.7601334. 7601334 [pii];10.1038/sj.emboj.7601334 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider A, Bursac D, Lithgow T. The direct route: a simplified pathway for protein import into the mitochondrion of trypanosomes. Trends Cell Biol. 2008;18:12–18. doi: 10.1016/j.tcb.2007.09.009. S0962-8924(07)00297-8 [pii];10.1016/j.tcb.2007.09.009 [doi] [DOI] [PubMed] [Google Scholar]
- 49.Osborne AR, Rapoport TA, van den Berg B. Protein translocation by the Sec61/SecY channel. Annu Rev Cell Dev Biol. 2005;21:529–550. doi: 10.1146/annurev.cellbio.21.012704.133214. 10.1146/annurev.cellbio.21.012704.133214 [doi] [DOI] [PubMed] [Google Scholar]
- 50.Clements A, Bursac D, Gatsos X, Perry AJ, Civciristov S, et al. The reducible complexity of a mitochondrial molecular machine. Proc Natl Acad Sci U S A. 2009;106:15791–15795. doi: 10.1073/pnas.0908264106. 0908264106 [pii];10.1073/pnas.0908264106 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simpson AG, Inagaki Y, Roger AJ. Comprehensive multigene phylogenies of excavate protists reveal the evolutionary positions of “primitive” eukaryotes. Mol Biol Evol. 2006;23:615–625. doi: 10.1093/molbev/msj068. msj068 [pii];10.1093/molbev/msj068 [doi] [DOI] [PubMed] [Google Scholar]
- 52.van der Laan M, Meinecke M, Dudek J, Hutu DP, Lind M, et al. Motor-free mitochondrial presequence translocase drives membrane integration of preproteins. Nat Cell Biol. 2007;9:1152–1159. doi: 10.1038/ncb1635. ncb1635 [pii];10.1038/ncb1635 [doi] [DOI] [PubMed] [Google Scholar]
- 53.Lev S, Ben HD, Peretti D, Dahan N. The VAP protein family: from cellular functions to motor neuron disease. Trends Cell Biol. 2008;18:282–290. doi: 10.1016/j.tcb.2008.03.006. S0962-8924(08)00120-7 [pii];10.1016/j.tcb.2008.03.006 [doi] [DOI] [PubMed] [Google Scholar]
- 54.Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. 10.1038/nature02188 [doi];nature02188 [pii] [DOI] [PubMed] [Google Scholar]
- 55.Reinders J, Zahedi RP, Pfanner N, Meisinger C, Sickmann A. The complete yeast mitochondrial proteome: Multidimensional separation techniques for mitochondrial proteomics. Journal of Proteome Research. 2006;5:1543–1554. doi: 10.1021/pr050477f. [DOI] [PubMed] [Google Scholar]
- 56.Waller RF, Jabbour C, Chan NC, Celik N, Likic VA, et al. Evidence of a reduced and modified mitochondrial protein import apparatus in microsporidian mitosomes. Eukaryot Cell. 2009;8:19–26. doi: 10.1128/EC.00313-08. EC.00313-08 [pii];10.1128/EC.00313-08 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pusnik M, Charriere F, Maser P, Waller RF, Dagley MJ, et al. The single mitochondrial porin of Trypanosoma brucei is the main metabolite transporter in the outer mitochondrial membrane. Mol Biol Evol. 2009;26:671–680. doi: 10.1093/molbev/msn288. msn288 [pii];10.1093/molbev/msn288 [doi] [DOI] [PubMed] [Google Scholar]
- 58.Dolezal P, Dagley MJ, Kono M, Wolynec P, Likic VA, et al. The essentials of protein import in the degenerate mitochondrion of Entamoeba histolytica. PLoS Pathog. 2010;6:e1000812. doi: 10.1371/journal.ppat.1000812. 10.1371/journal.ppat.1000812 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alcock F, Clements A, Webb C, Lithgow T. Evolution. Tinkering inside the organelle. Science. 2010;327:649–650. doi: 10.1126/science.1182129. 327/5966/649 [pii];10.1126/science.1182129 [doi] [DOI] [PubMed] [Google Scholar]
- 60.Macasev D, Whelan J, Newbigin E, Silva-Filho MC, Mulhern TD, et al. Tom22′, an 8-kDa trans-site receptor in plants and protozoans, is a conserved feature of the TOM complex that appeared early in the evolution of eukaryotes. Mol Biol Evol. 2004;21:1557–1564. doi: 10.1093/molbev/msh166. 10.1093/molbev/msh166 [doi];msh166 [pii] [DOI] [PubMed] [Google Scholar]
- 61.Kmita H, Budzinska M. Involvement of the TOM complex in external NADH transport into yeast mitochondria depleted of mitochondrial porin1. Biochim Biophys Acta. 2000;1509:86–94. doi: 10.1016/s0005-2736(00)00284-4. S0005273600002844 [pii] [DOI] [PubMed] [Google Scholar]
- 62.Gentle I, Gabriel K, Beech P, Waller R, Lithgow T. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J Cell Biol. 2004;164:19–24. doi: 10.1083/jcb.200310092. 10.1083/jcb.200310092 [doi];jcb.200310092 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kozjak V, Wiedemann N, Milenkovic D, Lohaus C, Meyer HE, et al. An essential role of Sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J Biol Chem. 2003;278:48520–48523. doi: 10.1074/jbc.C300442200. 10.1074/jbc.C300442200 [doi];C300442200 [pii] [DOI] [PubMed] [Google Scholar]
- 64.Paschen SA, Waizenegger T, Stan T, Preuss M, Cyrklaff M, et al. Evolutionary conservation of biogenesis of beta-barrel membrane proteins. Nature. 2003;426:862–866. doi: 10.1038/nature02208. 10.1038/nature02208 [doi];nature02208 [pii] [DOI] [PubMed] [Google Scholar]
- 65.Dolezal P, Likic V, Tachezy J, Lithgow T. Evolution of the molecular machines for protein import into mitochondria. Science. 2006;313:314–318. doi: 10.1126/science.1127895. [DOI] [PubMed] [Google Scholar]
- 66.Gatsos X, Perry AJ, Anwari K, Dolezal P, Wolynec PP, et al. Protein secretion and outer membrane assembly in Alphaproteobacteria. FEMS Microbiol Rev. 2008;32:995–1009. doi: 10.1111/j.1574-6976.2008.00130.x. FMR130 [pii];10.1111/j.1574-6976.2008.00130.x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kispal G, Csere P, Guiard B, Lill R. The ABC transporter Atm1p is required for mitochondrial iron homeostasis. Febs Letters. 1997;418:346–350. doi: 10.1016/s0014-5793(97)01414-2. [DOI] [PubMed] [Google Scholar]
- 68.Lange H, Lisowsky T, Gerber J, Mühlenhoff U, Kispal G, et al. An essential function of the mitochondrial sulfhydryl oxidase Erv1p/ALR in the maturation of cytosolic Fe/S proteins. Embo Reports. 2001;2:715–720. doi: 10.1093/embo-reports/kve161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan KW, Slotboom DJ, Cox S, Embley TM, Fabre O, et al. A novel ADP/ATP transporter in the mitosome of the microaerophilic human parasite Entamoeba histolytica. Current Biology. 2005;15:737–742. doi: 10.1016/j.cub.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 70.Keister DB. Axenic Culture of Giardia-Lamblia in Tyi-S-33 Medium Supplemented with Bile. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- 71.Dagley MJ, Dolezal P, Likic VA, Smid O, Purcell AW, et al. The protein import channel in the outer mitosomal membrane of Giardia intestinalis. Mol Biol Evol. 2009;26:1941–1947. doi: 10.1093/molbev/msp117. msp117 [pii];10.1093/molbev/msp117 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Likic VA, Dolezal P, Celik N, Dagley M, Lithgow T. Using hidden markov models to discover new protein transport machines. Methods Mol Biol. 2010;619:271–284. doi: 10.1007/978-1-60327-412-8_16. 10.1007/978-1-60327-412-8_16 [doi] [DOI] [PubMed] [Google Scholar]
- 73.Sun CH, Chou CF, Tai JH. Stable DNA transfection of the primitive protozoan pathogen Giardia lamblia. Mol Biochem Parasitol. 1998;92:123–132. doi: 10.1016/s0166-6851(97)00239-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence alignment of Giardia Nfu against ekaryotic and prokaryotic orthologues. Conserved thioredoxin-like CXXC motif is shown in green. Giardia, Giardia intestinalis EAA38809; Trichomonas, Trichomonas vaginalis, TVAG_146780; Trypanosoma, Trypanosoma brucei, XP_845796; Leishmania, Leishmania infantum, XP_001470367; Toxoplasma, Toxoplasma gondii, XP_002371042; Plasmodium, Plasmodium falciparum, CAX64255; Saccharomyces, Saccharomyces cerevisiae, NP_012884; Homo, Homo sapiens, AAI13695; Rickettsia, Rickettsia prowazekii, NP_221029; Stigmatella, Stigmatella aurantiaca, ZP_01463912.
(PDF)
Sequence alignment of Giardia IscA against eukaryotic and bacterial orthologs. The conserved cysteine residues are highlited in yellow. Organism names and accession numbers: Giardia, Giardia intestinalis GL50803_14821; Trichomonas, Trichomonas vaginalis TVAG_055320; Trypanosoma, Trypanosoma cruzi XP_806610; Saccharomyces, Saccharomyces cerevisiae Q12425; Homo, Homo sapiens NP_919255; Arabidopsis, Arabidopsis thaliana NP_179262; Chlamydomonas, Chlamydomonas reinhardtii XP_001697636; Rickettsia, Rickettsia conorii NP_360365; Escherichia, Escherichia coli CAQ32901; Mycobacterium, Mycobacterium leprae NP_301657.
(PDF)
Sequence alignment of Giardia Jac1 against eukaryotic and bacterial orthologs. The conserved HSP70 interactin site is highlited in green. Organism names and accession numbers: Giardia, Giardia intestinalis, GL50803_17030; Trichomonas, Trichomonas vaginalis, TVAG_422630; Trypanosoma, Trypanosoma brucei, XP_843770; Leishmania, Leishmania infantum, XP_001466207; Plasmodium, Plasmodium falciparum, CAX64223; Toxoplasma, Toxoplasma gondii, XP_002368309; Naegleria, Naegleria gruberi, EFC47366; Saccharomyces, Saccharomyces cerevisiae, NP_011497; Homo, Homo sapiens, AAN85282; Escherichia, Escherichia coli, YP_002408666.
(PDF)
Sequence alignment of Giardia Mge1 against eukaryotic (Mge1) and bacterial (GrpE) orthologs. The residues in yellow indicate a GrpE dimer interface. HSP70 binding sites are shown in green (Harrison CJ, Hayer-Hartl M, Di Liberto M, Hartl F, Kuriyan J, Crystal structure of the nucleotide exchange factor GrpE bound to the ATPase domain of the molecular chaperone DnaK, Science 1999, 276:431–435. Giardia intestinalis, GL50803_1376; Homo sapiens, NP_079472; Saccharomyces cerevisae, NP_014875; Escherichia coli, NP_417104; Arabidopsis thaliana, NP_567757; Trichomonas vaginalis, XP_001329309; Trypanosoma brucei, XP_845338; Dictyostelium discoideum, XP_638912; Bacillus subtilis, NP_390426; Halobacterium sp., NP_279548.
(PDF)
Sequence alignment of G. intestinalis mitosomal oxidoreductase OR-1 (GL50803_91252), against G. intestinalis non-mitosomal paralogue OR-2 (GL50803_15897) and structurally related proteins containing flavodoxin-like FMN-binding domain (conserved residua in blue), FAD binding pocket (residua involved in FAD binding in green) and NADP(H) binding site (residua involved in NADP(H) in red). Saccharomyces cerevisiae Tah18, DAA11472; Homo sapiens NDOR, NADPH dependent diflavin oxidoreductase, AAH15735; Rattus norvegicus NOS, nitric oxide synthase, AAC13747; Rattus norvegicus CPR, cytochrome P450 reductase, NP_113764; Escherichia coli SiR, sulfite reductase, YP_002330508; Homo sapiens MSR, methionine synthase reductase, NP_076915; Trichomonas vaginalis Hyd, hydrogenase, TVAG_136330; Leptospira interrogans FNR, ferredoxin reductase, YP_003372.
(PDF)
Conserved glycine which is present in all GroES and Cpn10 homologues is shown in green. Hsp60 binding site is shown in yellow (van der Giezen M, León-Avila G, Tovar J. (2005) Characterization of chaperonin 10 (Cpn10) from the intestinal human pathogen Entamoeba histolytica. Microbiology 151:3107-15). Giardia intestinalis GL50803_29500; Trichomonas vaginalis TVAG_191660; Saccharomyces cerevisiae NP_014663.1; Homo sapiens XP_001118014.1; Leishmania infantum XP_001470405.1; Plasmodium falciparum PFL0740c; Arabidopsis thaliana NP_563961.1; Dictyostelium discoideum XP_636819.1; Mycobacterium tuberculosis NP_217935.1; Escherichia coli NP_290775.1.
(PDF)
Sequence alignment of Giardia Pam16 against eukaryotic Pam 16 orthologues and giardial Pam 18 paralogue. Conserved leucin in an interacting hydrofobic pocket is shown in green (D'Silva PR, Schilke B, Hayashi M, Craig EA (2008) Interaction of the J-protein heterodimer Pam18/Pam16 of the mitochondrial import motor with the translocon of the inner membrane. Mol Biol Cell 19:424-32). The typical HPD motif (in blue) present in Pam18 is degenerated in Pam16, in yellow (Mokranjac D, Bourenkov G, Hell K, Neupert W, Groll M (2006) Structure and function of Tim14 and Tim16, the J and J-like components of the mitochondrial protein import motor. EMBO J 25:4675-85). Giardia intestinalis Pam 16 GL50803_19230; Trichomonas vaginalis TVAG_470110; Toxoplasma gondii XP_002367323.1; Saccharomyces cerevisiae NP_012431.1; Neurospora crassa XP_960477.1; Pediculus humanus XP_002428010.1; Schistosoma japonicum CAX74438.1; Homo sapiens NP_057153.8; Mus musculus NP_079847.1; Xenopus laevis NP_001084733.1; Giardia intestinalis Pam 18 XP_002364144.
(PDF)
Protein sequence alignment of VAP (VAMP-associated protein) homologues. Domain structure is depicted for each represented sequence according to HHPRED (http://toolkit.tuebingen.mpg.de/). Major sperm protein domain, yellow. Coiled-coil domain in green and dimerization motif GXXXG in red. The G. intestinalis VAP contains all protein characteristics as described for human homologue.
(PDF)
Sequence alignment of Giardia AbcB transporter against mitochondrial and bacterial orthologs. Giardia intestinalis AbcB, GL50803_17315; Saccharomyces cerevisiae Atm1, NP_014030; Saccharomyces cerevisae Mdl1, NP_013289; Homo sapiens AbcB7, NP_004290; Homo sapiens AbcB10, NP_036221; Arabidopsis thaliana Atm3, NP_200635; Naegleria gruberi Atm1,XP_002683195; Rhodobacter sphaeroides AbcB, YP_001168064; Halobacterium sp. AbcB, NP_279266. Walker A part of a conserved ATP-binding motif in yellow; Q-loop part of a conserved ATP-binding motif in green; ABC signature, a conserved sequence specific for ABC proteins in pink; Walker B part of a conserved ATP-binding motif in blue; D-loop part of a conserved ATP-binding motif in red; H-loop part of a conserved ATP-binding motif in purple; X-loop contains a conserved arginine in AbcB transporters (•), which is not present in Giardia sequence, in cyan (Dawson RJ, Locher KP (2006) Structure of a bacterial multidrug ABC transporter. Nature 443:180-185; Bernard DG, Cheng Y, Zhao Y, Balk J (2009) An allelic mutant series of ATM3 reveals its key role in the biogenesis of cytosolic iron-sulfur proteins in Arabidopsis. Plant Physiol 151: 590-602).
(PDF)
Complete list of proteins identified by LC MS/MS in mitosomal fractions labelled by iTRAQ reagents.
(PDF)
List of Giardia proteins within the mitosomal distribution range (MiD) identified by LC MS/MS.
(PDF)
Identification of protein families using PfamA+B databases.
(PDF)
Predictions of cellular localization.
(PDF)
Orthology phylogenetic profililng. Genomes of G. intestinalis and Rickettsia typhi were compared using orthology phylogenetic profile tool at GiardiaDB.
(PDF)
List of primers that were used for subcloning of genes into expression vector pONDRA to investigate subcellular localization of corresponding gene products in G. intestinalis.
(PDF)