Abstract
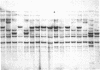
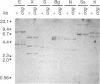
Rat thyroglobulin (TG) cDNA clones were used to identify DNA restriction fragment variants among inbred mouse strains. One of these variants was shown to be closely linked to the recessive mutation congenital goiter (cog), which had previously been mapped to mouse chromosome 15. These results indicate that the structural gene for thyroglobulin is on chromosome 15 and suggest that a mutation at the site of the TG gene is the basis of the cog defect. No differences were observed between cog/cog and +/+ DNA in Southern blots using TG cDNA probes corresponding to 88% of the coding sequences, suggesting that the cog mutation is not due to a large deletion of this portion of the gene. Neither was there any obvious qualitative or quantitative difference between mutant and normal TG mRNA as judged by blot hybridization of electrophoretically fractionated thyroid RNAs. The thyroglobulin gene locus (Tgn) was mapped near the glutamic-pyruvic transaminase isoenzyme locus Gpt-1. The Tgn locus is syntenic with the c-myc protooncogene locus (Myc) in the mouse as in the rat and man.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avvedimento V. E., Di Lauro R., Monticelli A., Bernardi F., Patracchini P., Calzolari E., Martini G., Varrone S. Mapping of human thyroglobulin gene on the long arm of chromosome 8 by in situ hybridization. Hum Genet. 1985;71(2):163–166. doi: 10.1007/BF00283375. [DOI] [PubMed] [Google Scholar]
- Baas F., Bikker H., Geurts van Kessel A., Melsert R., Pearson P. L., de Vijlder J. J., van Ommen G. J. The human thyroglobulin gene: a polymorphic marker localized distal to C-MYC on chromosome 8 band q24. Hum Genet. 1985;69(2):138–143. doi: 10.1007/BF00293284. [DOI] [PubMed] [Google Scholar]
- Baas F., van Ommen G. J., Bikker H., Arnberg A. C., de Vijlder J. J. The human thyroglobulin gene is over 300 kb long and contains introns of up to 64 kb. Nucleic Acids Res. 1986 Jul 11;14(13):5171–5186. doi: 10.1093/nar/14.13.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M., Wiener F., Spira J., Babonits M., Nilsson M. G., Sumegi J., Klein G. Mapping of the c-myc, pvt-1 and immunoglobulin kappa genes in relation to the mouse plasmacytoma-associated variant (6;15) translocation breakpoint. EMBO J. 1985 Dec 1;4(12):3183–3188. doi: 10.1002/j.1460-2075.1985.tb04063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer W. G., Maltais L. J., DeBaets M. H., Eicher E. M. Inherited congenital goiter in mice. Endocrinology. 1987 Feb;120(2):838–840. doi: 10.1210/endo-120-2-838. [DOI] [PubMed] [Google Scholar]
- Brocas H., Szpirer J., Lebo R. V., Levan G., Szpirer C., Cheung M. C., Vassart G. The thyroglobulin gene resides on chromosome 8 in man and on chromosome 7 in the rat. Cytogenet Cell Genet. 1985;39(2):150–153. doi: 10.1159/000132125. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautigny A., Mattei M. G., Morello D., Alliel P. M., Pham-Dinh D., Amar L., Arnaud D., Simon D., Mattei J. F., Guenet J. L. The structural gene coding for myelin-associated proteolipid protein is mutated in jimpy mice. 1986 Jun 26-Jul 2Nature. 321(6073):867–869. doi: 10.1038/321867a0. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Jacobsson A., Stadler U., Glotzer M. A., Kozak L. P. Mitochondrial uncoupling protein from mouse brown fat. Molecular cloning, genetic mapping, and mRNA expression. J Biol Chem. 1985 Dec 25;260(30):16250–16254. [PubMed] [Google Scholar]
- Johnson D. A., Bedigian H. G., Cherry M., Meier H. Leukemogenesis, immune responsiveness, and murine leukemia virus expression in congenic AKR/J mice differing at H-2. Infect Immun. 1980 Sep;29(3):1007–1012. doi: 10.1128/iai.29.3.1007-1012.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Inoko H., Katsuki M., Ando A., Sato T., Hirose T., Takashima H., Inayama S., Okano H., Takamatsu K. Molecular genetic analysis of myelin-deficient mice: shiverer mutant mice show deletion in gene(s) coding for myelin basic protein. J Neurochem. 1985 Mar;44(3):692–696. doi: 10.1111/j.1471-4159.1985.tb12870.x. [DOI] [PubMed] [Google Scholar]
- Lane P. W., Liu H. M. Association of megacolon with a new dominant spotting gene (Dom) in the mouse. J Hered. 1984 Nov-Dec;75(6):435–439. doi: 10.1093/oxfordjournals.jhered.a109980. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Lee S. M., Lewis S., Johnson F. M. Identification and biochemical analysis of mouse mutants deficient in cytoplasmic malic enzyme. Biochemistry. 1980 Oct 28;19(22):5098–5103. doi: 10.1021/bi00563a025. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. Distribution of crossing-over in mouse chromosomes. Genet Res. 1976 Dec;28(3):291–299. doi: 10.1017/s0016672300016980. [DOI] [PubMed] [Google Scholar]
- Marriq C., Rolland M., Lissitzky S. Structure-function relationship in thyroglobulin: amino acid sequence of two different thyroxine-containing peptides from porcine thyroglobulin. EMBO J. 1982;1(4):397–401. doi: 10.1002/j.1460-2075.1982.tb01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken L., Simons M. J., Swillens S., Massaer M., Vassart G. Primary structure of bovine thyroglobulin deduced from the sequence of its 8,431-base complementary DNA. Nature. 1985 Aug 15;316(6029):647–651. doi: 10.1038/316647a0. [DOI] [PubMed] [Google Scholar]
- Musti A. M., Avvedimento E. V., Polistina C., Ursini V. M., Obici S., Nitsch L., Cocozza S., Di Lauro R. The complete structure of the rat thyroglobulin gene. Proc Natl Acad Sci U S A. 1986 Jan;83(2):323–327. doi: 10.1073/pnas.83.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau J. H., Taylor B. A. Lengths of chromosomal segments conserved since divergence of man and mouse. Proc Natl Acad Sci U S A. 1984 Feb;81(3):814–818. doi: 10.1073/pnas.81.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Andrews S. J., Loutit J. F., Clegg J. B. A mouse beta-globin mutant that is an exact model of hemoglobin Rainier in man. Genetics. 1985 Aug;110(4):709–721. doi: 10.1093/genetics/110.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin M., Barker P. E., Ruddle F. H., Brocas H., Targovnik H., Vassart G. Proximity of thyroglobulin and c-myc genes on human chromosome 8. Somat Cell Mol Genet. 1985 Jul;11(4):397–402. doi: 10.1007/BF01534417. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts M. H., Pohl V., de Martynoff G., Boyd C. D., Bester A. J., Van Jaarsveld P. P., Vassart G. Defective splicing of thyroglobulin gene transcripts in the congenital goitre of the Afrikander cattle. EMBO J. 1985 Mar;4(3):731–737. doi: 10.1002/j.1460-2075.1985.tb03690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach A., Takahashi N., Pravtcheva D., Ruddle F., Hood L. Chromosomal mapping of mouse myelin basic protein gene and structure and transcription of the partially deleted gene in shiverer mutant mice. Cell. 1985 Aug;42(1):149–155. doi: 10.1016/s0092-8674(85)80110-0. [DOI] [PubMed] [Google Scholar]
- Skow L. C., Burkhart B. A., Johnson F. M., Popp R. A., Popp D. M., Goldberg S. Z., Anderson W. F., Barnett L. B., Lewis S. E. A mouse model for beta-thalassemia. Cell. 1983 Oct;34(3):1043–1052. doi: 10.1016/0092-8674(83)90562-7. [DOI] [PubMed] [Google Scholar]
- Sul H. S., Wise L. S., Brown M. L., Rubin C. S. Cloning of cDNA sequences for murine malic enzyme and the identification of aberrantly large malic enzyme mRNA in MOD-1 null mice. J Biol Chem. 1984 Jan 10;259(1):555–559. [PubMed] [Google Scholar]
- Targovnik H. M., Pohl V., Christophe D., Cabrer B., Brocas H., Vassart G. Structural organization of the 5' region of the human thyroglobulin gene. Eur J Biochem. 1984 Jun 1;141(2):271–277. doi: 10.1111/j.1432-1033.1984.tb08188.x. [DOI] [PubMed] [Google Scholar]
- Taylor B. A., Rowe L. Genes for serum amyloid A proteins map to Chromosome 7 in the mouse. Mol Gen Genet. 1984;195(3):491–499. doi: 10.1007/BF00341452. [DOI] [PubMed] [Google Scholar]
- Van Herle A. J., Vassart G., Dumont J. E. Control of thyroglobulin synthesis and secretion (second of two parts). N Engl J Med. 1979 Aug 9;301(6):307–314. doi: 10.1056/NEJM197908093010605. [DOI] [PubMed] [Google Scholar]
- Whitney J. B., 3rd, Martinell J., Popp R. A., Russell L. B., Anderson W. F. Deletions in the alpha-globin gene complex in alpha-thalassemic mice. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7644–7647. doi: 10.1073/pnas.78.12.7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vijlder J. J., van Ommen G. J., van Voorthuizen W. F., Koch C. A., Arnberg A. C., Vassart G., Dinsart C., Flavell R. A. Nonfunctional thyroglobulin messenger RNA in goats with hereditary congenital goiter. J Mol Appl Genet. 1981;1(1):51–59. [PubMed] [Google Scholar]