Abstract
Functional neuroimaging methods hold promise for the identification of cognitive function and communication capacity in some severely brain-injured patients who may not retain sufficient motor function to demonstrate their abilities. We studied seven severely brain-injured patients and a control group of 14 subjects using a novel hierarchical functional magnetic resonance imaging assessment utilizing mental imagery responses. Whereas the control group showed consistent and accurate (for communication) blood-oxygen-level-dependent responses without exception, the brain-injured subjects showed a wide variation in the correlation of blood-oxygen-level-dependent responses and overt behavioural responses. Specifically, the brain-injured subjects dissociated bedside and functional magnetic resonance imaging-based command following and communication capabilities. These observations reveal significant challenges in developing validated functional magnetic resonance imaging-based methods for clinical use and raise interesting questions about underlying brain function assayed using these methods in brain-injured subjects.
Keywords: blunt-head injury, brain stem, cognitive impairment, functional magnetic resonance imaging, minimally conscious state
Introduction
Many severely brain-injured patients have no clear or reliable motor output channel, often making accurate diagnosis or assessment of a capacity to communicate very difficult or impossible (Gill-Thwaites, 2006; Voss et al., 2006; Giacino and Smart, 2007; Magee, 2007; Schnakers et al., 2008). In this context, recent functional neuroimaging studies have explored the use of mental imagery tasks as proxies for an overt visible motor response to command when motor deficits may mask residual and occasionally significant cognitive abilities. Owen et al. (2006) pioneered the use of canonical MRI (functional MRI) paradigms for assessment of command-following (Boly et al., 2007). In their paradigms, subjects imagine performing a prespecified task in response to a prompt, such as, ‘Imagine playing tennis’. In a compelling case study, Owen’s group used these methods to demonstrate evidence of command-following capability in a patient meeting the criteria for vegetative state (Royal College of Physicians, 2003) who generated brain activation patterns that fit the criteria determined by studies in a group of normal subjects (Owen et al., 2006).
Recently, the use of this paradigm has been expanded for establishing communication, using two independent mental imagery tasks to independently signal ‘yes’ and ‘no’. This approach was successfully applied in a single severely brain-injured patient (Monti et al., 2010). However, the demands of this dual-task paradigm are significant, requiring the ability to follow the instructions to generate both types of mental imagery, the structural integrity of the reporting brain substructures to demonstrate a signal, and the ability to hold two pre-potent response types in memory while evaluating the questions presented. It is likely that some severely brain-injured patients could fail to accurately complete such a complicated task and yet have a readiness to communicate by simpler means. In addition, little is known about how such paradigms correlate with the actual capabilities of brain-injured subjects who can reliably follow commands or communicate using verbal or gestural outputs, a significant portion of the patient population, as all brain-injured subjects reported to successfully perform these tasks to date have had little or no motor control (Owen et al., 2006; Monti et al., 2010). To explore these related issues we developed and tested a hierarchical, single-response-type, functional MRI paradigm in normal subjects and in a convenience sample of seven severely brain-injured patients, two of whom demonstrated reliable verbal or gestural communication at the bedside. Our approach attempts to provide assessments of the subject’s capacity to utilize brain activity to follow commands and answer questions with graded levels of difficulty. It seeks to minimize the patient’s effort and afford the greatest flexibility for response output. Using this approach we identify several dissociations of patients’ bedside behaviour and their ability to perform functional MRI-based communication tasks. These findings provide important challenges for the systematic development of functional MRI paradigms to assess brain function and cognitive capacity after severe brain injuries.
Materials and methods
Subjects
Experiments were performed on 14 healthy adult subjects and seven severely brain-injured subjects. The Institutional Review Board of Weill Cornell Medical College approved all experiments and informed consent was obtained from the healthy volunteers and the legally authorized representatives of the brain-injured subjects. The brain-injured subjects made up a convenience sample, as we did not attempt to develop a sample that would represent the patient population characteristics as a whole. One of the severely brain-injured subjects was excluded from the study due to excessive head motion during functional MRI leading to a total of six (three female) subject data sets that remained for further analysis. Excessive head motion was defined first by a ‘head motion’ alarm native to the GE scanner during scanning. Subsequently, head motion parameters were calculated for each subject to verify the alarm. For details of measured head motion parameters for all subjects, refer to Supplementary Fig. 7. Demographic data and the functional MRI responses of all normal subjects are listed in Table 1. The Coma Recovery Scale––Revised (CRS-R) (Giacino et al., 2004) was conducted on the day of each functional MRI scan to provide behavioural data for each clinical subject. Demographic data of the clinical subjects, the nature of the clinical subjects’ injuries and diagnoses, and the functional MRI responses of all clinical subjects are listed in Table 2 and in the Supplementary Materials.
Table 1.
List of demographic data and functional scans for all normal subjects
| Subject | Sex/age | Task | Sports imagery | Response/correct answer |
|---|---|---|---|---|
| 1 | f, 40 | CF | Tennis | Clear response |
| 2 | m, 36 | CF | Swimming | Clear response |
| 3 | m, 33 | CF | Swimming | Clear response |
| CF | Tennis | Clear response | ||
| 4 | m, 34 | CF | Swimming | Clear response |
| CF | Tennis | Clear response | ||
| 5 | m, 33 | CF | Swimming | Clear response |
| CF | Tennis | Clear response | ||
| 6 | m, 26 | CF | Swimming | Clear response |
| CF | Tennis | Clear response | ||
| 7 | m, 40 | CF | Swimming | Clear response |
| BC––mother’s name | Swimming | Clear response/yes | ||
| MC—face (2) | Table tennis | Clear response/yes | ||
| MC—suit (2) | Table tennis | Clear response/yes | ||
| 8 | m, 40 | MC—face (2) | Racket ball | Clear response/yes |
| MC—suit (2) | Racket ball | Clear response/yes | ||
| 9 | f, 51 | CF | Swimming | Clear response |
| CF | Tennis | Clear response | ||
| BC—dining in/out | Swimming | Clear response/yes | ||
| MC—face (2) | Swimming | Clear response/yes | ||
| MC—suit (2) | Swimming | Clear response/yes | ||
| 10 | f, 35 | BC—dining in/out | Pushing legs | Clear response/yes |
| MC—face (2) | Volleyball | Clear response/yes | ||
| MC—suit (2) | Volleyball | Clear response/yes | ||
| 11 | m, 43 | CF | Swimming | Clear response |
| CF | Tennis | Clear response | ||
| BC—dining in/out | Karate | Clear response/yes | ||
| MC—face | Karate | Clear response/yes | ||
| MC—suit | Karate | Clear response/yes | ||
| 12 | f, 26 | CF | Swimming | Clear response |
| BC—dining in/out | Basketball | Clear response/yes | ||
| MC—face (2) | Basketball | Clear response/yes | ||
| MC—suit (2) | Basketball | Clear response/yes | ||
| 13 | m, 29 | BC—dining in/out | Rock climbing | Clear response/yes |
| MC—face (2) | Rock climbing | Clear response/yes | ||
| MC—suit (2) | Rock climbing | Clear response/yes | ||
| 14 | m, 23 | BC—dining in/out | Volleyball | Clear response/yes |
| MC—face (2) | Volleyball | Clear response/yes | ||
| MC—suit (2) | Volleyball | Clear response/yes |
The correct answer in the last column has been determined by a blinded analysis, using the same procedure for all subjects. CF = Command following; BC = Binary choice task (mother’s name or preference for dining in or out); MC = Multiple choice task (for the face and suits of playing cards).
Table 2.
Demographic data, diagnosis, time elapsed since injury, CRS-R scores, aetiology of injury and results for all clinical subjects
| Behaviour |
Imaging |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | Age/ gender | Diagnosis | Time elapsed since injury (months) | CRS-R | Oetiology of injury | Command following | Communication | Command following | Communication |
| 1 (Test 1) | 25/F | MCS | 29 | 7 | CVA | No | No | No | No |
| 1 (Test 2) | 25/F | MCS | 29 | 10 | CVA | Yes | No | Yes | ± |
| 2 | 25/M | LIS | 23 | NT | TBI | Yes | Yes | Yes | No |
| 3 (Test 1) | 19/F | MCS | 6 | 14 | TBI | Yes | No | Yes | NT |
| 3 (Test 2) | 19/F | EMCS | 10 | 19 | TBI | Yes | No | Yes | No |
| 4 (Test 1) | 58/F | MCS | 20 | 16–19 | HIE | Yes | No | No | No |
| 4 (Test 2) | 60/F | EMCS | 32 | 23 | HIE | Yes | Yes | No | No |
| 5 | 39/M | MCS | 60 | 10–13 | TBI | No | No | No | NT |
| 6 | 40/M | MCS | 62 | 14 | TBI | No | No | No | NT |
± = an ambiguous response; CVA = cerebrovascular accident (stroke); EMCS = emerged from minimally conscious state; HIE = hypoxic-ischaemic encephalopathy; LIS = locked-in state; MCS = minimally conscious state; NT = not tested, describes a test that was not performed; TBI = traumatic brain injury.
Functional magnetic resonance paradigm
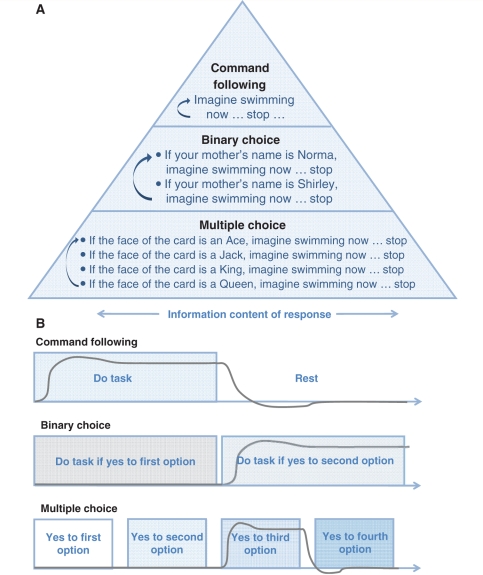
Our hierarchical functional MRI paradigm begins with a command-following task, in which the subject is asked to imagine themselves performing a physical activity such as swimming or playing tennis. Next, in all normal subjects and four of the clinical subjects, we asked the subject to use the same imagined activity to respond to one of two options in a simple two-part question. Finally, we introduced a ‘multiple-choice’ task (Sorger et al., 2009), in which all normal subjects picked and four of the clinical subjects were taught one of the face cards from a deck of playing cards before the scan, and then asked the subjects to respond again using the imagined physical activity when either the correct face or suit of the chosen card is named during the scan (Bardin et al., 2009). In the following, we detail these tasks in hierarchical order, beginning with the simplest one (Fig. 1).
Command following: the command-following task was validated on normal subjects (n = 10). The normal subjects heard instructions to imagine themselves swimming or playing tennis with their right hand, starting with a command ‘imagine yourself swimming’ and stopping with a ‘stop’ command. In the interim, subjects were required to relax and think of nothing in particular. All scans and the type of imagery are listed in Table 1. The timing for the first two subjects was similar to the one used by Owen et al. (2006). The task consisted of four 30 s blocks of rest alternating with three 30 s blocks of sports imagery. Task/rest blocks were repeated three times. The timing for the other eight subjects was adjusted to resemble the subsequent scans of the patients: eight blocks of 16 s rest alternating with 16 s sports imagery. Instructions were part of the task blocks and required ∼4 s. For the injured subjects, the same timing was used but the instructions during scanning were ‘Imagine yourself swimming … stop’.
Binary choice: The binary choice task was validated on normal subjects (n = 7). Normal subjects were asked to respond to the question ‘do you prefer making dinner at home or eating out at a restaurant?’ (n = 6) or to identify the name of their mother from being provided a correct and incorrect choice (n = 1). The latter question was added to match the question asked to the clinical subjects. In the first case, the task consisted of eight 12 s blocks of response to ‘preparing dinner yourself’ preceded by 4 s of auditory instruction, alternating with eight 12 s blocks of response to ‘dining out’, again preceded by 4 s of instruction. In the second case, the block lengths for the task were the same but an additional 4 s were added before each task to allow for the wording ‘if your mother’s name is Sarah, imagine swimming now … stop’ and ‘if your mother’s name is (actual name), imagine swimming now … stop’. The brain-injured subjects’ question asked whether one of two options was their mother’s name.
Multiple choice: the multiple choice task was validated on normal subjects (n = 8). In this paradigm, we started with four alternatives along two dimensions, the suit and face of a playing card. Before the scan, normal control subjects picked a card from a covered stack of 16 cards representing all of the face cards of a common deck of cards and noted their chosen card on a questionnaire. After the scan, subjects noted their chosen card on a new questionnaire a second time to ensure that they did not forget the card during the experiment (none of the normal subjects did). The injured subjects who performed this task were shown and told the card by an investigator who was not involved in the data analysis. The multiple-choice question was the same for both the normal and injured subjects. Normal subjects were instructed to only respond to the verbal presentation of the correct suit (club/diamond/heart/spade) or face of the card (ace/jack/king/queen) by imagining their favourite sport until the next suit or face was mentioned. The task consisted of 12 s of response followed by 4 s of rest, for each suit or face, repeated four times. Each normal subject (n = 8) imagined playing his or her favourite sport as listed in Table 1. The wording in the case of the brain-injured subjects was ‘if your card is a (club/diamond/heart/spade or ace/jack/king/queen), imagine swimming now … stop’. To accommodate the more detailed explanations, an additional 4 s of no-task was added to each block.
Figure 1.
Paradigm. (A) Different levels of functional MRI to evaluate different levels of awareness. (B) Our timing paradigm is based on the time of response for one established brain area, as obtained for example from the command-following step. A schematic response time course of the blood-oxygen-level-dependent signal is shown in grey for a response to the second alternative in the binary answer and the third alternative in the multiple choice answer.
Magnetic resonance imaging data acquisition
Before the MRI scan, the subjects were instructed to lay still with their eyes closed. Soft padding was placed around the head and anchored by the head coil caging to limit motion. All tasks performed during functional MRI scanning were verbally explained to the subjects before the experiments and repeated immediately before each corresponding scan. The subjects used foam earplugs for noise protection and headphones with additional noise protection capability. During data acquisition, pre-recorded auditory instructions were played out on a PC with an MRI compatible audio system (Resonance Technology Inc.). The volume of the headphones was adjusted to the comfort level of the normal subjects. For the brain-injured subjects, the volume was set at the comfort level of one of the investigators. Data were acquired on a 3.0 T General Electric Signa Excite HDx MRI scanner with an eight-channel head receive-only coil. For functional MRI, a Gradient-Echo Echo Planar Imaging sequence was used (repetition time = 2 s, echo time = 30 ms, flip angle 70o, acquisition and reconstruction matrix 64 × 64, 28 slices of 5 mm thickness, 24 cm field of view). The resulting voxel size is thus 3.75 × 3.75 × 5 mm. The number of repetitions depended on the stimulation paradigm used. Specifically, the command-following paradigm was the same for normal and brain-injured subjects (128 repetitions total, 4:16 min). The communication paradigms differed slightly by group as clinical subjects had elongated instructions (normal subjects, binary choice/multiple choice 128 repetitions, 4:16 min; clinical subjects, binary choice/multiple choice 160 repetitions, 5:20 min). At least four acquisitions at the beginning of each scan were discarded before starting the tasks to ensure saturation of the signal. In addition, anatomical high-resolution scans of the whole head were acquired. An axial 3D-IRFSPGR sequence (BRAVO) was acquired (acquisition and reconstruction matrix 256 × 256, 120 slices of 1.2 mm thickness, 24 cm field of view, repetition time = 8.864 ms, echo time = 3.524 ms, inversion time = 400 ms, flip angle = 13o, parallel imaging acceleration factor = 2, surface coil intensity correction filter).
Functional magnetic resonance imaging data analysis
We used three different types of data analysis: (i) blinded off-line data analysis of the binary choice and multiple-choice tasks; (ii) off-line analysis for producing group statistical parametric maps, the example figures, and for further analysis of response latencies; and (iii) a region of interest-based analysis of patient data.
The off-line analysis was first performed for all normal subjects who were tested with the binary choice (n = 7) and multiple choice tasks (n = 8), using the functional MRI Expert Analysis Tool version 5.92, part of the FMRIB Software Library software package (Smith et al., 2004). Statistical parametric maps were computed using FMRIB's Improved Linear Model in FMRIB Software Library. For the binary choice and multiple choice conditions, the analysis was blinded so that the investigator determining a subject’s answer to the question did not know the correct response. The following preprocessing was applied: motion correction, non-brain removal, spatial smoothing, grand-mean intensity normalization, local autocorrelation correction and high-pass temporal filtering (32 s cut off). Z-statistic images were thresholded using clusters determined by Z > 2.3 and a multiple-test-corrected cluster-significance threshold of P = 0.05 (Worsley et al., 1996). These statistical parametric maps were computed for each potential response, which were each then interrogated in a hierarchical fashion. First, the statistical maps were analysed for significant activation in the brain area recorded during the command following or, in the cases where command following was omitted, the binary choice task. For all normal subjects, this area was congruent with the supplementary motor area as defined by the Harvard–Oxford Cortical Atlas. If no area or multiple areas appeared significantly active in the supplementary motor area, the time course and overall brain activity were analysed for evidence of event-related activity within a larger region of interest. However, this analysis step was not necessary for any normal subjects as the brain area approach alone was sufficient to blindly identify the correct answers in all cases. Demographic data for all normal subjects and their results for the blinded off-line analysis are listed in Table 1. Data analysis for the clinical subjects was performed in a similar hierarchical fashion; however, interpretation of this data required further analysis (refer to ‘Results’ section).
Group analysis of command following in normal subjects (n = 9 for swimming, n = 7 for tennis), group analysis of binary choice in normal subjects (n = 4 out of 7 subjects who gave the same answer) and individual subjects analysis of command following, binary choice and multiple choice (n = 10 controls, n = 6 clinical subjects for command following; n = 7 controls, n = 4 clinical subjects for binary choice; n = 8 controls, n = 4 clinical subjects for multiple choice. See Tables 1 and 2 for which task was used for each subject) was performed using BrainVoyagerQX version 1.10.4.1250 (Goebel et al., 2006), which was also used in Figs 2–5. For all tasks, volumes of time series were corrected for slice scan-time differences and for motion, spatially filtered with a 3D Gaussian smoothing kernel, corrected for linear trends, and high-pass filtered with a cut-off of three cycles over the whole time span. The time-series volumes were then coregistered to the 3D anatomical volumes and these were normalized to Talairach coordinates. For the computation of statistical parametric maps, general linear models were used (Friston et al., 1995), taking the six motion-correction-parameter time-series into account as nuisance variables, which were regressed out. In patient subject data sets where we identified no activation, the analysis was repeated without including motion as regressor and we found no qualitative differences in the results. Thus in this report data is always shown with motion variables regressed out. The haemodynamic response function was modelled as a gamma function (we found that for the shorter block lengths of 16 s as described above the data was better modelled than with using the difference of two gamma functions). The results of all single-subject and group analyses were thresholded using the false-discovery rate, which was calculated for P < 0.05 (Benjamini and Hochberg, 1995; Genovese et al., 2002). The group analyses of the command following and binary choice tasks were fixed-effects analyses.
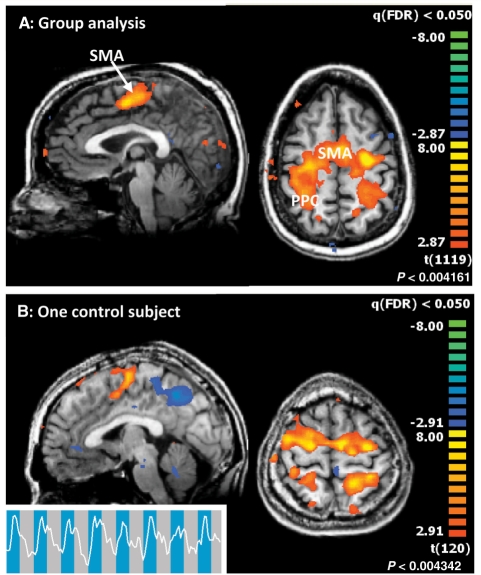
Figure 2.
Command-following task. (A) Group analysis of the command-following task in normal subjects. The nine subjects imagined themselves swimming. Areas of activation are predominantly in the supplementary motor area, partially extending laterally into the premotor areas, and parts of the posterior parietal cortex. (x = −2 mm, z = 48 mm in Talairach space). (B) Bottom panel: Single subject result for command-following (swimming, Subject 5). All individual control subject results for ‘swimming’ imagery are shown in Supplementary Fig. 1. (x = −2 mm, z = 60 mm in Talairach space.).
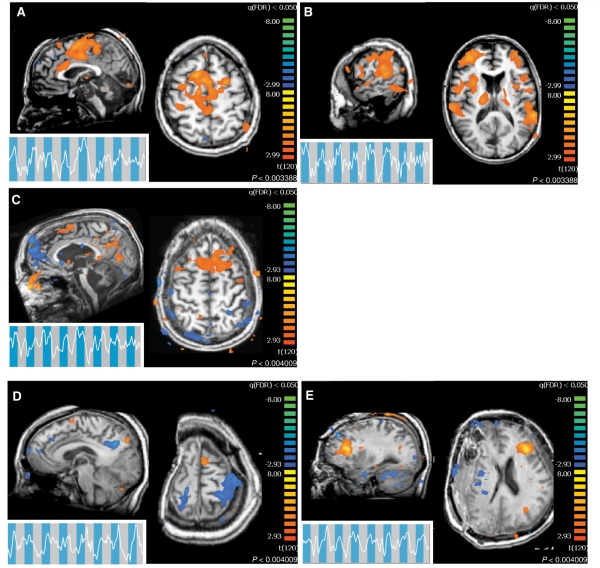
Figure 3.
Clinical subject command-following task. Command-following task results for the subjects with brain injury who demonstrated a statistically significant response. Time series are local spatial averages around the maximum activation in the supplementary motor area and are overlaid onto the task blocks: blue, sports imagery; grey, rest. In all activation maps, P < 0.05 false discovery rate. For all subjects, the task was the auditory presentation of ‘imagine yourself swimming’ (Fig. 1). All coordinates are given in Talairach space. (A) Subject 1 command-following response, midline slice (x = −2 mm, z = 52 mm). (B) Subject 1 command-following response, lateral slice (x = −58 mm, z = 14 mm). (C) Subject 2 command-following response (x = −4 mm, z = 58 mm). (D) Subject 3, visit 1, command-following response (x = −1 mm, z = 65 mm). (E) Subject 3, visit 2, command-following response (x = −30 mm, z = 21 mm).
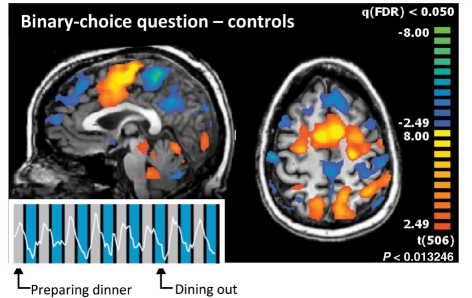
Figure 4.
Binary choice communication task. The six subjects each imagined their favourite sports activity to answer one of the two questions ‘do you prefer to prepare dinner yourself’ or ‘do you prefer eating out’ with ‘yes’. Shown here is the average activation map for the four subjects who preferred to prepare dinner themselves and a representative time course of one of these subjects (Subject 10), overlaid onto blue colour for ‘dining out’ and grey colour for ‘preparing dinner’. Individual control subject results are shown in Supplementary Fig. 2. (P < 0.05 false discovery rate, fixed-effects analysis. The time series is a local spatial average around the maximum activation in the supplementary motor area. x = −2 mm, z = 48 mm in Talairach space).
Figure 5.
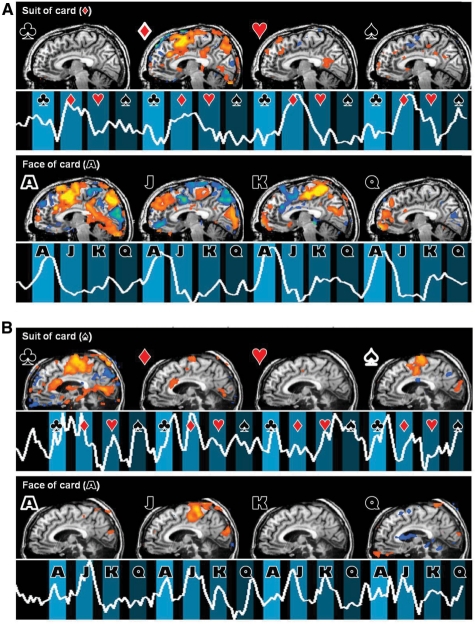
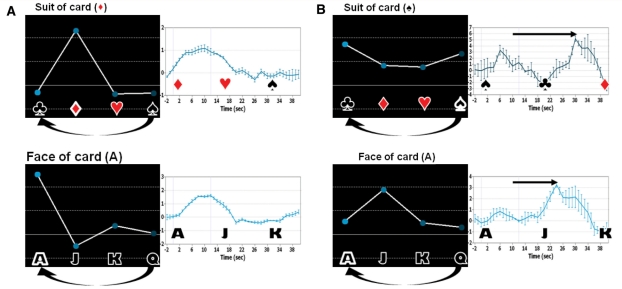
Multiple choice communication task. (A) Multiple-choice communication task results from one normal subject who picked the Ace of Diamonds card. The subject (Subject 13) responded with rock climbing imagery. The time course represents the blood-oxygen-level-dependent signal in the supplementary motor area cluster (suit) and the posterior parietal cortex (face), each in the region with the strongest blood-oxygen-level-dependent response. The symbols next to the brain indicate the used contrast for generating the SPM [club, diamond, heart, spade (suit) and ace, jack, king, queen (face)]. Individual control subject results are shown in Supplementary Fig. 2. (B) Same for Clinical Subject 1. The card picked for the clinical subject is ace of spades, and the patient was instructed to respond with swimming imagery. (In all activation maps, Z = −6, P < 0.05 false discovery rate. Time series are local spatial averages around the maximum activation in the supplementary motor area x = −6 mm in Talairach space).
In addition, a separate analysis contrasting imagery blocks and rest blocks was conducted in FSL using a region of interest based approach, limited to the supplementary motor area as defined by the Harvard-Oxford Cortical Structural Atlas. This analysis was conducted in order to allow for comparisons of our results with the results of previous studies that conducted region of interest based analyses (Monti et al., 2010). The region of interest was transformed to fit an individual subject’s brain using a matrix transformation with 12 degrees of freedom (applyxfm, FMRIB Software Library). A significance threshold was established at a cluster-level Z-value of more than 2.3 (corrected P < 0.05). Percent signal change across the region of interest was also calculated (Supplementary Figs 4 and 5).
Results
Command following: normal subjects
In the command-following paradigm, we asked normal subjects to imagine themselves swimming (n = 9) or playing tennis with their right hand (n = 7) when prompted by the investigator’s voice (Fig. 2). All normal subjects demonstrated statistically significant blood-oxygen-level-dependent signals in a whole-brain analysis comparing task performance to resting baseline. The responses showed significant supplementary motor area activations, sometimes extending laterally into the premotor areas, both on a group level as well as in single-subject scans (Fig. 2; P < 0.05 false discovery rate; all single-subject results for the ‘swimming’ imagery task are provided in Supplementary Fig. 1). Additional activation can be seen in some, mainly posterior, parts of the parietal cortex and occasionally in other parts of the brain. Although considerable variability can be observed across individual subjects, supplementary motor areas are active in all subjects and all scans, demonstrating consistency with prior assessments of successful completion of the task (Boly et al., 2007). A separate analysis using a specific region of interest for the supplementary motor area (Monti et al., 2010) demonstrated significant activation of the predefined region of interest for all normal subjects (Supplementary Fig. 5).
Command following: subjects with brain injury
Three of the six clinical subjects showed a statistically significant blood-oxygen-level-dependent signal in response to the command ‘imagine yourself swimming’ in a whole-brain analysis (Fig. 3A–E). In addition, two of these three subjects satisfied the supplementary motor area region of interest analysis (Supplementary Figs 4 and 5). Two of the subjects demonstrating task performance fulfilled diagnostic criteria for being in minimally conscious state and one subject fulfilled clinical criteria for being in locked-in state. Of the subjects who failed to produce a signal, two fulfilled diagnostic criteria for being in minimally conscious state and one had emerged from minimally conscious state with severe cognitive disability. For basic clinical information for all clinical subjects, as well as a summary of bedside behavioural and functional MRI assessments of command following and communication see Table 2. Additional details of clinical qualitative and quantitative behaviour assessments are provided in the Supplementary Material.
Assessment of Subject 1, who had a diagnosis of minimally conscious state, occurred over 2 days. The subject’s arousal state varied considerably over the two functional MRI experimental sessions. On the first day the patient was unable to follow simple commands at the bedside and the command-following functional MRI results were negative. On this day the patient was unable to receive her regular dose of an arousal modulating agent, amantadine, secondary to an incidental obstruction of her percutaneous gastrostomy tube. On the second day, however, the patient demonstrated the ability to occasionally follow commands at the bedside throughout the day and at the time of this morning testing session, the patient showed consistent downward vertical eye movements to command and appeared alert. Results of the functional MRI command-following study showed clear task-dependent activation within the supplementary motor area (Fig. 3A), as well as regions of the parietal cortex (Fig. 3B). Comparison of medicated and unmedicated functional MRI response from the first day is shown in Supplementary Fig. 3. Activations in the parietal cortex, though outside of the standard region of interest for this task, were also highly significant. A third region of interest-based analysis of the supplementary motor area region of activation demonstrated a level of activation consistent with that found in normal subjects (refer to ‘Materials and Methods’ section and Supplementary Figs 4 and 5).
Subject 2 had a diagnosis of locked-in state in the setting of severe diffuse axonal injury following traumatic brain injury and could reliably follow commands and communicate using head movements. During functional MRI command following, this subject demonstrated a task-dependent activation within the supplementary motor area, as well as significant noise corruption due to sporadic head motion in the scanner. The supplementary motor area activation was similar in statistical significance to the activation of Subject 1 (Fig. 3C). Region of interest-based analysis of the supplementary motor area region of activation demonstrated a level of activation similar to that of normal subjects (Supplementary Figs 4 and 5).
Subject 3 was scanned on two separate visits, 4 months apart. During the first visit, the subject’s diagnosis was minimally conscious state. During the second visit, the subject’s diagnosis was emerged from minimally conscious state. During both visits, the subject demonstrated the ability to follow commands at the bedside. During the first visit, the subject produced a statistically significant activation in the whole brain analysis and regional activation within the supplementary motor area (Fig. 3D). Subsequently, the subject underwent a second cranioplasty that correlated with significant improvements in behavioural responsiveness (including emergence from a minimally conscious state), global cerebral metabolism and changes of resting state network patterns across the brain (Voss et al., 2010, see Supplementary Materials for additional clinical history). During the post-cranioplasty follow-up visit, Subject 3 again demonstrated a statistically significant activation in the whole brain analysis; however, regional activation did not appear in the supplementary motor area. The whole brain analysis revealed statistically significant activity in regions frontal and lateral to the supplementary motor area (Fig. 3E). At the time of these studies, the patient could reliably follow commands and met the clinical criteria for reliable communication on the Coma Recovery Scale (Revised) (Giacino et al., 2004). The region of interest based analysis of the supplementary motor area region showed greater activation for the first compared with the second assessment but neither demonstrated activation levels similar to that of normal subjects (Supplementary Fig. 5).
Subject 4 was scanned on two separate visits ∼2 years apart. On the first visit, the subject was diagnosed as in minimally conscious state and was able to follow commands but was unable to communicate. On the second visit, the subject had emerged from a minimally conscious state and exhibited appropriate and fluent expressive language. However, on both visits this subject failed to demonstrate statistically significant task-dependent functional MRI activations during the command-following task, despite verbally confirming on the second visit her attempt to perform the task during the imaging session (Supplementary Materials).
Subjects 5 and 6 met diagnostic criteria for minimally conscious state but could not reliably follow commands at the bedside. Neither subject demonstrated a statistically significant activation during the command-following task.
Peak voxel statistics for brain-injured Subjects 1–3 are reported in Table 3.
Table 3.
Detailed results of peak voxel statistics from the command-following paradigm in subjects with brain injury
| Subject | x | y | z | T-value | P-value |
|---|---|---|---|---|---|
| S1 (Fig. 3A) | 17 | 13 | 52 | 5.355 | 0.00002 |
| S1 (Fig. 3B) | −60 | −40 | 14 | 5.552 | 0.004 |
| S2 (Fig. 3C) | −6 | 14 | 58 | 4.09 | 0.0069 |
| S3 (Fig. 3D) | 1 | 1 | 64 | 4.49 | 0.0022 |
| S3 (Fig. 3E) | −31 | 28 | 18 | 7.15 | 0.0022 |
As noted, the voxels correspond to blood-oxygen-level-dependent activity in the voxels shown in Fig. 3. P-values listed are false discovery rate values.
Communication: normal subjects
The first communication task, binary choice, asked normal subjects to respond to the question ‘do you prefer making dinner at home or eating out at a restaurant?’ (n = 6) or to respond to a question asking for their mother’s name (n = 1). Subjects were asked to imagine performing some physical activity, such as swimming, after they heard the correct answer, thus conveying their answer to the investigator (Fig. 4). A blinded investigator was able to determine the correct answers to all binary-choice questions, through a single analysis per question, as detailed in the ‘Materials and Methods’ section. For each subject, statistically significant activations obtained by the whole-brain analysis occurred during the response period for the correct answer (P < 0.05 false discovery rate). In all subjects, blood-oxygen-level-dependent activations were found predominantly in the supplementary motor area, partially extending laterally into the pre-motor areas, and parts of the posterior parietal cortex. A group-level analysis of the binary-choice paradigm is shown in Fig. 4. To demonstrate variability across individuals, all single-subject results for the binary-choice task are provided in Supplementary Fig. 2.
In the second task, multiple choice, subjects chose a playing card from a covered stack of 16 cards (faces jack, queen, king and ace) and recorded it on a questionnaire (n = 8). In the functional MRI scanner, subjects heard the investigator’s voice ask them to identify the suit and face of their card. The results for the multiple-choice task are presented for a single normal control subject in Fig. 5A. All single-subject results for the multiple-choice task are provided in Supplementary Fig. 2. A blinded investigator was able to determine the correct answers to all multiple-choice questions, thus correctly identifying the playing card for all subjects, through a single analysis per question, as detailed in the ‘Methods’ section. In all subjects, statistically significant activations occurred during the response period for the correct answers (P < 0.05 false discovery rate), in similar areas as in the two other imagery paradigms.
In summary, command-following and communication tasks were trivial to perform in all normal subjects. A blinded investigator was able to determine the correct answer to both the binary-choice question and the multiple-choice question.
Communication: subjects with brain injury
We tested the binary choice and multiple choice communication paradigms in four clinical subjects (Subjects 1–4).
For Subject 1, we observed significant activation in the supplementary motor area during the performance of the multiple-choice task. Similar to the results of the command-following task, Subject 1’s neuroimaging results from the multiple choice card paradigm differed based on day of exam. We presented the subject with the ace of spades card at the bedside over the course of 2 days prior to the scanning period. At times when she was able to move her left eye, she provided accurate ‘yes’ responses with downward eye movement when shown her card among a series of choices of distracter cards. On the first day of scanning, the patient was unable at the time of scan to signal reliably with the left eye to any spoken command. Imaging results from the multiple choice study on this day showed no statistically significant blood-oxygen-level-dependent activation during the response periods for any of the questions. The same was true for a binary-choice question to identify her mother’s name. On the second day of scanning, the patient could reliably signal the identity of her card (ace of spades) with a downward left-eye movement prior to scanning (the subject had increased general arousal on this day, see Supplementary Materials for clinical history). The first two multiple choice runs of the second day demonstrate significant activations in the motor cortex for both club and spade, in suits, and for jack, in faces (Fig. 5B). The signal strength and pattern showed an apparent effort that produced an incorrect answer to both suit (partially correct as spade and club both appear to have been signalled) and face (a clear but incorrect signal for jack as opposed to ace).
Subjects 2–4 showed no statistically significant functional MRI responses for the binary choice or multiple choice communication assessments. Of note, Subjects 2 and 4 both demonstrated consistent and fluent gestural or verbal communication [Subjects 2 and 4 (second assessment), respectively] at the bedside and indicated that they attempted to perform the tasks. Subject 2 performed correct gestural identification of the target card after functional MRI imaging session when presented among distracters; Subject 4 gave verbal attestation that she would try to carry out mental imagery during the scanning session. The functional MRI signal obtained from Subject 3 during the attempt to perform communication showed excessive movement artefact that limited interpretation of her negative findings (data not shown). Of note, motion did not corrupt the signal during command following and it is unclear whether the task performance demands might have interacted with the behavioural response in this instance, creating a motor overflow. More surprisingly, the functional MRI signal during Subject 2’s attempts at communication were not motion degraded but simply did not reveal a time-locked functional MRI signal indicating a response despite the accurate bedside communication with the subject before and after the imaging session and significant command-following response (Fig. 3C).
Discussion
Adapting neuroimaging techniques to assess capacity to communicate and establish direct communication with brain-injured subjects is a major ethical (Fins, 2003, 2009; Fins et al., 2007) and diagnostic goal (Laureys et al., 2004; Schiff et al., 2005; Laureys and Boly, 2007; Monti et al., 2009). However, the use of functional MRI-based paradigms to specify levels of residual cognitive capacity, as well as the extent to which they have a clear relationship to traditional bedside evaluations, are still major open questions (Fins and Schiff, 2010). Here we have tested a novel hierarchical functional MRI methodology to assess residual cognitive capacity in patients with severe brain injury combined with impaired motor function. Having positively validated this paradigm in normal subjects, with no failure in more than 50 experimental runs, it is striking to see the wide variance in measured response from our population of subjects with brain injury. While carrying out the command-following task guaranteed an accurate assessment of simple and multiple choice communication in the normal subjects, the subjects with brain injury demonstrated all possible dissociations. That is, the presence of reliable gestural command following and communication with mixed functional MRI-based responses (Subjects 2 and 4), functional MRI-based attempts at communication without a behavioural communication channel (Subject 1), and fluent verbal command following and communication with no functional MRI-based signals (Subject 4). These findings challenge the immediate translation of neuroimaging assessments into clinical use and raise interesting questions about the underlying neurophysiological substrates of response generation in the brain-injured versus normal control subjects as discussed below.
Normal subjects
Earlier research has shown that command-following with an imagined motor activity produces robust activation in the motor cortices even while the subject is immobile in the functional MRI scanner (Jeannerod and Frak, 1999; Ross et al., 2003). In agreement with these earlier findings, in our various sports imagery tasks we were able to clearly identify signals in those regions that are associated with production of motor imagery. Based on activation in these regions, we could utilize brain activity to communicate with all normal subjects. More importantly, if reliable brain activations could be obtained from a severely brain-injured subject, the multiple-choice framework could be adapted to probe responses on standard psychometric scales, interrogating issues such as grading the subjective experience of pain (Likert, 1932). Given the uniform effectiveness of the multiple choice communication methodology in normal subjects, such a paradigm seems a reasonable benchmark for efficiently assessing how similarly subjects with brain injury may comprehend, integrate and respond to external information.
Subjects with severe brain injury: command following
Three of the six clinical subjects successfully performed the command-following task. Of these three, two were diagnosed as in minimally conscious state at the time of scanning and one was diagnosed as in locked-in state. At a minimum, this result reinforces the diagnosis of the two minimally conscious state cases, as one of the clinical behavioural reporters of the minimally conscious state is the ability to follow commands (Giacino et al., 2002). Of the three subjects who did not successfully perform the command-following task, two had a diagnosis of minimally conscious state and one was emerged from minimally conscious state, with severe cognitive disability. This last subject demonstrates fluent verbal communication and appropriate responses to humorous stimuli but remains disoriented and behaviourally within the cognitive range of ‘confusional state’ (Sherer et al., 2005, see Supplementary Materials). The fact that this subject was unable to perform the command-following task over multiple experimental scans is troubling and raises significant concerns regarding the use of these methods in the brain-injured population. In particular, this subject was able to verbally confirm her attempt to do the task while in the scanner after receiving the instructions. It is unclear whether this failure is due to damage to motor imagery networks, to an inability to understand the task to be performed, or to some other poorly understood cause. We believe this subject presents a demonstration proof that the relationships between pattern of injury, cognitive ability and capacity for motor imagery are as yet too poorly understood to draw conclusions regarding the reason for failure to perform such tasks.
Subjects with severe brain injury: communication
Of the six clinical subjects analysed, five demonstrated no statistically significant response in either the binary-choice or multiple-choice communication paradigms. Of particular note, Subjects 3 and 4 failed at these tasks even though they demonstrate the ability to communicate fluently (Subject 3 through head movements and Subject 4 verbally), and were able to indicate post hoc that they attempted to perform the tasks. As described above, the failure of subjects who can reliably communicate behaviourally to perform the functional MRI tasks suggests major limitations in the sensitivity of these experimental tasks for identifying a range of cognitive functions from minimally conscious state to moderate cognitive disability.
In Subject 1, we identified statistically significant responses in the expected brain regions that were reliably generated by the patient in response to items repeated over time within the task. However, the information communicated was incorrect. We considered the possibility that this subject may have attempted to respond accurately but with a delay that might confound our planned analyses based on our clinical observation of reliable signalling with the downward eye movement at the time of the imaging session and accurate indication of the ace of spades as the target card. The likelihood of such a confounding factor is increased by the known clinical correlation of bilateral paramedian thalamic injuries with response delays (Segarra, 1970; Katz et al., 1987). To further investigate the patient’s response to the multiple-choice paradigm we developed an additional analysis. Figure 6 shows averaged haemodynamic responses with onset beginning at the time of display of the correct face and suit of the card for a normal subject (Fig. 6A) and the injured subject (Fig. 6B). A representative normal subject shows an immediate response beginning at ∼2 s with a broad peak centred ∼10 s. In contrast, for both the face and suit response data the brain-injured subject's time series show a clear late peak beginning ∼18 s after the spoken command and peaking at ∼24–30 s for the two runs, in addition to other earlier, shallower peaks. As noted above, the most robust activations for Subject 1 during the playing card task occurred during response periods for club and jack. Because the cards are read out in alphabetical order and the paradigm repeats, these two responses both fall directly after the correct answers, spade and ace (Fig. 6B). Therefore, taking a peak response latency of 14–20 s relative to normal subjects in the data analysis into account could provide an explanation for the wrong responses in both the face and the suit tasks; if such a latency is present, it may mask a correct response of the subject to both face and suits. Since this paradigm used 12 s response windows preceded by 4 s of instruction and followed by 4 s rest periods, the peak latency of 14–20 s overlaps with the anticipated response window for the following choice. Therefore, further experiments would be necessary to determine whether the observed incorrect responses are caused by the subject’s slow response. Of note, our response window is shorter than the 30 s window used by Monti et al. (2010) and several of the time series shown for their patient responses to command following have a similar profile of a late peak around 30 s, as seen in our patient, differing from those of normal subjects. The latter observation suggests the potential role of a more general phenomenon related to response window and not necessarily linked to the paramedian thalamic injuries present in this particular clinical subject.
Figure 6.
Interpretation of Clinical Subject 1 data as delayed response. Relative magnitudes of the fit coefficients of the general linear model, in dependence of stimuli, for one normal subject (A) and Clinical Subject 1 (B) left panels of each column, and corresponding trial-averaged haemodynamic response curves for an area of strong activation, right panels. Since the stimuli are applied cyclically (four times), club follows spade and ace follows queen. Both the face and suit causing maximal activations in the patient’s time series (i.e. jack, club) immediately follow the correct suit and face options (ace, spade). The trial averages in the right panels start at the time when the correct suit or face of the selected card is played out. The subject with brain injury shows a peak response ∼14–20 s delayed relative to the peak latency of ∼10 s seen in both time series obtained from the normal subject. These time intervals are indicated by arrows. For the suit time series, a weak initial response at ∼6 s is seen in the brain injured subject’s response, which also showed significant activations for both the club and ace.
In our view Subject 1’s communication results have at least three possible interpretations: (i) the subject gave the wrong answers in the scanner despite performing accurate identification of the target at the bedside; (ii) the subject perceived the card differently in her own mental image or incompletely, so that when asked to identify the card by name rather than sight she chose different descriptors and (iii) the subject tried to signal the correct responses but a possible pronounced response latency produced a significant delay that when measured could effectively account for her giving the correct responses. The most parsimonious choice in our view, considering the results of the time-averaging of the data and using post hoc reasoning, is that Subject 1 was able to carry out the mental imagery to communicate. It should be noted, however, that the functional MRI task places a memory demand ahead of the motor imagery response stage not included in bedside presentation of the visual stimulus target; therefore the cognitive demands of the functional MRI task are different and may also elicit failure modes not evident on bedside testing (e.g. goal neglect or impaired visual working memory).
Thus, at a minimum we established communication of responses independent of their accuracy. These findings indicate a communication readiness but not the capacity to functionally communicate. As such, the observations are directly comparable with the bedside examination of many patients in a minimally conscious state who demonstrate inconsistent communication through reliable channels for producing one-bit responses (Giacino et al., 2002). Emergence from a minimally conscious state requires that such communication channels convey consistently accurate information, confirmed by sequential and accurate (at least six in a row) responses to questions directed to immediately verifiable situational conditions, e.g. ‘am I clapping my hands?’, over a minimum of two different and serially repeated examinations (Giacino et al., 2004). There are a number of questions raised by this case, including whether the brain-injured subject has the cognitive ability to fully communicate, whether she fluctuated in level of awareness during these scans, and whether utilizing a different neural network, such as imagining a spatial-navigation event (Owen et al., 2006), would provide improved data. The findings illustrate that simply achieving communication using a single session of functional MRI questioning is a far step from using these tools for diagnostic purposes or clinical decision-making. Establishing that the patient’s cognitive level is above a minimally conscious state would require the same operational methodology employed in bedside neuropsychological examinations and based on the data presented here, will require considerable further development of both methodology and understanding of sources of variance in the measured response arising within the distributed neuronal systems used to generate specific brain activations during task performance.
Do interactions of cognitive task demands and motor imagery demands confound assessments?
An important question raised by these findings is whether brain injuries that extend into CNS structures involved in motor control produce resource allocation problems when using overt or imagined motor responses to provide an indication of the result of another cognitively mediated behaviour. Motor preparation or imagery may share overlapping working memory or task maintenance resources in the brain with systems required to carry-out any given communication task (not just the complicated visual–spatial memory and verbal matching task imposed by card naming used here). Some evidence for such trade-offs have been described in the patterns of recovery of cognitive versus motor functions following traumatic brain injuries (Green et al. 2006). In all four subjects tested here using the communication paradigm widespread brain injury is present, albeit with varying aetiologies, raising the possibility that such resource allocation problems may limit harnessing an intact motor imagery command-following response system when additional cognitive demands are introduced.
Perhaps the most general pattern of injury that might produce such a trade-off and evidence of behavioural impairment consistent with a minimally conscious state at the bedside is that of brainstem and bilateral thalamic injuries produced by basilar artery occlusion present in Subject 1. This combination of injuries is well-known and can be seen to present a major concern for interpretation of cognitive capacity in this class of patients based even on this single case. Bilateral paramedian thalamic injuries in isolation may produce a well-described clinical syndrome (Segarra, 1970; Katz et al., 1987) that combines arousal regulation impairment, marked response delay (with clinical experience suggesting a range of delay from 5 to ∼45 s) and most importantly vertical gaze paresis. Prolonged latencies for cognitive task performance are also a common finding in less severe brain injuries than seen in our clinical subject, albeit with comparably smaller latencies for actual movements (Stuss et al., 1989; Loken et al., 1995; Mathias and Wheaton, 2007). As such, this pattern of structural injuries may present a high risk for obscuring a latent capacity to communicate by providing only limited time periods of alertness and directly injuring the only motor pathways (vertical gaze/neck control) typically well-preserved in the locked-in syndromes and variably slowing responses obscuring their contingency with environmental events (Smart et al., 2008; Carrai et al., 2009).
As Subject 1 has inconsistently shown an ability to communicate through a single sporadic motor-output channel, an ability to respond to mental imagery commands and intermittently communicate while on amantadine, and clinical evidence of direct communication with those around her including formal assessments of personal biographical knowledge, these findings are strongly suggestive of a higher level of cognitive function beyond a minimally conscious state. Operationally, however, neither functional MRI-based nor formal bedside assessment in this clinical subject can consistently establish a functional level above the minimally conscious state diagnostic category. Here our findings do not change the clinical diagnosis of minimally conscious state but raise considerable concerns about tracking the further recovery of cognitive function for this subject beyond the minimally conscious state and developing more reliable and robust assessments of communication and expression vectors. The present findings and patient history (long-term and possibly incorrect diagnosis of vegetative state or low-level minimally conscious state) suggest a particular ethical obligation to affirmatively interrogate survivors of severe basilar artery occlusive disease to ascertain whether or not communication is possible. At minimum these data demonstrate a higher risk of dissociation of cognitive capacity and expressed behaviour than may be appreciated generally for survivors of basilar artery occlusions.
Here we have developed and field-tested a hierarchical, single-response-type, functional MRI paradigm that utilizes command following, binary choice and multiple choice task frameworks to vary levels of difficulty and obtain a graded assessment of a patient’s ability to utilize brain activity to follow commands and communicate. This method minimizes the patient’s effort to learn and express the internal mental imagery necessary for such assessments. Compared with the methods of Monti et al. (2010), our approach offloads the requirement for the patient to prepare two potential mental imagery responses using the multiple choice design of the experiments to provide a statistical disambiguation, if accurate communication is possible. Thus it is possible that this approach may reveal more intermediate levels of communication readiness or capacity than the more complex task sets. Importantly, as shown above, this approach presents opportunities to identify and quantify intermediate levels of response that do not represent functional communication. Nonetheless, such limited responses do reflect communication readiness and, when present, indicate a need for repeated testing and efforts to seek alternative response types (both in functional MRI and other neuroimaging modalities). The demonstration of the use of the functional MRI signal by the patient for communication (i.e. a repeated identifiable activation pattern to one of the choices for suit and face), independent of the accuracy of the responses, is very important. Intermittent communication is a feature of some patients in a minimally conscious state who may fail to demonstrate reliable communication not because of response failure but due to the inconsistent accuracy of clear visible responses. Compared with the signal strength and ‘obviousness’ of the normal subject responses, patient responses are likely to have a more pronounced spatial or temporal variability as well as session-to-session variability than the already present functional MRI variability observed in healthy control subjects.
Conclusion
As demonstrated here, using functional MRI reliably and consistently will need to overcome numerous challenges, including variations in data quality obtained in different sessions due to arousal state, motion, medication effects and other uncontrolled or injury-specific signal properties and variables (Monti et al., 2009). In addition, our data clearly demonstrate that absence of proof of communication using functional MRI does not provide evidence of the absence of an ability to communicate, as demonstrated by our two subjects who can communicate successfully outside of the scanner but did not perform our functional MRI tasks. Although this could represent specific damage to areas required to perform the task, it may also suggest that performance of these tasks, i.e. functional MRI-based command following with mental imagery, demands higher cognitive ability than required for reliably establishing simple gestural or verbal communication. Collecting large samples of data within and across brain-injured subjects will be necessary to calibrate these methods for further development and clinical uses. In addition, because of the heterogeneity of the clinical population under study, studies with small sample sizes are likely to demonstrate variable levels of dissociation between imaging and behavioural results (Kotchubey et al., 2005; Perrin et al., 2006). For example, Schnakers et al. (2008) showed no obvious dissociation between behavioural and event related response assessments of command following in a word counting paradigm tested in 22 patients.
In conclusion, our results demonstrate that, despite a successful application of communication-based functional MRI paradigms in healthy subjects, the application to patients with severe loss of motor function requires more research to understand the general and subject-specific constraints on the design of tasks. In particular, the observed dissociation between the behavioural and neuroimaging results of our behaving subjects with brain-injury suggests a level of unexplained variance that at present confounds the potential wider use of this technique. We believe that a prospective study with a large sample size of both normal and subjects with brain injury is required to fully understand the implications of the successful or unsuccessful application of this method.
Funding
National Institutes of Health NIH-NICHD; James S. McDonnell Foundation (to N.D.S.); the Dana Foundation (to N.D.S. and J.J.F.); the Robert Wood Johnson Foundation (to J.J.F.); the Lounsbery Foundation (to J.J.F., N.D.S. and J.H.); the Jerold B. Katz Foundation (to N.D.S. and J.J.F.), the Buster Foundation (to J.J.F. and J.H.); Deutsche Forschungsgemeinschaft DFG-Research Centre MATHEON (to K.T.); the Weill Cornell Center for Translational Science Activity (to N.D.S.).
Supplementary material
Supplementary material is available at Brain online.
References
- Bardin JC, Fins JJ, Hersh J, Heier LA, Schiff ND, Voss HU. Multiple choice fMRI in severely brain injured patients. Neuroscience. 2009 -S-1557-SfN. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate––a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Boly M, Coleman MR, Davis MH, Hampshire A, Bor D, Moonen G, et al. When thoughts become action: an fMRI paradigm to study volitional brain activity in non-communicative brain injured patients. Neuroimage. 2007;36:979–92. doi: 10.1016/j.neuroimage.2007.02.047. [DOI] [PubMed] [Google Scholar]
- Boly M, Faymonville ME, Peigneux P, Lambermont B, Damas P, Del Fiore G, et al. Auditory processing in severely brain injured patients - differences between the minimally conscious state and the persistent vegetative state. Arch Neurol. 2004;61:233–8. doi: 10.1001/archneur.61.2.233. [DOI] [PubMed] [Google Scholar]
- Carrai R, Grippo A, Fossi S, Campolo MC, Lanzo G, Pinto F, et al. Transient post-traumatic locked-in syndrome: A case report and a literature review. Clin Neurophysiol. 2009;39:95–100. doi: 10.1016/j.neucli.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Fins JJ. Constructing an ethical stereotaxy for severe brain injury: balancing risks, benefits and access. Nat Rev Neurosci. 2003;4:323–7. doi: 10.1038/nrn1079. [DOI] [PubMed] [Google Scholar]
- Fins JJ. Being conscious of their burden: severe brain injury and the two cultures challenge. Ann N Y Acad Sci. 2009;1157:131–47. doi: 10.1111/j.1749-6632.2009.04473.x. [DOI] [PubMed] [Google Scholar]
- Fins JJ, Schiff ND. In the Blink of the Mind’s Eye. Hastings Center Report. 2010;40 doi: 10.1353/hcr.0.0257. No. 3, May–June 2010. 1–23. [DOI] [PubMed] [Google Scholar]
- Fins JJ, Schiff ND, Foley KM. Late recovery from the minimally conscious state––ethical and policy implications. Neurology. 2007;68:304–7. doi: 10.1212/01.wnl.0000252376.43779.96. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RSJ, Turner R. Characterizing dynamic brain responses with fMRI––a multivariate approach. Neuroimage. 1995;2:166–72. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, et al. The minimally conscious state––definition and diagnostic criteria. Neurology. 2002;58:349–53. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Hirsch J, Schiff N, Laureys S. Functional neuroimaging applications for assessment and rehabilitation planning in patients with disorders of consciousness. Arch Phys Med Rehabil. 2006;87:S67–76. doi: 10.1016/j.apmr.2006.07.272. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K, Whyte J. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–9. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Smart CM. Recent advances in behavioral assessment of individuals with disorders of consciousness. Curr Opin Neurol. 2007;20:614–9. doi: 10.1097/WCO.0b013e3282f189ef. [DOI] [PubMed] [Google Scholar]
- Gill-Thwaites H. Lotteries, loopholes and luck: misdiagnosis in the vegetative state patient. Brain Inj. 2006;20:1321–8. doi: 10.1080/02699050601081802. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with BrainVoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RE, Christensen B, Melo B, Monette G, Bayley M, Hebert D, et al. Is there a trade-off between cognitive and motor recovery after traumatic brain injury due to competition for limited neural resources? Brain Cogn. 2006;60:199–201. [PubMed] [Google Scholar]
- Jeannerod M, Frak V. Mental imaging of motor activity in humans. Curr Opin Neurobiol. 1999;9:735–9. doi: 10.1016/s0959-4388(99)00038-0. [DOI] [PubMed] [Google Scholar]
- Katz DI, Alexander MP, Mandell AM. Dementia following strokes in the mesencephalon and diencephalon. Arch Neurol. 1987;44:1127–33. doi: 10.1001/archneur.1987.00520230017007. [DOI] [PubMed] [Google Scholar]
- Kotchoubey B, Lang S, Mezger G, Schmalohr D, Schneck M, Semmler A, et al. Information processing in severe disorders of consciousness: vegetative state and minimally conscious state. Clin Neurophysiol. 2005;116:2441–53. doi: 10.1016/j.clinph.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Laureys S, Boly M. What is it like to be vegetative or minimally conscious? Curr Opin Neurol. 2007;20:609–13. doi: 10.1097/WCO.0b013e3282f1d6dd. [DOI] [PubMed] [Google Scholar]
- Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–46. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;140:1–55. [Google Scholar]
- Loken WJ, Thornton AE, Otto RL, Long CJ. Sustained attention after severe closed-head injury. Neuropsychology. 1995;9:592–8. [Google Scholar]
- Magee WL. Music as a diagnostic tool in low awareness states: considering limbic responses. Brain Inj. 2007;21:593–9. doi: 10.1080/02699050701426907. [DOI] [PubMed] [Google Scholar]
- Mathias JL, Wheaton P. Changes in attention and information-processing speed following severe traumatic brain injury: a meta-analytic review. Neuropsychology. 2007;21:212–23. doi: 10.1037/0894-4105.21.2.212. [DOI] [PubMed] [Google Scholar]
- Monti MM, Coleman MR, Owen AM. Neuroimaging and the vegetative state: resolving the behavioral assessment dilemma? Ann N Y Acad Sci. 2009;1157:81–9. doi: 10.1111/j.1749-6632.2008.04121.x. [DOI] [PubMed] [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L, et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med. 2010;362:579–89. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- Perrin F, Schnakers C, Schabus M, Degueldre C, Goldman S, Bredart S, et al. Brain response to one’s own name in vegetative state, minimally conscious state, and locked-in syndrome. Arch Neurol. 2006;63:562–9. doi: 10.1001/archneur.63.4.562. [DOI] [PubMed] [Google Scholar]
- Ross JS, Tkach J, Ruggieri PM, Lieber M, Lapresto E. The mind's eye: functional MR imaging evaluation of golf motor imagery. AJNR Am J Neuroradiol. 2003;24:1036–44. [PMC free article] [PubMed] [Google Scholar]
- Royal College of Physicians. The Vegetative State: Guidance on Diagnosis and Management. Report of a Working Party, Royal College of Physicians, London, 2003. [Google Scholar]
- Schiff ND, Rodriguez-Moreno D, Kamal A, Kim KHS, Giacino JT, Plum F, et al. fMRI reveals large-scale network activation in minimally conscious patients. Neurology. 2005;64:514–23. doi: 10.1212/01.WNL.0000150883.10285.44. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Perrin F, Schabus M, Majerus S, Ledoux D, Damas P, et al. Voluntary brain processing in disorders of consciousness. Neurology. 2008;71:1614–20. doi: 10.1212/01.wnl.0000334754.15330.69. [DOI] [PubMed] [Google Scholar]
- Segarra JM. Cerebral vascular disease and behavior. I. The syndrome of the mesencephalic artery (basilar artery bifurcation) Arch Neurol. 1970;22:408–18. doi: 10.1001/archneur.1970.00480230026003. [DOI] [PubMed] [Google Scholar]
- Sherer M, Nakase-Thompson R, Yablon SA, Gontkovsky ST. Multidimensional assessment of acute confusion after traumatic brain injury. Arch Phys Med Rehabil. 2005;86:896–904. doi: 10.1016/j.apmr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Smart CM, Giacino JT, Cullen T, Moreno DR, Hirsch J, Schiff ND, et al. A case of locked-in syndrome complicated by central deafness. Nat Clin Pract Neurol. 2008;4:448–53. doi: 10.1038/ncpneuro0823. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sorger B, Dahmen B, Reithler J, Gosseries O, Maudoux A, Laureys S, et al. Another kind of ‘BOLD response’: answering multiple-choice questions via online decoded single-trial brain signals. Prog Brain Res. 2009;177:275–92. doi: 10.1016/S0079-6123(09)17719-1. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Stethem LL, Hugenholtz H, Picton T, Pivik J, Richard MT. Reaction time after head injury: fatigue, divided and focused attention, and consistency of performance. J Neurol Neurosurg Psychiatr. 1989;52:742–8. doi: 10.1136/jnnp.52.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss HU, Heier LA, Schiff ND. Multimodal imaging of recovery of functional networks associated with reversal of paradoxical herniation after cranioplasty. Clin Imaging. 2010 doi: 10.1016/j.clinimag.2010.07.008. doi:10.1016/j.clinimag.2010.07.008 Epub ahead of print 1 September 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss HU, Ulug AM, Dyke JP, Watts R, Kobylarz EJ, McCandliss BD, et al. Possible axonal regrowth in late recovery from the minimally conscious state. J Clin Invest. 2006;116:2005–11. doi: 10.1172/JCI27021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]