Abstract
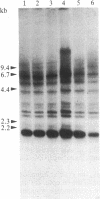
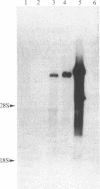
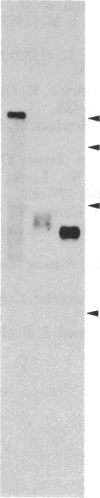
Following carcinogen exposure in vitro, normal rat tracheal epithelial cells are transformed in a multistage process in which the cultured cells become immortal and, ultimately, neoplastic. Five cell lines derived from tumors produced by neoplastically transformed rat tracheal epithelial cells were examined for the expression of 11 cellular oncogenes previously implicated in pulmonary or epithelial carcinogenesis. RNA homologous to fms was expressed at a level 5-19 times higher than normal tracheal epithelial cells in three of five of the tumor-derived lines. All three lines expressing high levels of fms-related RNA gave rise to invasive tumors of epithelial origin when injected into nude mice. Increased expression of the fms-related mRNA was not due to gene amplification, and no gene rearrangement was detected by Southern analyses. RNA blot analysis using a 3' v-fms probe detected a 9.5-kilobase message in the three tumor-derived lines, whereas both normal rat alveolar macrophages and the human choriocarcinoma line BeWo expressed a fms transcript of approximately 4 kilobases. We conclude from these data that the gene expressed as a 9.5-kilobase transcript in these neoplastic epithelial cells is a member of a fms-related gene family but may be distinct from the gene that encodes the macrophage colony-stimulating factor (CSF-1) receptor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. Oncogenes in human cancers and in chemically induced animal tumors. Prog Med Virol. 1985;32:86–100. [PubMed] [Google Scholar]
- Bilello J. A., Colletta G., Warnecke G., Koch G., Frisby D., Pragnell I. B., Ostertag W. Analysis of the expression of spleen focus-forming virus (SFFV)-related RNA and gp55, a Friend and Rauscher virus-specific protein. Virology. 1980 Dec;107(2):331–344. doi: 10.1016/0042-6822(80)90301-3. [DOI] [PubMed] [Google Scholar]
- Browning P. J., Bunn H. F., Cline A., Shuman M., Nienhuis A. W. "Replacement" of COOH-terminal truncation of v-fms with c-fms sequences markedly reduces transformation potential. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7800–7804. doi: 10.1073/pnas.83.20.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Donner L., Fedele L. A., Garon C. F., Anderson S. J., Sherr C. J. McDonough feline sarcoma virus: characterization of the molecularly cloned provirus and its feline oncogene (v-fms). J Virol. 1982 Feb;41(2):489–500. doi: 10.1128/jvi.41.2.489-500.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Parker P., Waterfield M. D. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984 Oct 4;311(5985):483–485. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Griffin C. A., Baylin S. B. Expression of the c-myb oncogene in human small cell lung carcinoma. Cancer Res. 1985 Jan;45(1):272–275. [PubMed] [Google Scholar]
- Hunter T. Oncogenes and proto-oncogenes: how do they differ? J Natl Cancer Inst. 1984 Oct;73(4):773–786. [PubMed] [Google Scholar]
- Koury M. J., Balmain A., Pragnell I. B. Induction of granulocyte-macrophage colony-stimulating activity in mouse skin by inflammatory agents and tumor promoters. EMBO J. 1983;2(11):1877–1882. doi: 10.1002/j.1460-2075.1983.tb01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzan S., Brody A. R., Nettesheim P., Eling T. Production of arachidonic acid metabolites by macrophages exposed in vitro to asbestos, carbonyl iron particles, or calcium ionophore. Am Rev Respir Dis. 1985 Apr;131(4):624–632. doi: 10.1164/arrd.1985.131.4.624. [DOI] [PubMed] [Google Scholar]
- Mark G. E., Rapp U. R. Primary structure of v-raf: relatedness to the src family of oncogenes. Science. 1984 Apr 20;224(4646):285–289. doi: 10.1126/science.6324342. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., Xu Y. H., Ishii S., Clark A. J., Semba K., Toyoshima K., Yamamoto T., Pastan I. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. Science. 1984 Apr 27;224(4647):417–419. doi: 10.1126/science.6200934. [DOI] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Adamson E. D., Tremblay J. M., Müller D., Cline M. J., Verma I. M. Transcription of c-onc genes c-rasKi and c-fms during mouse development. Mol Cell Biol. 1983 Jun;3(6):1062–1069. doi: 10.1128/mcb.3.6.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Tremblay J. M., Adamson E. D., Verma I. M. Tissue and cell type-specific expression of two human c-onc genes. Nature. 1983 Aug 4;304(5925):454–456. doi: 10.1038/304454a0. [DOI] [PubMed] [Google Scholar]
- Nau M. M., Brooks B. J., Battey J., Sausville E., Gazdar A. F., Kirsch I. R., McBride O. W., Bertness V., Hollis G. F., Minna J. D. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature. 1985 Nov 7;318(6041):69–73. doi: 10.1038/318069a0. [DOI] [PubMed] [Google Scholar]
- Neckameyer W. S., Shibuya M., Hsu M. T., Wang L. H. Proto-oncogene c-ros codes for a molecule with structural features common to those of growth factor receptors and displays tissue specific and developmentally regulated expression. Mol Cell Biol. 1986 May;6(5):1478–1486. doi: 10.1128/mcb.6.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Reynolds F. H., Jr, Stephenson J. R. New mammalian transforming retrovirus: demonstration of a polyprotein gene product. J Virol. 1983 Mar;45(3):914–924. doi: 10.1128/jvi.45.3.914-924.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Josephs S. F., Wong-Staal F. Oncogenes: their role in neoplastic transformation. Annu Rev Microbiol. 1985;39:419–449. doi: 10.1146/annurev.mi.39.100185.002223. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Chen J. H., Roussel M. F., Sherr C. J. The product of the c-fms proto-oncogene: a glycoprotein with associated tyrosine kinase activity. Science. 1985 Apr 19;228(4697):320–322. doi: 10.1126/science.2580348. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Rettenmier C. W., Look A. T., Sherr C. J. Cell surface expression of v-fms-coded glycoproteins is required for transformation. Mol Cell Biol. 1984 Oct;4(10):1999–2009. doi: 10.1128/mcb.4.10.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariban E., Mitchell T., Kufe D. Expression of the c-fms proto-oncogene during human monocytic differentiation. Nature. 1985 Jul 4;316(6023):64–66. doi: 10.1038/316064a0. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Varmus H. E., Bishop J. M., George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., deKernion J. B., Verma I. M., Cline M. J. Expression of cellular oncogenes in human malignancies. Science. 1984 Apr 20;224(4646):256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Terzaghi M., Nettesheim P. Dynamics of neoplastic development in carcinogen-exposed tracheal mucosa. Cancer Res. 1979 Oct;39(10):4003–4010. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toftgard R., Roop D. R., Yuspa S. H. Proto-oncogene expression during two-stage carcinogenesis in mouse skin. Carcinogenesis. 1985 Apr;6(4):655–657. doi: 10.1093/carcin/6.4.655. [DOI] [PubMed] [Google Scholar]
- Wheeler E. F., Rettenmier C. W., Look A. T., Sherr C. J. The v-fms oncogene induces factor independence and tumorigenicity in CSF-1 dependent macrophage cell line. 1986 Nov 27-Dec 3Nature. 324(6095):377–380. doi: 10.1038/324377a0. [DOI] [PubMed] [Google Scholar]