Abstract
Activation of Nrf2 by covalent modifications that release it from its inhibitor protein Keap1 has been extensively documented. In contrast, covalent modifications that may regulate its action after its release from Keap1 have received little attention. Here we show that CREB-binding protein induced acetylation of Nrf2, increased binding of Nrf2 to its cognate response element in a target gene promoter, and increased Nrf2-dependent transcription from target gene promoters. Heterologous sirtuin 1 (SIRT1) decreased acetylation of Nrf2 as well as Nrf2-dependent gene transcription, and its effects were overridden by dominant negative SIRT1 (SIRT1-H355A). The SIRT1-selective inhibitors EX-527 and nicotinamide stimulated Nrf2-dependent gene transcription, whereas resveratrol, a putative activator of SIRT1, was inhibitory, mimicking the effect of SIRT1. Mutating lysine to alanine or to arginine at Lys588 and Lys591 of Nrf2 resulted in decreased Nrf2-dependent gene transcription and abrogated the transcription-activating effect of CREB-binding protein. Furthermore, SIRT1 had no effect on transcription induced by these mutants, indicating that these sites are acetylation sites. Microscope imaging of GFP-Nrf2 in HepG2 cells as well as immunoblotting for Nrf2 showed that acetylation conditions resulted in increased nuclear localization of Nrf2, whereas deacetylation conditions enhanced its cytoplasmic rather than its nuclear localization. We posit that Nrf2 in the nucleus undergoes acetylation, resulting in binding, with basic-region leucine zipper protein(s), to the antioxidant response element and consequently in gene transcription, whereas deacetylation disengages it from the antioxidant response element, thereby resulting in transcriptional termination and subsequently in its nuclear export.
Keywords: CBP, Nicotinamide, Resveratrol, Sirtuins, Transcription Termination, Nrf2, Acetylation-Deacetylation, Nucleocytoplasmic Localization
Introduction
The transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) is a key oxidative stress response modifier that induces transcription of a variety of genes through binding to the antioxidant response element (ARE)4 in target gene promoters (1–3). Nrf2-dependent activation of ARE-driven gene promoters is generally understood to lead to induction of cytoprotective proteins, which enable cells to combat oxidative insult (2–4). However, overexpression of Nrf2 in cancerous cells may actually be deleterious because it may enable them to sustain growth and become chemo-resistant to various pro-oxidant chemotherapeutic drugs (5–7).
In nonstressed cells, Nrf2 activity is thought to be repressed by Keap1 (Kelch-like ECH-associated protein 1) (8–10), a cytoskeleton-associated protein that, when complexed with Nrf2, promotes ubiquitin-mediated degradation of Nrf2. In one model for the activation of Nrf2 in stressed cells, electrophile- or reactive oxygen species-induced release of Nrf2 is proposed to involve covalent modifications of Keap1 and/or Nrf2 in the cytoplasm. Such modifications include oxidation of key cysteine residues in Keap1 (11), phosphorylation of Nrf2 at Ser40 by protein kinase C (12, 13), and switching of Cullin-3-dependent ubiquitylation from Nrf2 to Keap1, leading to the degradation of Keap1 and stabilization and activation of Nrf2 (14–18). The released Nrf2 is then translocated into the nucleus via classical nuclear import-targeting sequences in the protein (19, 20). Productive binding of Nrf2 to its DNA response element, the ARE, requires heterodimeric interaction between Nrf2 and basic-region leucine zipper-containing proteins, especially small Maf proteins (2–4).
Another model of the regulation of Nrf2 activity proposes that Nrf2 is constitutively expressed and is directly translocated into the nucleus following its synthesis on ribosomes (21, 22). According to proponents of this model, Keap1 is independently shuttled into the nucleus, where it interacts with Nrf2 to target it for degradation. The model interprets the activating effects of electrophiles and other stimuli of Nrf2 activation as resulting from interference with the Keap1-Nrf2 interaction within the nucleus (22).
In contrast to the enormous amount of work on the activation of Nrf2, covalent modification(s) that may regulate its action after it is separated from Keap1 have not been as well studied. In HEK293T cells, Salazar et al. (23) showed that Nrf2 is phosphorylated by GSK-3β and that this phosphorylation localizes it to the cytoplasmic compartment; according to their report, diminished entry of Nrf2 into the nucleus resulted in impaired Nrf2-induced activation of ARE-driven gene promoters. In contrast, in neuroblastoma cells phosphorylation of the Neh4 (Nrf2-ECH homology 4) and Neh5 domains of Nrf2 positively influenced its nuclear translocation as well as its transactivation activity (24). In another study, Fyn kinase-mediated phosphorylation of Tyr568 in Nrf2 was reported to regulate its nuclear export and degradation (25). Other than the recently published report that Nrf2 undergoes reversible acetylation in HEK293T cells subjected to sodium arsenite [As(III)]-induced stress, primarily in its Neh1 domain (26), only phosphorylation had previously been reported to modulate activity of Nrf2 downstream of its activation. Here we show that acetylation/deacetylation plays a crucial role in the nucleocytoplasmic shuttling of Nrf2 and that acetylatable lysine residues in its Neh3 domain participate in modulating its transcriptional activity.
EXPERIMENTAL PROCEDURES
Reporter Gene Assays
K562 cells obtained from the American Type Culture Collection (Manassas, VA) were maintained in culture as described previously (27, 28). For co-transfection of expression plasmids, cells (1 × 105 cells in 1 ml of medium/well) seeded in 24-well plates for 24 h were co-transfected with 0.2 or 0.3 μg of luciferase reporter plasmids (human Gαi2-luc gene promoter, minimal promoters HO-1-ARE-luc (29) or hNQO1-ARE-luc (30)) along with pCI-Nrf2, and plasmid harboring the cDNA for CBP, E1A (adenovirus early protein 1A), or SIRT1 (as indicated in legends to figures), using FuGENETM 6 transfection reagent (Roche Applied Science) at a 3:1 ratio of FuGENETM 6 reagent (μl) to DNA (μg) or a 4:1 ratio of polyethylenimine (catalog number 23966-2; Polysciences, Inc., Warrington, PA) (μl of 1 μg/μl solution) to DNA (μg). The total amount of DNA was adjusted, if necessary, by adding the empty vector plasmid. The cells were harvested 20–24 h later, by centrifugation at 12,000 × g (45 s) in 1.5-ml microcentrifuge tubes and processed for luciferase assay as described previously (20, 28, 31, 32).
Localization of Nrf2 in HepG2 Cells by Fluorescence Microscopy
HepG2 cells, obtained from the American Type Culture Collection, were grown in minimum essential medium supplemented with 1 mm sodium pyruvate, 2 mm l-glutamine, 1× minimum essential medium nonessential amino acids (Invitrogen), 10% fetal bovine serum, 100 units of penicillin/ml of medium, and 100 μg of streptomycin/ml of medium. Approximately 4 × 105 cells/well were seeded onto poly-d-lysine-coated coverslips in six-well plates in 2 ml of medium and incubated overnight at 37 °C. The cells were then transfected with 1.5–2 μg of plasmid pEGFP-Nrf2 construct, using FuGENE HD transfection reagent (Roche Applied Science) at a 3:1 ratio of FuGENE HD (μl) to DNA (μg). Twenty-four hours after transfection, the cells were either incubated with or without tBHQ (20 μm) for 1 h to induce activation and nuclear accumulation of Nrf2 or with resveratrol (50 μm). In cells incubated with tBHQ and resveratrol, resveratrol was added 30 min before the addition of tBHQ. In parallel experiments, the cells were also transfected with 0.25 μg of expression plasmid for CBP with or without expression plasmid for E1A. All of the cells were harvested by removing the medium and rinsing once with 1× PBS, followed by fixation with 1 ml of 3.7% paraformaldehyde for 10 min and rinsing with PBS. The cells were processed for fluorescence imaging analysis as described previously (20). Briefly, after fixation, the cells were incubated with 100 μg/ml RNase A (Sigma) for 20 min at 37 °C and rinsed three times with 1× PBS. To stain the nuclei, the cells were incubated for 2 min at room temperature in a solution of propidium iodide (3 μg/ml), rinsed with PBS, and then rinsed once with H2O. The coverslips were then mounted onto slides using Aqua-Poly/Mount (Polysciences, Inc., Warrington, PA), kept overnight at 4 °C, and visualized under a fluorescence microscope at excitation and emission wavelengths, respectively, of 536 and 617 nm for red fluorescence (propidium iodide) and 485 and 530 nm for GFP. Using Adobe Photoshop, images of the propidium iodide and GFP fluorescence patterns were merged to visualize nuclear localization.
EMSA
Nuclear extracts used for EMSA were prepared as described previously (28, 31, 32). For EMSA, annealed 5′-overhang oligonucleotide sequence of the Gαi2 gene promoter containing the ARE-binding motif in the promoter was radiolabeled with [α-32P]dCTP by using the Klenow fill-in reaction and purified as described previously (28). After electrophoresis, the gel was dried and then exposed to Classic Blue Autoradiography Film BX (Molecular Technologies, St. Louis, MO) at −80 °C. The radiolabeled bands were detected by autoradiography.
Detection of Acetylated Nrf2 in Cells
K562 cells were transfected with CBP with or without E1A or SIRT1 with or without dominant negative SIRT1 (SIRT1-H355A), along with pCI-Neo or pCI-Nrf2. To enhance detection of acetylated species (33–35) in CBP-treated cells, the cells were treated with 66 nm trichostatin A and 10 mm NAM (to inhibit deacetylases) 6 h before harvesting. After harvesting, whole cell lysates were prepared using nondenaturing lysis buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 1 mm sodium vanadate, 5 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, and 10 μl/ml protease inhibitor mixture (Sigma)); co-immunoprecipitation was performed with the whole cell lysates. Briefly, the lysates (200 μg of protein) were diluted with the lysis buffer to 100 μl and then incubated with 1 μg each of either normal rabbit IgG or antibody to Nrf2 overnight at 4 °C with gentle rotation. The immunocomplexes were collected by incubation at 4 °C for 2 h to overnight with 20 μl of protein A/G Plus-agarose (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), followed by centrifugation at 1,000 × g for 1 min. The agarose beads were washed four times with 1 ml of wash buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 0.5 mm sodium vanadate, 0.2% Nonidet P-40, 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, and 1 μg/ml pepstatin A) and then boiled for 5 min with 30–40 μl of 2× SDS sample buffer. After centrifugation, the supernatant solution was subjected to Western blotting using anti-acetyl-lysine antibody (Cell Signaling Technology, Danvers, MA).
Site-directed Mutagenesis
Nrf2 was mutated at putative acetylation sites by using the QuikChangeTM site-directed mutagenesis kit from Stratagene (La Jolla, CA) to replace the lysine residues with alanine, arginine, or glutamine residues. Mutations were confirmed by DNA sequencing at the Molecular Biology Core Facility at Meharry Medical College.
Western Blotting
Western blotting analyses, using various antibodies, were performed as described previously (20, 28, 31, 32).
RESULTS
Effect of CBP on Nrf2-induced Transcription
CBP and p300 proteins are common co-activators for a variety of transcription factors (36, 37). Because they possess acetyltransferase activity (38–40) and because CBP had previously been shown in a yeast two-hybrid system to bind to Nrf2 (41), we reasoned that CBP might modulate Nrf2-induced transcription, perhaps through acetylation of Nrf2 itself. Based on our previous findings that the Gαi2 gene promoter activity is profoundly regulated by Nrf2 (20, 32, 42), we initially monitored Nrf2-induced transcription from this promoter in K562 cells in the absence or presence of exogenously added CBP. Heterologous CBP enhanced Nrf2-induced transcription from this promoter (Fig. 1A); the action of CBP was completely abrogated by the adenovirus protein E1A, which also completely abrogated Nrf2-induced transcription, indicating involvement of E1A-binding protein(s) in the transcriptional action of Nrf2. This effect of E1A on Nrf2-induced transcription is consistent with at least two previous reports that ARE-mediated gene transcription is sensitive to E1A (41, 43). E1A binds to a variety of proteins including CBP/p300, and when bound to CBP or p300, it blocks the acetyltransferase activity of these proteins (44–48). It seems reasonable to conclude that the inhibitory action of E1A resulted not only from its inhibition of the acetyltransferase activity of CBP (45, 46) (Fig. 2A) but also from its binding to other proteins (44, 49).
FIGURE 1.
Effect of CBP on Nrf2-dependent gene transcription. A, Nrf2-dependent transcription from the Gαi2 gene promoter is enhanced by CBP and inhibited by E1A. K562 cells (1 × 105 cells in 1 ml of medium/well) seeded in 24-well plates for 24 h were co-transfected with 0.2 μg each of empty vector (pCI-Neo) or heterologous Nrf2 (pCI-Nrf2) as inducer and 0.2 μg of pGαi2(−1214/+115)-luc with or without expression plasmid for CBP (0.2 μg) or E1A (0.2 μg). The total amount of DNA in each well was 0.8 μg; the empty vector (pCMV5) was used to make up the total to this amount, as needed. The cells were harvested 24 h after transfection to measure promoter activity (28, 31, 32). The data are the means ± S.E. of duplicate assays from four separate cell cultures. *, significantly different (p < 0.02) from control (vector only); **, significantly different (p < 0.02) from the Nrf2 effect; ***, significantly different (p < 0.02) from Nrf2 + CBP. B, Nrf2-induced transcription from HO-1-ARE-luc and hNQO1-ARE-luc reporters is enhanced by CBP and inhibited by E1A. The HO-1-ARE-luc contains three copies of ARE (5′-CGGACCTTGACTCAGCAGAAAA-3′) cloned upstream of the mouse HO-1 minimal promoter (−32 to +72 bp) (29). The hNQO1-ARE-luc (human NAD(P)H:quinone oxidoreductase 1-ARE-luc) contains a single copy of ARE, derived from the human NQO1 promoter, placed upstream of a minimal promoter containing a TATA box fused to the luciferase gene (30). All of the plasmids were transfected at 0.2 μg each, and the total amount of DNA was adjusted to 0.8 μg as in A. The data are the means ± S.E. of duplicate assays from four separate cell cultures. *, significantly different (p < 0.05) from control (vector only); **, significantly different (p < 0.02) from Nrf2-induced activity; ***, significantly different (p < 0.01) from Nrf2 + CBP. C, whole cell content of Nrf2 in samples used for the luciferase assays shown in B. Western blotting analysis (10% SDS-PAGE) was performed with 15 μg of protein. Nrf2 was detected with anti-Nrf2 antibody (sc-13032; Santa Cruz Biotechnology, Inc.). A nonspecific band in the same blot was used as loading control. The Western blot shown is representative of three such blots from three different cell cultures.
FIGURE 2.
Nrf2 is acetylated in cells during Nrf2-induced gene transcription. K562 cells (8 × 105 in 8 ml of medium) were seeded in T25 flasks. After 24 h, the cells were transfected with expression plasmid for CBP (1.6 μg) with or without E1A (1.6 μg) (A) or SIRT1 (1.6 μg) with or without dominant negative SIRT1 (SIRT1-H355A) (1.6 μg) or treated with NAM (10 mm) (B), along with 0.2 μg of pCI-Nrf2 for 24 h. Cell cultures in A were treated with trichostatin A (66 nm) and NAM (10 mm) for 6 h before harvesting to inhibit deacetylase(s) (33–35). Acetylation was measured in whole cell lysates by a co-immunoprecipitation assay. The whole cell lysates (200 μg of protein) were first precipitated with anti-Nrf2 antibody (1 μg, sc-13032; Santa Cruz Biotechnology, Inc.), as described under “Experimental Procedures,” followed by Western blotting (WB) of the IP samples, using anti-acetyl-lysine antibody (antibody 9441; Cell Signaling Technology). The blots shown in A and B are representative of three different experiments. C, whole cell content of Nrf2 in CBP- and SIRT1-treated cells. Western blotting analysis (8% SDS-PAGE) was performed with 10 μg of protein. Nrf2 was detected with anti-Nrf2 antibody (sc-13032; Santa Cruz Biotechnology, Inc.). A nonspecific band in the same blot was used as loading control. The Western blot shown is representative of three such blots. D, SIRT1 inhibits Nrf2-induced transcription from prototypic ARE-driven reporter gene. K562 cells were transfected with 0.2 μg of the ARE-driven reporter minimal promoter HO-1-ARE-luc along with pCI-Nrf2 (0.2 μg) and plasmid harboring the cDNA for wild-type SIRT1 (0.2 μg) with or without dominant negative SIRT1 (SIRT1-H355A) (0.2 μg). The total amount of DNA in each well was 0.8 μg, made up by the addition of empty vector. The cells were harvested 24 h after transfection, and promoter activity was measured as in Fig. 1. The values plotted are the means ± S.E. of duplicate assays from four different cell cultures. *, significantly different (p < 0.05) from Nrf2 treatment.
The somewhat small increase in Nrf2-induced transcription (1.8-fold over basal) shown for the Gαi2 gene promoter (Fig. 1A) is a reflection of the reporter gene construct used. In our experience (32, 42), two widely used prototypic ARE-driven minimal promoters (HO-1-ARE-luc (29) and hNQO1-ARE-luc (30)) usually result in much higher fold increases in promoter activity than the Gαi2-luc used in Fig. 1A. This is evident from the data in Fig. 1B in which the use of the HO-1-ARE-luc resulted in promoter activity that was ∼4-fold over basal; with the hNQO1-ARE-luc reporter, Nrf2-induced transcription was 2–3-fold over basal. With these prototype ARE-driven reporter gene constructs, the CBP effect on Nrf2-induced transcription was much more robust than with the Gαi2-luc reporter, and with both prototype promoter constructs, promoter activity was completely abrogated by E1A (Fig. 1B), as with the Gαi2 gene promoter (Fig. 1A).
To rule out the possibility that the treatment with CBP or E1A may have altered the cellular content of Nrf2, we blotted for Nrf2 in whole cell lysates used for the luciferase assays in Fig. 1B. As shown in Fig. 1C, the whole cell content of Nrf2 was not changed by the treatment with CBP ± E1A. This indicates that their effects on transcription did not result from changes in the cellular amounts of Nrf2.
Nrf2 Is Acetylated in Cells
We investigated whether Nrf2 was acetylated under the conditions of the experiments shown in Fig. 1. To enhance detection of acetylated species, cell cultures were treated with trichostatin A and NAM 6 h before harvesting, to inhibit deacetylase(s) (33–35). Whole cell lysates were first immunoprecipitated with antibody to Nrf2 to generate IP samples; the IP samples were then used for Western blotting with anti-acetyl-lysine antibody. These co-immunoprecipitation assays (Fig. 2A) showed increases in acetylated Nrf2 protein in CBP-treated cells (compare lanes 2 and 1), and the increase was inhibited in the presence of E1A (lane 3).
To further demonstrate that Nrf2 is acetylatable, we measured its acetylation status in cells treated with expression plasmid for the NAD+-dependent Class III histone/protein deacetylase SIRT1. These cultures were not treated with either trichostatin A or NAM, so as not to interfere with deacetylation. As shown in Fig. 2B, treatment with expression plasmid for SIRT1 markedly diminished the endogenous acetylation of Nrf2 (Fig. 2B, compare lanes 2 and 1). This effect was overridden by co-transfection with plasmid harboring the cDNA for dominant negative SIRT1 (SIRT1-H355A (50, 51)) (Fig. 2B, compare lanes 3 and 2), indicating that the observed inhibitory effect was specific to SIRT1. The addition of NAM, a known inhibitor of SIRT1 (52), blocked SIRT1-induced deacetylation of Nrf2 (Fig. 2B, compare lanes 4 and 2).
In Fig. 2C, we show that the whole cell content of Nrf2 was not changed by the treatment with CBP (or CBP plus E1A) (lanes 2 and 3 versus lane 1 (control)) or the treatments with SIRT1, or NAM (lanes 4 and 5 versus lane 1). These results provide strong support for our interpretation that the band densities seen in Fig. 2 (A and B) resulted from changes in the acetylation status of Nrf2, not changes in the whole cell content of Nrf2.
To demonstrate that the deacetylation activity of SIRT1 impacted Nrf2-dependent gene transcription, we performed transfection assays to measure Nrf2-induced gene transcription in cells transfected with expression plasmid for SIRT1. Transfection of expression plasmid for SIRT1 markedly inhibited Nrf2-induced transcription from the prototype ARE-driven reporter gene construct HO-1-ARE-luc (Fig. 2D). This effect was overridden by dominant negative SIRT1 (SIRT1-H355A).
Effects of Small Molecule Modulators of SIRT1 on Transcriptional Activity of Nrf2
We extended the experiments in Fig. 2D by using small molecule modulators of SIRT1, the rationale being that small molecule inhibitors of SIRT1 (e.g. NAM and EX-527 (52–55)) would sustain acetylation conditions within the nucleus, whereas activators such as the phytochemical resveratrol (56–58) would cause deacetylation conditions.
At 0.1–1.0 μm, EX-527, a potent inhibitor of SIRT1 activity (53–55, 59), caused a dose-dependent increase in Nrf2-induced transcription from the prototype ARE-driven gene promoter construct HO-1-ARE-luc (Fig. 3A). NAM, a widely used inhibitor of SIRT1 activity (52–55), also induced an increase in Nrf2-dependent transcription from this promoter (Fig. 3B). Conversely, when the cells were treated for 90 min with resveratrol, which is arguably the most widely used small molecule activator of SIRT1, both tBHQ-induced and Nrf2-dependent gene transcription were drastically diminished, mimicking the effect of SIRT1. This is clearly evident from the data in Fig. 3 (C and D), in which three different ARE-driven gene promoter constructs (Gαi2-luc, HO-1-ARE-luc, and hNQO1-ARE-luc reporters) were used. The results are presented as relative promoter activity (i.e. activity in the presence of resveratrol as a percentage of activity in the absence of resveratrol). The pattern of inhibition of transcription by resveratrol was similar, irrespective of the reporter gene construct used. Although the resveratrol effect could be indirect, rather than directly targeting SIRT1 (58, 59), the fact that SIRT1 was inhibitory (Fig. 2D) implied that the observed inhibitory effect of resveratrol, at least in part, involved SIRT1. Taken together with the effects of EX-527, NAM, SIRT1, and CBP, these data lend support to the conclusion that acetylation-deacetylation regulates the transcriptional activity of Nrf2.
FIGURE 3.
Effect of small molecule modulators of SIRT1 on Nrf2-dependent gene transcription. In A, K562 cells were transfected with 0.2 μg of HO-1-ARE-luc reporter construct, along with 0.3 μg of pCI-Nrf2 or empty vector (pCI-Neo). After 24 h, various concentrations of EX-527 were added, and the cells were harvested for promoter analysis (28, 31, 32) 2 h later (left panel). In B, cells were treated with NAM (10 mm) at the time of transfection and harvested along with cells treated with EX-527. The data are the means ± S.E. of duplicate assays from three different cell cultures. In C, K562 cells transfected with 0.3 μg of Gαi2 gene promoter construct (pGαi2(−1214/+115)-luc) or 0.2 μg each of either HO-1-ARE-luc or hNQO1-ARE-luc reporter were treated with resveratrol 24 h after transfection, followed by tBHQ (20 μm) 30 min later. The cells were harvested for promoter analysis 60 min after the addition of tBHQ. In D, cells transfected with 0.2 μg each of empty vector pC1-Neo or heterologous Nrf2 (pCI-Nrf2) as inducer, along with 0.3 μg of pGαi2(−1214/+115)-luc, or 0.2 μg each of either HO-1-ARE-luc or hNQO1-ARE-luc reporter were treated with resveratrol 24 h after transfection. The cells were harvested for promoter activity 90 min after the addition of resveratrol. In C and D, the effects of resveratrol on promoter activities are presented as percentage plots, taking “no resveratrol treatment” as 100%. The values plotted are the means ± S.E. of duplicate assays from three or four experiments; error bars are not indicated if the number of experiments was less than three. Con, control.
Acetylation/Deacetylation Conditions Alter Nrf2 Binding to Its Cognate DNA Response Element, the ARE
We hypothesized that the results shown in Figs. 1–3 would reflect the binding of Nrf2 to its DNA response element, the ARE. To test this idea, we performed EMSAs, using a 31-mer 32P-labeled double-stranded DNA probe containing the ARE motif that maps at −85/−76 in the Gαi2 gene promoter (32). We have shown in previous gel shift studies that nuclear complexes formed with this probe contained Nrf2, as confirmed by supershift assays (32, 42). As shown in Fig. 4A, the band density of the complex formed by nuclear extracts with this probe was depressed by anti-Nrf2 antibody (lanes 6 and 7 versus lane 4), as well as by antibody to its binding partner (i.e. small Maf) (lanes 8 and 9 versus lane 4), indicating that Nrf2 was present in the complex. As controls, antibody to Sp1, which does not bind to the probe (42), had no effect (lane 10), and the unlabeled probe abolished the binding of nuclear proteins to the labeled DNA (lane 11).
FIGURE 4.
EMSA of Nrf2-DNA binding activity of nuclear extracts. A, K562 cells were transfected with pCI-Nrf2 or pCI-Neo (control). After 24 h the cells were incubated for 1 h with or without tBHQ (20 μm) in the absence or presence of resveratrol (50 μm). B, cells were co-transfected with expression plasmid for CBP with or without expression plasmid for E1A; the cells were harvested after 24 h. For both A and B, nuclear extracts were prepared and used for EMSA as described previously (20, 28, 32), using 32P-labeled double-stranded DNA probe 5′-GCCCGCCCCGGCCCAGTCACAGGCTTGGTTC-3′, which contains the ARE (underlined) motif that maps at −84/−76 in the Gαi2 gene promoter. The reactions were carried out with 2 μg of nuclear extract protein for each lane. When antibodies were used, the nuclear extract was incubated with the labeled probe for 30 min at 25 °C prior to the addition of each antibody and then incubated for an additional 30 min, followed by electrophoresis. Antibodies against Nrf2 and small Maf (Maf F/G/K (C-18) from Santa Cruz Biotechnology, Inc.) were used at 2 or 4 μg for lanes 6 and 7 and at 1 or 2 μg for lanes 8 and 9, respectively. Anti-Sp1 antibody was used at 2 μg. Nrf2-DNA binding complex is indicated by the arrow. Unlabeled probe (lane 11) was used at 20-fold excess. Ab, antibody; NE, nuclear extract; pCI-Neo, empty vector; pCI-Nrf2, expression plasmid for Nrf2.
Consistent with our hypothesis, we noted that nuclear extracts from cells treated with tBHQ plus resveratrol (to impart deacetylation conditions) exhibited less binding activity to the probe than nuclear extracts from cells treated with tBHQ alone (Fig. 4A, compare lanes 5 and 4). Furthermore, the binding intensity was greater in nuclear extracts from cells transfected with expression plasmid for the acetyltransferase CBP than under basal conditions (Fig. 4B, compare lanes 2 and 1). The CBP-induced binding activity was completely abrogated in the presence of E1A (Fig. 4B, compare lanes 3 and 2). The E1A effect mimicked the result obtained with resveratrol in Fig. 4A, indicating that deacetylation conditions, or inhibition of acetylation, resulted in decreased interaction of Nrf2 with its DNA response element, the ARE.
Acetylation Sites in Nrf2
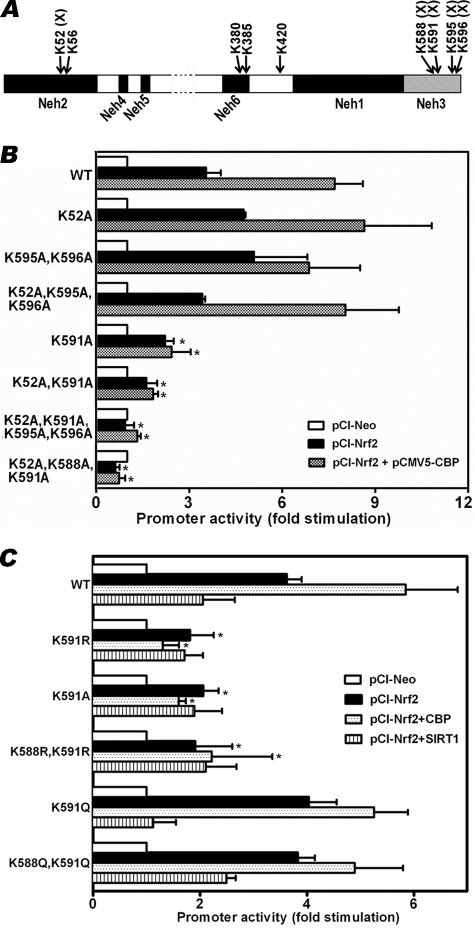
Having demonstrated that Nrf2 is acetylatable in the intact cell (Fig. 2) and that acetylation-deacetylation impacts binding of Nrf2 to its DNA response element (Fig. 4), we used in silico and mutagenesis approaches to reveal acetylatable sites in Nrf2 that might be relevant to the results shown in Figs. 1–4. Because Sun et al. (26) recently identified acetylatable lysyl residues in the Neh1 domain, which influenced transactivation potential of Nrf2, we restricted our search to other domains. We found nine putative acetylatable lysyl residues in these domains in mouse Nrf2 (Fig. 5A) by using the Prediction of Acetylation on Internal Lysines (PAIL) program, which predicts acetylation sites in proteins (60). These sites are conserved among 16 different species, including humans. We considered the Neh3 domain to be most interesting because of the clustering of four of these putative sites in that domain. We were also interested in the Neh3 domain because previous structural studies on Nrf2 (8, 61) had not clearly delineated the functional significance of this domain.
FIGURE 5.
Mutation (Lys → Ala or Lys → Arg) of Lys591 and Lys588 residues in the Neh3 domain of Nrf2 impairs Nrf2-dependent transactivation of ARE-driven reporter gene. A, schematic of putative acetylation sites outside the Neh1 domain of Nrf2, identified with the acetylation site prediction algorithm PAIL (60). Lysine to alanine substitution mutations were created at five of these sites (×) by using the QuikChangeTM site-directed mutagenesis kit from Stratagene (La Jolla, CA), and the mutants were used for the transfection experiments summarized in B. In separate experiments, lysine to arginine (Lys → Arg) or lysine to glutamine (Lys → Gln) substitution mutations were created at Lys588 and Lys591 and used for the transfection experiments summarized in C. Transactivation activities of WT and mutant Nrf2 were assessed in transient transfection assays using HO-1-ARE-luc as the reporter construct (42). The values shown are the means ± S.E. of triplicate assays from four (B) or five (C) different experiments. *, significantly different (p < 0.05) from the WT.
In the first series of experiments, we mutated (Lys → Ala) five of the nine PAIL program-predicted sites (Fig. 5A, mutated Lys residues are identified with the symbol ×). We then monitored Nrf2-induced gene transcription using these mutants. To ascertain whether any of these sites was relevant to the observed effect of CBP, we assessed Nrf2-dependent gene transcription in the absence or presence of CBP. As shown in Fig. 5B, mutant K591A resulted in robust inhibition (>52%) of Nrf2-dependent gene transcription, indicating the importance of Lys591 in the transcriptional activity of Nrf2. The inhibitory effect of mutating Lys591 could be further increased by additional mutation at Lys588, suggesting that Lys588 may also be important. Mutating three other putative acetylation sites (Lys595 and Lys596 in the Neh3 domain and Lys52 in the Neh2 domain) had no effects on Nrf2-dependent gene transcription. However, combining mutation(s) at these sites with mutation at Lys591 always resulted in potent inhibition of promoter activity, further emphasizing the importance of Lys591 in the transcriptional activity of Nrf2. Compared with the wild-type Nrf2, there was little or no effect of CBP on reporter gene activity when the Lys591 locus was mutated to alanine along with Lys52, Lys52 and Lys588, or the Lys595 and Lys596 loci. Together, these data strongly suggest that Lys591 and perhaps Lys588 are CBP-acetylatable sites.
In additional experiments, we made conservative mutations (Lys → Arg) at some of these sites and compared their effects with the Lys → Ala mutations. In this second set of mutations, we also made acetylation mimics (Lys → Gln) at the same lysine residues that were mutated to arginine residues. The Lys → Gln substitution is often used to mimic the acetylated form of the Lys residue (62–66). As shown in Fig. 5C, lysine to arginine mutation at Lys591 (K591R) resulted in robust inhibition of Nrf2-dependent gene transcription, much like the results obtained with K591A mutants. The results with the double mutant K588R/K591R were essentially similar to those with the single mutant K591R. Similar to K591A and K588A/K591A mutants and unlike the wild type, promoter activities induced by Lys → Arg (K591R and K588R/K591R) mutants were insensitive to the activating effect of CBP, confirming that Lys588 and Lys591 are CBP-acetylatable sites. Based on the data with CBP, the results with the acetylation mimics (K591Q and K588Q/K591Q) resembled the results with wild-type Nrf2.
In Fig. 5C, the lack of effect of SIRT1 on K591R, K591A, and K588R/K591R resembles the data with CBP and supports the idea that these sites are acetylatable sites. However, SIRT1 inhibited the transcriptional effect of the K591Q single mutant, and the K588Q/K591Q double mutant, similar to the wild type. This was rather surprising, given the fact that the K591Q and K588Q/K591Q sites cannot be deacetylated. Conceptually, the Lys → Gln substitution is understood to mimic the acetylated form of the Lys residue, whereas the Lys → Arg substitution only eliminates the ϵ-amino group of the acetylation site without a neutralization of positive charges (62–66). However, the Lys → Gln substitution does not always faithfully mimic the acetylated form of the Lys residue. For example, in their studies on DNA damage-induced acetylation of lysine 3016 of ataxia-telangiectasia mutated kinase, a modification that activates ataxia-telangiectasia mutated kinase, Sun et al. (65) found that glutamine did not substitute for acetyl-lysine in ataxia-telangiectasia mutated kinase. In another example, Manohar et al. (66) showed in studies on nucleosome dyad acetylation that the Lys → Gln substitution did not fully replicate the role of lysine acetylation; they found that the Lys → Gln substitution mimicked the change in charge but was a very poor mimic of the steric effect of acetylation. Thus, our findings may indicate that in addition to deacetylation of wild-type Nrf2, thereby inhibiting its transcriptional activity, SIRT1 expression may also affect Nrf2 activity by an additional mechanism when acetylatable lysines are mutated to glutamine.
Impact of Acetylation-Deacetylation on Nucleocytoplasmic Localization of Nrf2
Given that subcellular localization is a major determining factor of the function of Nrf2, we used several approaches to study additional functional impact of acetylation/deacetylation of Nrf2, vis à vis its subcellular localization. First, we used fluorescence imaging to localize the fusion protein GFP-Nrf2 (20) in cells treated with reagents that impart either acetylation or deacetylation. Second, we used Western blotting to assess the content of Nrf2 in nuclear and cytoplasmic fractions prepared from similarly treated cells. Third, we used site-directed mutagenesis to assess the localization of GFP-Nrf2 mutated at key acetylatable lysine residues.
HepG2 cells were used for fluorescence imaging because, unlike K562 cells, the cytoplasmic and nuclear compartments of HepG2 cells are easily discernible (20). In the first approach, the cells were transfected with expression plasmid encoding the fusion protein GFP-Nrf2 (20). After 24 h, the cells were incubated with tBHQ for 1 h to induce nuclear accumulation of Nrf2 in the absence or presence of resveratrol (Fig. 6A). Consistent with the fact that tBHQ induces nuclear accumulation of Nrf2 (2, 32), incubation with tBHQ induced intense GFP fluorescence in the nucleus (Fig. 6A, compare columns 3 and 4 to columns 1 and 2). In contrast, in cells incubated with tBHQ plus resveratrol (columns 7 and 8), GFP fluorescence in the nucleus (caused by GFP-Nrf2) was severely diminished, and most of the green fluorescence was cytoplasmic. Also in cells incubated with resveratrol alone (no tBHQ, columns 5 and 6), most of the GFP fluorescence was concentrated outside the nucleus, indicating that the resultant effect of resveratrol was re-distribution of GFP-Nrf2 between the nuclear and cytoplasmic compartment, in favor of nuclear exclusion of Nrf2.
FIGURE 6.
Nucleocytoplasmic localization of Nrf2 in cells, as assessed by fluorescence imaging. HepG2 cells were grown on coverslips in six-well plates to 50–80% confluence, then transfected with plasmid harboring cDNA for EGFP linked to the coding sequence for Nrf2 (EGFP-Nrf2, also designated GFP-Nrf2) (20), and then examined by wide field microscopy. A, effect of resveratrol on tBHQ-induced GFP-Nrf2 localization. Resveratrol (50 μm) and/or tBHQ (20 μm) were/was added to some cells 24 h after transfection. In cells treated with both resveratrol and tBHQ, resveratrol was added 30 min before the addition of tBHQ. All of the cells were harvested 1 h after the addition of tBHQ and processed for fluorescence imaging (20). Propidium iodide (red) was used to counterstain the nuclei. The data presented are representative of three experiments. B, E1A induces cytoplasmic localization of GFP-Nrf2 in cells treated with CBP. HepG2 cells grown as described in A were transfected with 1.5 μg of GFP-Nrf2 (panel a) along with an expression plasmid for the acetyltransferase CBP (0.25 μg) in the absence (panel b) or presence of an expression plasmid for E1A (0.25 μg) (panel c) or E1A plus LMB (panel d). The cells were harvested 24 h after transfection and processed for fluorescence imaging (20). LMB (10 ng/ml) was added 30 min before harvest. The data presented are representative results from three or four separate cultures. C, CBP increases nuclear fluorescence of GFP-Nrf2 compared with basal. Quantification of green fluorescence intensity in B was performed as described previously (20), using Nikon Elements Advanced Research Software (Melville, NY). ResV, resveratrol.
If the nuclear exclusion of GFP-Nrf2 observed in cells treated with resveratrol resulted from resveratrol-induced deacetylation of Nrf2, then mimicking deacetylation by other means, such as by inhibiting the acetyltransferase activity of CBP, should also yield similar results as with resveratrol. As shown in Fig. 6B, treatment with CBP altered the nuclear-cytoplasmic distribution of GFP-Nrf2 such that the fluorescence signal in the nucleus was more intense than in control cells (compare panel b to panel a). The presence of E1A (panel c) reversed this distribution to that observed in control cells. Quantification of these data (Fig. 6C) indicated a distribution ratio of nuclear:cytoplasmic content of GFP-Nrf2 of 65:35 in cells treated with CBP compared with 38:62 in the controls (basal). In cells treated with E1A or CBP plus E1A, the ratio was identical to that in the basal condition.
Detection of GFP-Nrf2 in both the cytoplasm and nucleus of CBP-treated cells and in cells treated with CBP + E1A suggested that these treatments did not prevent nuclear-cytoplasmic shuttling of Nrf2, which is known to contain two nuclear export sequences (67, 68). This suggestion was confirmed by the fact that when we used leptomycin B (LMB) to block nuclear export of Nrf2, the E1A-induced cytoplasmic localization of GFP-Nrf2 was abolished (Fig. 6, B, compare panels d and c, and C). LMB interferes with the CRM1 export machinery (69, 70) that recognizes the nuclear export sequences in nuclear export sequence-containing proteins (71). In our experiments, LMB alone caused an almost total (∼90%) nuclear retention of GFP-Nrf2 (data not shown), indicating that it blocked nuclear export of GFP-Nrf2. Overall, the data in Fig. 6 suggest that deacetylation, or the inhibition of CBP-mediated acetylation, results in relocalization of GFP-Nrf2 to the cytoplasmic compartment.
In the second approach to assess the nucleocytoplasmic redistribution of Nrf2 under conditions of acetylation-deacetylation, we used Western blotting to determine the relative levels of Nrf2 in cytoplasmic and nuclear fractions from K562 cells transfected with the expression plasmid for SIRT1 or cells incubated with EX-527, a potent small molecule inhibitor of SIRT1. In addition, to mimic the experimental set-up for Fig. 6A, the effect of resveratrol (a putative stimulator of SIRT1 activity) on tBHQ-induced nuclear accumulation of Nrf2 (2, 32) was also assessed. The whole cell content of Nrf2 was not affected by these treatments (Fig. 7A; see also Fig. 2C).
FIGURE 7.
Nucleocytoplasmic distribution of Nrf2 in cells, as assessed by Western blotting. K562 cells (4 × 106/8 ml in T25 flask) were transfected with 4 μg each of expression plasmid for Nrf2 (pCI-Nrf2) or the empty vector (pCI-Neo) along with expression plasmid for SIRT1 (4 μg) with or without dominant negative SIRT1 (SIRT1-H355A) (4 μg) or treated with tBHQ (20 μm) for 1 h in the absence or presence of small molecule modulators (1 μm EX-527 or 50 μm resveratrol) of SIRT1. When used, EX-527 or resveratrol was added 30 min before the addition of tBHQ. Western blotting analysis (8% SDS-PAGE) was performed with 10 μg of protein. Cytoplasmic and nuclear fractions were prepared as described previously (32). Nrf2 was detected with anti-Nrf2 antibody (sc-13032; Santa Cruz Biotechnology, Inc.). A, total cell content of Nrf2 is not changed by small molecule modulators of SIRT1. B, assessment of relative purity of cytoplasmic and nuclear fractions, using antibodies against protein markers for these fractions. Markers for nuclear (p300/CβP-associated factor) and cytoplasmic (β-tubulin) fractions were used to assess the degree of potential cross-contamination between the two fractions. C and D, relative content of Nrf2 in nuclear (upper panels) and cytoplasmic fractions (lower panels) from cells treated with small molecule modulators of SIRT1 (C) or expression plasmid for SIRT1 (D). Western blotting analysis (8% SDS-PAGE) was performed with 10 μg of protein for each fraction. Quantification of the Western blots was done by densitometric scanning using UN-SCAN-IT software (Silk Scientific, Inc, Orem, UT). The results were calculated relative to the corresponding loading controls. The ratios obtained are plotted as histograms (three or four different experiments), setting the ratios obtained for the empty vector treatment as 100%. D, SIRT1-induced changes in the nucleocytoplasmic distribution of Nrf2. C, cytoplasmic fraction; W, whole cell lysate; N, nuclear fraction; Neo, empty vector (pCI-Neo); Nrf2, pCI-Nrf2; ResV, resveratrol; dnSIRT1, dominant negative SIRT1.
The Western blot shown in Fig. 7B is an indicator of the general quality of our nuclear and cytoplasmic preparations, as judged by the use of nuclear (p300/CβP-associated factor) and cytoplasmic (β-tubulin) markers. As shown in Fig. 7C, resveratrol, a putative activator of SIRT1, decreased tBHQ-induced nuclear accumulation of Nrf2 by ∼73%, but EX-527, an inhibitor of SIRT1 activity, actually induced a modest increase. The resveratrol-induced 73% decrease in Nrf2 level in the nuclear fraction was accompanied by a 27% increase in the cytoplasmic fraction. On the other hand, the EX-527-induced modest increase in Nrf2 level in the nucleus was accompanied by a 10% decrease in Nrf2 level in the cytoplasmic fraction. Thus, the effects of resveratrol on cytoplasmic and nuclear levels of Nrf2 levels were clearly opposite to those of EX527. These reciprocal relationships suggest that treatment with these small molecule modulators of SIRT1 altered nucleocytoplasmic localization of Nrf2.
Given that EX-527 and resveratrol have opposing effects on the deacetylase SIRT1, we further investigated the nucleocytoplasmic redistribution of Nrf2 by transfecting the cells with an expression plasmid for SIRT1. Transfection with SIRT1 resulted in decreased content of Nrf2 in the nuclear fraction (Fig. 7D, upper panel), and this effect was overridden by dominant negative SIRT1 (SIRT1-H355A). The decreased content of Nrf2 in the nucleus in cells transfected with SIRT1 was accompanied by an increased content of Nrf2 in the cytoplasmic fraction compared with cells treated with Nrf2 alone (Fig. 7D, lower panel). These results mimic those obtained with resveratrol. Overall these immunoblotting data are consistent with our fluorescence imaging data (Fig. 6) and suggest that deacetylation results in relocalization of GFP-Nrf2 to the cytoplasmic compartment, whereas acetylation conditions favor its nuclear retention.
In the third approach to assess the impact of acetylation-deacetylation on subcellular localization of Nrf2, we used fluorescence imaging and confocal microscopy to localize GFP-Nrf2 molecules that were mutated at key acetylatable lysine residues in the Neh3 domain. As shown in Fig. 8, wild-type GFP-Nrf2 (panel a) exhibited intense green fluorescence in the nucleus, whereas green fluorescence exhibited by K591R mutants (panel b) was dispersed all over the cell. The double mutant K588R/K591R (panel d) exhibited even less nuclear fluorescence than the K591R alone. In both cases, it is clear that mutation at these sites engendered an even distribution of GFP fluorescence throughout the cell, indicating relocalization of GFP-Nrf2 to the cytoplasmic compartment. In contrast, both the single Lys → Gln mutant (K591Q) (panel c) and the double mutant K588Q/K591Q (panel e) exhibited a pattern of green fluorescence that mimicked that of the wild-type GFP-Nrf2. Altogether, these data strongly suggest that deacetylation (i.e. Lys → Arg mutants) results in relocalization of GFP-Nrf2 to the cytoplasmic compartment, a phenomenon consistent with our Western blotting data in Fig. 7 as well as the imaging data in Fig. 6, whereas acetylation conditions favor its nuclear retention.
FIGURE 8.
Mutation (Lys → Arg) of Lys591 and Lys588 residues in the Neh3 domain of Nrf2 alters nucleocytoplasmic localization of GFP-Nrf2. HepG2 cells grown on coverslips in six-well plates to 50–80% confluence, as in the legends to Fig. 6, were transfected with wild-type pEGFP-Nrf2 or Lys → Arg or Lys → Gln mutants described in the legends to Fig. 5. The cells were processed for fluorescence imaging analysis as described under “Experimental Procedures.” To stain the nuclei, the cells were incubated for 2 min at room temperature in 3 μg/ml propidium iodide and then rinsed with PBS. The coverslips were then mounted onto the slides using Aqua/Polymount (Polysciences, Inc. Warrington, PA), kept overnight at 4 °C, and visualized under a Nikon TE2000-U C1 confocal laser scanning microscope at excitation/emission wavelengths of 488/505–550 nm (for green fluorescence) and 543/560–615 nm (for red fluorescence). Using Adobe Photoshop, images of the propidium iodide (PI) and GFP fluorescence patterns were merged to visualize nuclear localization.
DISCUSSION
Covalent modifications that may impact the biology of Nrf2 after its separation from Keap1 have not been extensively studied. Here we show that Nrf2 can undergo acetylation-deacetylation, corroborating a recent report (26) that measured reversible acetylation of Nrf2 (primarily in its Neh1 domain) in HEK293T cells subjected to sodium arsenite [As(III)]-induced stress. We also show that acetylation conditions (use of CBP or use of inhibitors of SIRT1 activity) enhance binding of Nrf2 to its DNA response element (the ARE), thereby increasing Nrf2-induced gene transcription. Deacetylation conditions (use of activators of SIRT1) resulted in a decrease in Nrf2-dependent gene transcription. Use of activators of SIRT1 or use of acetylation mutants (Lys → Arg) of Nrf2 resulted in redistribution of Nrf2 in favor of relocalization to the cytoplasmic compartment. Use of E1A to inhibit the action of CBP also resulted in redistribution of Nrf2 in favor of relocalization to the cytoplasmic compartment. To our knowledge, the present work is the first study demonstrating that acetylation/deacetylation conditions modulate nucleocytoplasmic localization of Nrf2.
The Neh1 domain of Nrf2 is responsible for mediating binding to the ARE (3, 8), but the contribution of the Neh3 domain to the transactivation potential of Nrf2 has only been described as permissive (73), based on experiments that deleted the last 16 amino acid residues in the carboxyl terminus of that domain (74). Mutational analysis of the Neh1 domain led Sun et al. (26) to conclude that acetylation-dependent modulation of the transcriptional activity of Nrf2 is primarily due to the lysyl residues in the Neh1 domain. Inspection of their data, however, indicates that only a 40–45% decrease in Nrf2-induced transcription was achieved by mutating all 18 Lys residues in that domain (26). We show here that lysyl residues elsewhere in the protein are relevant to acetylation-dependent regulation of the transactivation potential of Nrf2.
The computer program PAIL (60) predicts nine putative acetylation sites outside the Neh1 domain in mouse Nrf2 (depicted in Fig. 5A). Using the HO-1 gene reporter construct, we show that mutating the lysine residues at positions 588 and 591 in the Neh3 domain to either alanine or arginine drastically (>52%) impaired Nrf2-dependent gene transcription. Furthermore, such mutations completely obliterated the ability of CBP to enhance Nrf2-induced gene transcription that was observed with the wild-type Nrf2, an indication that those sites are targets for CBP. These results are distinctly different from those of Sun et al. (26), who studied the impact of acetylation in the Neh1 domain, by p300 acetyltransferase after introducing Lys → Arg mutations in that domain. Interestingly, they also found that such mutations did not influence transcription from the HO-1 gene, leading them to conclude that Nrf2-induced transcription of the HO-1 gene is not regulated by acetylation. In contrast, our results show that mutating (Lys → Ala or Lys → Arg) two acetylatable lysyl residues (Lys588 and Lys591) in the Neh3 domain impairs Nrf2-dependent transcription of the HO-1 gene. The differences between our results and those of Sun et al. (26) most likely reflect domain-specific effects. More importantly, they emphasize that multiple acetylation sites in multiple structural domains of Nrf2 impact the transcriptional activity of Nrf2.
Our results here are particularly novel because they reveal, for the first time, that the Neh3 domain, the function of which had previously been suggested as only permissive (73), actually modulates the transcriptional activity of Nrf2 through acetylation-dependent regulation that can be ascribed to Lys591 and perhaps Lys588 located in that domain. Thus, Neh3 can now unequivocally be regarded, alongside Neh4 and Neh5 (41), as making an important contribution to the transactivation potential of Nrf2.
That deacetylation favors cytoplasmic localization of Nrf2 is quite intriguing in the context of potential mechanism(s) for terminating the transcriptional activity of Nrf2. Brown et al. (75) demonstrated that TGFβ-induced activation of ATF3 caused ATF3 to displace CBP from the CBP-Nrf2 complex at the ARE, leading to repression of Nrf2-induced transcription. Kong and co-workers (76) showed that sustained interaction between Nrf2 and small Maf proteins prevented nuclear exit of Nrf2; they suggested that this interaction masks the nuclear export signals in Nrf2, preventing Nrf2 from interacting with the CRM1 export machinery. However, their data do not rule out other mechanisms such as covalent modifications. In a totally unrelated system, Soutoglou et al. (77) demonstrated that acetylation of HNF-4 by CBP is crucial for the proper nuclear retention of HNF-4, which is otherwise transported out to the cytoplasm via the CRM1 pathway. Recently, Lan et al. (78) showed that SIRT1-dependent deacetylation of the serine-threonine protein kinase LKB1 resulted in its relocalization to the cytoplasm. Deacetylation of NF-κB by histone deacetylase 3 decreases its transcriptional activity and results in its nuclear export through the CRM1 pathway (36, 79). Thus, it is intriguing to speculate that deacetylation of Nrf2 could cause or facilitate exposure of the relevant nuclear export signal, allowing interaction of Nrf2 with the CRM1 export machinery, leading to subsequent relocalization of Nrf2 to the cytoplasmic compartment. However, SIRT-dependent deacetylation of transcription factors does not always lead to their cytoplasmic localization. This is clearly evident from the report that SIRT-dependent deacetylation of the forkhead transcription factor FoxO1, a phenomenon that promotes expression of gluconeogenic genes, results in nuclear trapping of FoxO1 (80).
The work presented here provides insight into the question of mechanisms of the transcriptional action of Nrf2 and termination of such activity. We posit that Nrf2 in the nucleus undergoes acetylation by acetyltransferase(s) such as CBP, resulting in binding, with bZIP protein(s), to the ARE, and consequently in gene transcription. Inhibition of acetylation results in decreased Nrf2-mediated gene transcription. Deacetylation of key lysine residues in Nrf2 (for example, by SIRT1) could disengage Nrf2 from its DNA-binding partner(s) and/or the ARE, thereby resulting in transcriptional termination and subsequently in nuclear export of deacetylated Nrf2 through the CRM1 export machinery. Our fluorescence imaging data, as well as our immunoblotting results with SIRT1 and those with modulators of SIRT1, are consistent with this idea.
Acknowledgments
We thank Dr. Gerd Blobel (Children's Hospital, Philadelphia, PA) for providing us with plasmid harboring the cDNA for CBP, Dr. Richard Hanson (Case Western Reserve University School of Medicine, Cleveland, OH) for providing us with plasmid harboring the cDNA for E1A, and Dr. Pere Puigserver (Harvard Medical School, Boston, MA) for gifts of expression plasmids for SIRT1 and dominant negative SIRT1 (SIRT1-H355A).
This work was supported, in whole or in part, by National Institutes of Health Grants SO6-GM08037 and SC1CA143985. This work was also supported by the Meharry Medical College Morphology Core Laboratory, which is supported in part by National Institutes of Health Grants U54NS041071, U54CA9140, U54RR026140, and G12RR03032. This work was presented in poster form at the Experimental Biology Meeting, April 18–22, 2009, New Orleans, LA (Garduño, L., Theodore, M., Kawai, Y., and Arinze, I. J. (2009) FASEB J. 23, A495.13).
- ARE
- antioxidant response element
- CREB
- cAMP-responsive element-binding protein
- CBP
- CREB-binding protein
- CRM1
- chromosome region maintenance-1 protein (also called exportin-1)
- EGFP
- enhanced green fluorescent protein
- EX-527
- 6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide
- LMB
- leptomycin B
- NAM
- nicotinamide
- SIRT1
- silent information regulator of transcription 1 (mammalian ortholog of yeast sir2, also known as mammalian sirtuin 1)
- tBHQ
- tert-butylhydroquinone
- PAIL
- Prediction of Acetylation on Internal Lysines
- bZIP
- basic-region leucine zipper.
REFERENCES
- 1. Kwak M. K., Wakabayashi N., Itoh K., Motohashi H., Yamamoto M., Kensler T. W. (2003) J. Biol. Chem. 278, 8135–8145 [DOI] [PubMed] [Google Scholar]
- 2. Nguyen T., Sherratt P. J., Pickett C. B. (2003) Annu. Rev. Pharmacol. Toxicol. 43, 233–260 [DOI] [PubMed] [Google Scholar]
- 3. Motohashi H., Yamamoto M. (2004) Trends Mol. Med. 10, 549–557 [DOI] [PubMed] [Google Scholar]
- 4. Jaiswal A. K. (2004) Free Radic. Biol. Med. 36, 1199–1207 [DOI] [PubMed] [Google Scholar]
- 5. Wang X. J., Sun Z., Villeneuve N. F., Zhang S., Zhao F., Li Y., Chen W., Yi X., Zheng W., Wondrak G. T., Wong P. K., Zhang D. D. (2008) Carcinogenesis 29, 1235–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayes J. D., McMahon M. (2009) Trends Biochem. Sci. 34, 176–188 [DOI] [PubMed] [Google Scholar]
- 7. Kensler T. W., Wakabayashi N. (2010) Carcinogenesis 31, 90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J. D., Yamamoto M. (1999) Genes Dev. 13, 76–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kang M. I., Kobayashi A., Wakabayashi N., Kim S. G., Yamamoto M. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 2046–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watai Y., Kobayashi A., Nagase H., Mizukami M., McEvoy J., Singer J. D., Itoh K., Yamamoto M. (2007) Genes Cells 12, 1163–1178 [DOI] [PubMed] [Google Scholar]
- 11. Kobayashi A., Kang M. I., Watai Y., Tong K. I., Shibata T., Uchida K., Yamamoto M. (2006) Mol. Cell. Biol. 26, 221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang H. C., Nguyen T., Pickett C. B. (2002) J. Biol. Chem. 277, 42769–42774 [DOI] [PubMed] [Google Scholar]
- 13. Bloom D. A., Jaiswal A. K. (2003) J. Biol. Chem. 278, 44675–44682 [DOI] [PubMed] [Google Scholar]
- 14. Cullinan S. B., Gordan J. D., Jin J., Harper J. W., Diehl J. A. (2004) Mol. Cell. Biol. 24, 8477–8486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang D. D., Lo S. C., Cross J. V., Templeton D. J., Hannink M. (2004) Mol. Cell. Biol. 24, 10941–10953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang D. D., Lo S. C., Sun Z., Habib G. M., Lieberman M. W., Hannink M. (2005) J. Biol. Chem. 280, 30091–30099 [DOI] [PubMed] [Google Scholar]
- 17. Furukawa M., Xiong Y. (2005) Mol. Cell. Biol. 25, 162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hong F., Sekhar K. R., Freeman M. L., Liebler D. C. (2005) J. Biol. Chem. 280, 31768–31775 [DOI] [PubMed] [Google Scholar]
- 19. Jain A. K., Bloom D. A., Jaiswal A. K. (2005) J. Biol. Chem. 280, 29158–29168 [DOI] [PubMed] [Google Scholar]
- 20. Theodore M., Kawai Y., Yang J., Kleshchenko Y., Reddy S. P., Villalta F., Arinze I. J. (2008) J. Biol. Chem. 283, 8984–8994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nguyen T., Sherratt P. J., Nioi P., Yang C. S., Pickett C. B. (2005) J. Biol. Chem. 280, 32485–32492 [DOI] [PubMed] [Google Scholar]
- 22. Nguyen T., Nioi P., Pickett C. B. (2009) J. Biol. Chem. 284, 13291–13295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salazar M., Rojo A. I., Velasco D., de Sagarra R. M., Cuadrado A. (2006) J. Biol. Chem. 281, 14841–14851 [DOI] [PubMed] [Google Scholar]
- 24. Apopa P. L., He X., Ma Q. (2008) J. Biochem. Mol. Toxicol. 22, 63–76 [DOI] [PubMed] [Google Scholar]
- 25. Jain A. K., Jaiswal A. K. (2007) J. Biol. Chem. 282, 16502–16510 [DOI] [PubMed] [Google Scholar]
- 26. Sun Z., Chin Y. E., Zhang D. D. (2009) Mol. Cell. Biol. 29, 2658–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davis M. G., Kawai Y., Arinze I. J. (2000) Biochem. J. 346, 455–461 [PMC free article] [PubMed] [Google Scholar]
- 28. Yang J., Kawai Y., Hanson R. W., Arinze I. J. (2001) J. Biol. Chem. 276, 25742–25752 [DOI] [PubMed] [Google Scholar]
- 29. Papaiahgari S., Kleeberger S. R., Cho H. Y., Kalvakolanu D. V., Reddy S. P. (2004) J. Biol. Chem. 279, 42302–42312 [DOI] [PubMed] [Google Scholar]
- 30. Moehlenkamp J. D., Johnson J. A. (1999) Arch. Biochem. Biophys. 363, 98–106 [DOI] [PubMed] [Google Scholar]
- 31. Arinze I. J., Kawai Y. (2003) J. Biol. Chem. 278, 17785–17791 [DOI] [PubMed] [Google Scholar]
- 32. Arinze I. J., Kawai Y. (2005) J. Biol. Chem. 280, 9786–9795 [DOI] [PubMed] [Google Scholar]
- 33. Luo J., Nikolaev A. Y., Imai S., Chen D., Su F., Shiloh A., Guarente L., Gu W. (2001) Cell 107, 137–148 [DOI] [PubMed] [Google Scholar]
- 34. Li K., Casta A., Wang R., Lozada E., Fan W., Kane S., Ge Q., Gu W., Orren D., Luo J. (2008) J. Biol. Chem. 283, 7590–7598 [DOI] [PubMed] [Google Scholar]
- 35. Rajamohan S. B., Pillai V. B., Gupta M., Sundaresan N. R., Birukov K. G., Samant S., Hottiger M. O., Gupta M. P. (2009) Mol. Cell. Biol. 29, 4116–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen L. F., Fischle W., Verdin E., Greene W. C. (2001) Science 293, 1653–1657 [DOI] [PubMed] [Google Scholar]
- 37. Ceseña T. I., Cardinaux J. R., Kwok R., Schwartz J. (2007) J. Biol. Chem. 282, 956–967 [DOI] [PubMed] [Google Scholar]
- 38. Bannister A. J., Kouzarides T. (1996) Nature 384, 641–643 [DOI] [PubMed] [Google Scholar]
- 39. Yang X. J., Ogryzko V. V., Nishikawa J., Howard B. H., Nakatani Y. (1996) Nature 382, 319–324 [DOI] [PubMed] [Google Scholar]
- 40. Goodman R. H., Smolik S. (2000) Genes Dev. 14, 1553–1577 [PubMed] [Google Scholar]
- 41. Katoh Y., Itoh K., Yoshida E., Miyagishi M., Fukamizu A., Yamamoto M. (2001) Genes Cells 6, 857–868 [DOI] [PubMed] [Google Scholar]
- 42. Kawai Y., Arinze I. J. (2006) Cancer Res. 66, 6563–6569 [DOI] [PubMed] [Google Scholar]
- 43. Orino K., Tsuji Y., Torti F. M., Torti S. V. (1999) FEBS Lett. 461, 334–338 [DOI] [PubMed] [Google Scholar]
- 44. Flint J., Shenk T. (1997) Annu. Rev. Genet. 31, 177–212 [DOI] [PubMed] [Google Scholar]
- 45. Chakravarti D., Ogryzko V., Kao H. Y., Nash A., Chen H., Nakatani Y., Evans R. M. (1999) Cell 96, 393–403 [DOI] [PubMed] [Google Scholar]
- 46. Hamamori Y., Sartorelli V., Ogryzko V., Puri P. L., Wu H. Y., Wang J. Y., Nakatani Y., Kedes L. (1999) Cell 96, 405–413 [DOI] [PubMed] [Google Scholar]
- 47. Perissi V., Dasen J. S., Kurokawa R., Wang Z., Korzus E., Rose D. W., Glass C. K., Rosenfeld M. G. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 3652–3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Forsberg E. C., Johnson K., Zaboikina T. N., Mosser E. A., Bresnick E. H. (1999) J. Biol. Chem. 274, 26850–26859 [DOI] [PubMed] [Google Scholar]
- 49. Isobe T., Hattori T., Kitagawa K., Uchida C., Kotake Y., Kosugi I., Oda T., Kitagawa M. (2009) J. Biol. Chem. 284, 27766–27779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alcendor R. R., Kirshenbaum L. A., Imai S., Vatner S. F., Sadoshima J. (2004) Circ. Res. 95, 971–980 [DOI] [PubMed] [Google Scholar]
- 51. Qin W., Yang T., Ho L., Zhao Z., Wang J., Chen L., Zhao W., Thiyagarajan M., MacGrogan D., Rodgers J. T., Puigserver P., Sadoshima J., Deng H., Pedrini S., Gandy S., Sauve A. A., Pasinetti G. M. (2006) J. Biol. Chem. 281, 21745–21754 [DOI] [PubMed] [Google Scholar]
- 52. Bitterman K. J., Anderson R. M., Cohen H. Y., Latorre-Esteves M., Sinclair D. A. (2002) J. Biol. Chem. 277, 45099–45107 [DOI] [PubMed] [Google Scholar]
- 53. Mai A., Massa S., Lavu S., Pezzi R., Simeoni S., Ragno R., Mariotti F. R., Chiani F., Camilloni G., Sinclair D. A. (2005) J. Med. Chem. 48, 7789–7795 [DOI] [PubMed] [Google Scholar]
- 54. Solomon J. M., Pasupuleti R., Xu L., McDonagh T., Curtis R., DiStefano P. S., Huber L. J. (2006) Mol. Cell. Biol. 26, 28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ellis L., Atadja P. W., Johnstone R. W. (2009) Mol. Cancer Ther. 8, 1409–1420 [DOI] [PubMed] [Google Scholar]
- 56. Kaeberlein M., McDonagh T., Heltweg B., Hixon J., Westman E. A., Caldwell S. D., Napper A., Curtis R., DiStefano P. S., Fields S., Bedalov A., Kennedy B. K. (2005) J. Biol. Chem. 280, 17038–17045 [DOI] [PubMed] [Google Scholar]
- 57. Borra M. T., Smith B. C., Denu J. M. (2005) J. Biol. Chem. 280, 17187–17195 [DOI] [PubMed] [Google Scholar]
- 58. Hou X., Xu S., Maitland-Toolan K. A., Sato K., Jiang B., Ido Y., Lan F., Walsh K., Wierzbicki M., Verbeuren T. J., Cohen R. A., Zang M. (2008) J. Biol. Chem. 283, 20015–20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pacholec M., Bleasdale J. E., Chrunyk B., Cunningham D., Flynn D., Garofalo R. S., Griffith D., Griffor M., Loulakis P., Pabst B., Qiu X., Stockman B., Thanabal V., Varghese A., Ward J., Withka J., Ahn K. (2010) J. Biol. Chem. 285, 8340–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li A., Xue Y., Jin C., Wang M., Yao X. (2006) Biochem. Biophys. Res. Commun. 350, 818–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McMahon M., Thomas N., Itoh K., Yamamoto M., Hayes J. D. (2004) J. Biol. Chem. 279, 31556–31567 [DOI] [PubMed] [Google Scholar]
- 62. Li M., Luo J., Brooks C. L., Gu W. (2002) J. Biol. Chem. 277, 50607–50611 [DOI] [PubMed] [Google Scholar]
- 63. Simonsson M., Kanduri M., Grönroos E., Heldin C.-H., Ericsson J. (2006) J. Biol. Chem. 281, 39870–39880 [DOI] [PubMed] [Google Scholar]
- 64. Blackwell J. S., Jr., Wilkinson S. T., Mosammaparast N., Pemberton L. F. (2007) J. Biol. Chem. 282, 20142–20150 [DOI] [PubMed] [Google Scholar]
- 65. Sun Y., Xu Y., Roy K., Price B. D. (2007) Mol. Cell. Biol. 27, 8502–8509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Manohar M., Mooney A. M., North J. A., Nakkula R. J., Picking J. W., Edon A., Fishel R., Poirier M. G., Ottesen J. J. (2009) J. Biol. Chem. 284, 23312–23321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li W., Jain M. R., Chen C., Yue X., Hebbar V., Zhou R., Kong A. N. (2005) J. Biol. Chem. 280, 28430–28438 [DOI] [PubMed] [Google Scholar]
- 68. Li W., Yu S. W., Kong A. N. (2006) J. Biol. Chem. 281, 27251–27263 [DOI] [PubMed] [Google Scholar]
- 69. Wolff B., Sanglier J. J., Wang Y. (1997) Chem. Biol. 4, 139–147 [DOI] [PubMed] [Google Scholar]
- 70. Kudo N., Matsumori N., Taoka H., Fujiwara D., Schreiner E. P., Wolff B., Yoshida M., Horinouchi S. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 9112–9117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fornerod M., Ohno M., Yoshida M., Mattaj I. W. (1997) Cell 90, 1051–1060 [DOI] [PubMed] [Google Scholar]
- 72. Deleted in proof.
- 73. Li W., Kong A. N. (2009) Mol. Carcinog. 48, 91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nioi P., Nguyen T., Sherratt P. J., Pickett C. B. (2005) Mol. Cell. Biol. 25, 10895–10906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brown S. L., Sekhar K. R., Rachakonda G., Sasi S., Freeman M. L. (2008) Cancer Res. 68, 364–368 [DOI] [PubMed] [Google Scholar]
- 76. Li W., Yu S., Liu T., Kim J. H., Blank V., Li H., Kong A. N. (2008) Biochim. Biophys. Acta 1783, 1847–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Soutoglou E., Katrakili N., Talianidis I. (2000) Mol. Cell 5, 745–751 [DOI] [PubMed] [Google Scholar]
- 78. Lan F., Cacicedo J. M., Ruderman N., Ido Y. (2008) J. Biol. Chem. 283, 27628–27635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen L. F., Greene W. C. (2003) J. Mol. Med. 81, 549–557 [DOI] [PubMed] [Google Scholar]
- 80. Frescas D., Valenti L., Accili D. (2005) J. Biol. Chem. 280, 20589–20595 [DOI] [PubMed] [Google Scholar]