Abstract
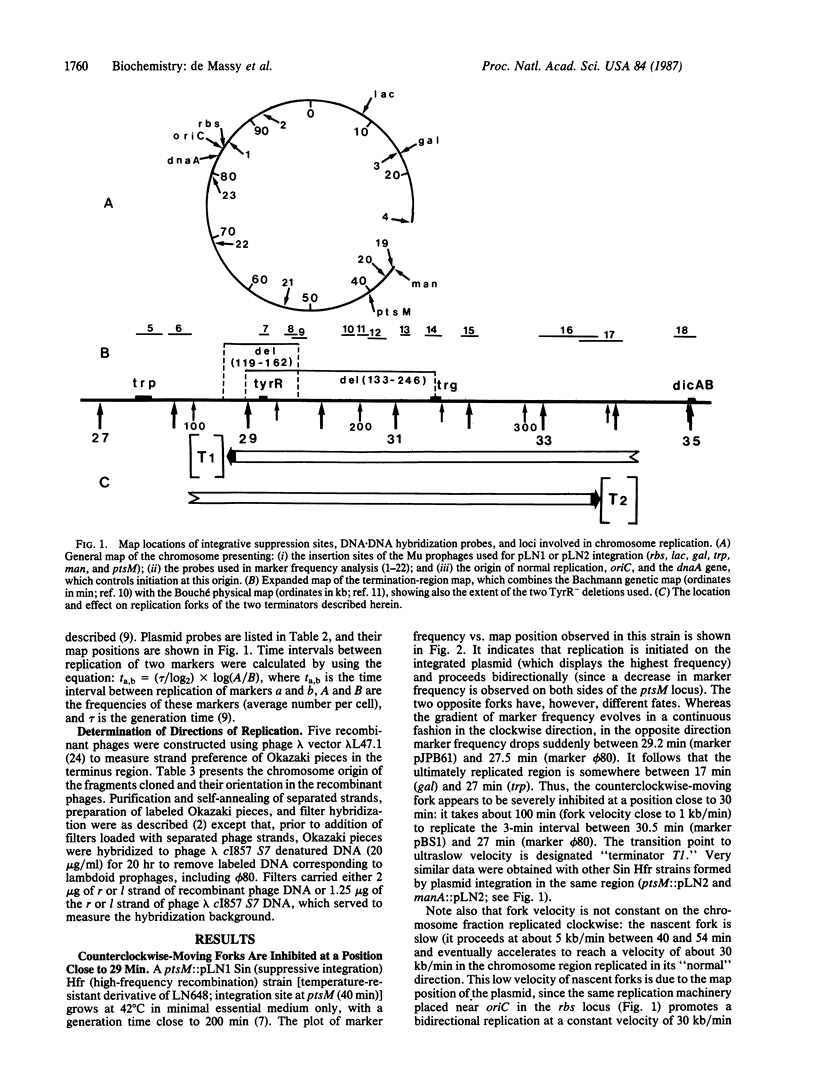
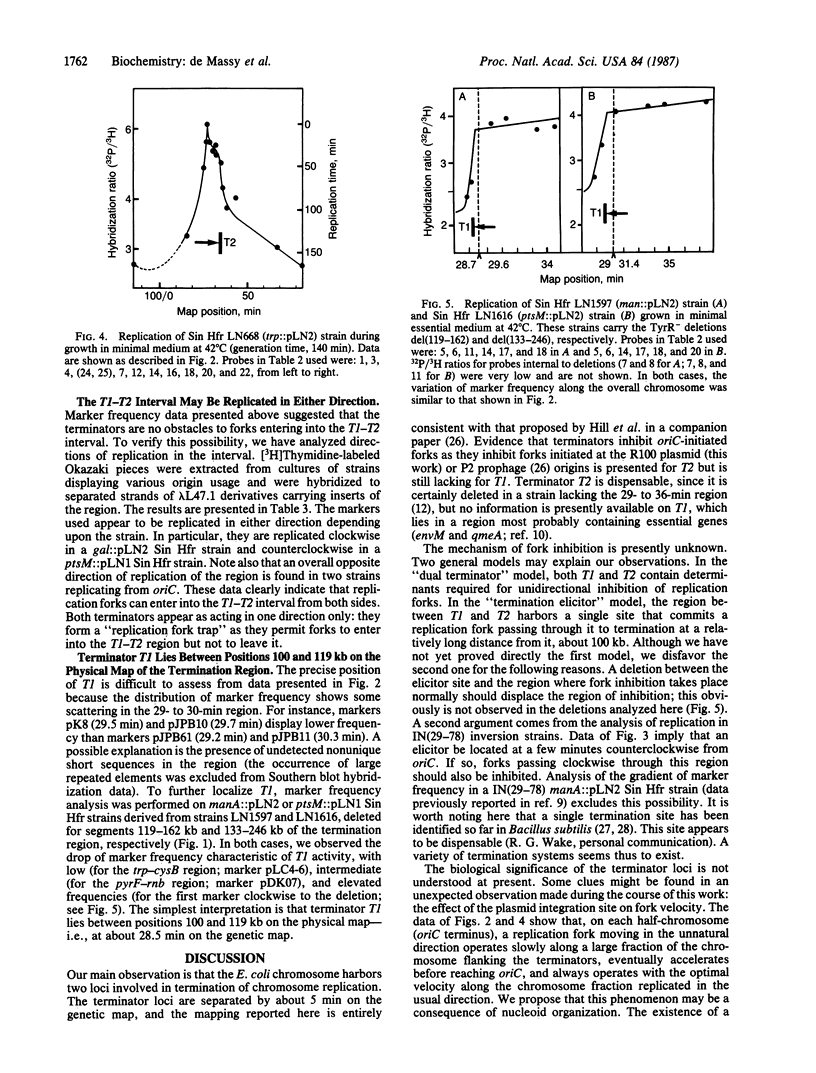
The replication cycle of Escherichia coli strains duplicating their chromosome from the same plasmid origin placed at various locations or of strains having undergone a major inversion event along the origin-to-terminus axis was studied by marker-frequency analysis. It was observed that replication forks are unidirectionally inhibited at two loci of the termination region: counterclockwise-moving forks are inhibited at terminator T1 (28.5 min), and forks moving in the opposite direction are inhibited at terminator T2 (33.5 min). By determining the strand preference of Okazaki fragments that are specific for markers from the T1-T2 interval, it was shown that this interval is replicated in either direction, depending upon the strain analyzed. In addition, we also observed that forks moving in the "unnatural" direction along each oriC-T1 or -T2 arm are very slow, especially in the one-third portion of the chromosome around the terminators. We propose that this phenomenon is a consequence of nucleoid organization, which is proposed to be symmetrical on the two oriC-T1 or -T2 arms and polarized with respect to the direction of replication. We also propose that T1 and T2 are the terminal limits of these two polarized half-nucleoid bodies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C., Ritzenthaler P., Mata-Gilsinger M. Cloning and endonuclease restriction analysis of uidA and uidR genes in Escherichia coli K-12: determination of transcription direction for the uidA gene. J Bacteriol. 1982 Feb;149(2):587–594. doi: 10.1128/jb.149.2.587-594.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché J. P., Gélugne J. P., Louarn J., Louarn J. M., Kaiser K. Relationships between the physical and genetic maps of a 470 x 10(3) base-pair region around the terminus of Escherichia coli K12 DNA replication. J Mol Biol. 1982 Jan 5;154(1):21–32. doi: 10.1016/0022-2836(82)90414-4. [DOI] [PubMed] [Google Scholar]
- Béjar S., Bouché J. P. A new dispensable genetic locus of the terminus region involved in control of cell division in Escherichia coli. Mol Gen Genet. 1985;201(2):146–150. doi: 10.1007/BF00425651. [DOI] [PubMed] [Google Scholar]
- Béjar S., Bouché J. P. Molecular cloning of the region of the terminus of Escherichia coli K-12 DNA replication. J Bacteriol. 1983 Feb;153(2):604–609. doi: 10.1128/jb.153.2.604-609.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Diaz R., Kaiser K. Rac-E. coli K12 strains carry a preferential attachment site for lambda rev. Mol Gen Genet. 1981;183(3):484–489. doi: 10.1007/BF00268769. [DOI] [PubMed] [Google Scholar]
- Donovan W. P., Kushner S. R. Cloning and physical analysis of the pyrF gene (coding for orotidine-5'-phosphate decarboxylase) from Escherichia coli K-12. Gene. 1983 Nov;25(1):39–48. doi: 10.1016/0378-1119(83)90165-8. [DOI] [PubMed] [Google Scholar]
- Eisenbeis S. J., Parker J. Strains of Escherichia coli carrying the structural gene for histidyl-tRNA synthetase on a high copy-number plasmid. Mol Gen Genet. 1981;183(1):115–122. doi: 10.1007/BF00270148. [DOI] [PubMed] [Google Scholar]
- Frey J., Chandler M., Caro L. The initiation of chromosome replication in a dnaAts46 and a dnaA+ strain at various temperatures. Mol Gen Genet. 1981;182(2):364–366. doi: 10.1007/BF00269686. [DOI] [PubMed] [Google Scholar]
- Harayama S., Engström P., Wolf-Watz H., Iino T., Hazelbauer G. L. Cloning of trg, a gene for a sensory transducer in Escherichia coli. J Bacteriol. 1982 Oct;152(1):372–383. doi: 10.1128/jb.152.1.372-383.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J. M., Kuempel P. L. Deletion of the terminus region (340 kilobase pairs of DNA) from the chromosome of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3766–3770. doi: 10.1073/pnas.82.11.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. M., Henson J. M., Kuempel P. L. The terminus region of the Escherichia coli chromosome contains two separate loci that exhibit polar inhibition of replication. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1754–1758. doi: 10.1073/pnas.84.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser K. The origin of Q-independent derivatives of phage lambda. Mol Gen Genet. 1980;179(3):547–554. doi: 10.1007/BF00271744. [DOI] [PubMed] [Google Scholar]
- Kuempel P. L., Duerr S. A. Chromosome replication in Escherichia coli is inhibited in the terminus region near the rac locus. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):563–567. doi: 10.1101/sqb.1979.043.01.062. [DOI] [PubMed] [Google Scholar]
- Kuempel P. L., Duerr S. A., Maglothin P. D. Chromosome replication in an Escherichia coli dnaA mutant integratively suppressed by prophage P2. J Bacteriol. 1978 Jun;134(3):902–912. doi: 10.1128/jb.134.3.902-912.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuempel P. L., Duerr S. A., Seeley N. R. Terminus region of the chromosome in Escherichia coli inhibits replication forks. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3927–3931. doi: 10.1073/pnas.74.9.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Louarn J. M., Bouché J. P., Legendre F., Louarn J., Patte J. Characterization and properties of very large inversions of the E. coli chromosome along the origin-to-terminus axis. Mol Gen Genet. 1985;201(3):467–476. doi: 10.1007/BF00331341. [DOI] [PubMed] [Google Scholar]
- Louarn J., Patte J., Louarn J. M. Evidence for a fixed termination site of chromosome replication in Escherichia coli K12. J Mol Biol. 1977 Sep 25;115(3):295–314. doi: 10.1016/0022-2836(77)90156-5. [DOI] [PubMed] [Google Scholar]
- Louarn J., Patte J., Louarn J. M. Map position of the replication terminus on the Escherichia coli chromosome. Mol Gen Genet. 1979 Apr 17;172(1):7–11. doi: 10.1007/BF00276208. [DOI] [PubMed] [Google Scholar]
- Louarn J., Patte J., Louarn J. M. Suppression of Escherichia coli dnaA46 mutations by integration of plasmid R100.1. derivatives: constraints imposed by the replication terminus. J Bacteriol. 1982 Aug;151(2):657–667. doi: 10.1128/jb.151.2.657-667.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. A., Howe J. G., Springer M., Touati-Schwartz D., Hershey J. W., Grunberg-Manago M. Cloning and mapping of a gene for translational initiation factor IF2 in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5033–5037. doi: 10.1073/pnas.79.16.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M., Graffe M., Goursot R., Grunberg-Manago M. Physical localisation and cloning of the structural gene for E. coli initiation factor IF3 from a group of genes concerned with translation. Gene. 1980 Oct;11(1-2):33–42. doi: 10.1016/0378-1119(80)90084-0. [DOI] [PubMed] [Google Scholar]
- Sancar A., Wharton R. P., Seltzer S., Kacinski B. M., Clarke N. D., Rupp W. D. Identification of the uvrA gene product. J Mol Biol. 1981 May 5;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Pettijohn D. E. Chromosomes in living Escherichia coli cells are segregated into domains of supercoiling. Proc Natl Acad Sci U S A. 1981 Jan;78(1):224–228. doi: 10.1073/pnas.78.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. T., Aynsley C., Wake R. G. Cloning and localization of the Bacillus subtilis chromosome replication terminus, terC. Gene. 1985;38(1-3):9–17. doi: 10.1016/0378-1119(85)90198-2. [DOI] [PubMed] [Google Scholar]
- Weiss A. S., Wake R. G. A unique DNA intermediate associated with termination of chromosome replication in Bacillus subtilis. Cell. 1984 Dec;39(3 Pt 2):683–689. doi: 10.1016/0092-8674(84)90475-6. [DOI] [PubMed] [Google Scholar]
- de Massy B., Patte J., Louarn J. M., Bouché J. P. oriX: a new replication origin in E. coli. Cell. 1984 Jan;36(1):221–227. doi: 10.1016/0092-8674(84)90092-8. [DOI] [PubMed] [Google Scholar]



