Abstract
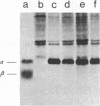
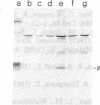
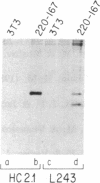
Retrovirus vectors [direct orientation (DO) vectors] that permit the simultaneous expression of an inserted protein-coding sequence and a dominant-acting selectable marker have been constructed. In these vectors, an internal simian virus 40 or human metallothionein promoter sequence serves to drive the expression of the bacterial neomycin phosphotransferase or guanine-xanthine phosphoribosyltransferase genes, whereas the viral long terminal repeat sequences are utilized to promote expression of inserted sequences. In some of the vectors, the viral 5' splice site, normally used in the biogenesis of the subgenomic env-encoding mRNA, has been eliminated. These vectors yield high transient and stable titers of virus after transfection of viral packaging cell lines, show little or no depression of virus titer with a variety of inserts, and faithfully transmit recombinant proviral sequences to recipient cells. To characterize the expression potential of these vectors, a variety of inserts encoding the alpha and beta subunits of the human major histocompatibility complex class II antigen HLA-DR have been introduced into these vectors. NIH 3T3 cells infected by viruses containing HLA-DR alpha or beta cDNAs express these proteins as shown by immunoprecipitation of metabolically labeled extracts. In addition, through the sequential infection of cells with retrovirus constructions expressing two different selectable markers, both subunits of the class II antigen have been introduced into NIH 3T3 cells. Such infected cells express HLA-DR molecules at the cell surface.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Chumakov I., Stuhlmann H., Harbers K., Jaenisch R. Cloning of two genetically transmitted Moloney leukemia proviral genomes: correlation between biological activity of the cloned DNA and viral genome activation in the animal. J Virol. 1982 Jun;42(3):1088–1098. doi: 10.1128/jvi.42.3.1088-1098.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn L. E., Glimcher L. H., Waldmann R. A., Smith J. A., Ben-Nun A., Seidman J. G., Choi E. Identification of functional regions on the I-Ab molecule by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1986 Feb;83(3):747–751. doi: 10.1073/pnas.83.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. D., Mulligan R. C. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6349–6353. doi: 10.1073/pnas.81.20.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitcher D. L., Neutra M. R., Mostov K. E. Functional expression of the polymeric immunoglobulin receptor from cloned cDNA in fibroblasts. J Cell Biol. 1986 Mar;102(3):911–919. doi: 10.1083/jcb.102.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell. 1984 Dec;39(3 Pt 2):449–467. [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Quantitative analysis of gene suppression in integrated retrovirus vectors. Mol Cell Biol. 1986 Mar;6(3):792–800. doi: 10.1128/mcb.6.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom V., Gay D., Tonegawa S. The beta 1 domain of the mouse E beta chain is important for restricted antigen presentation to helper T-cell hybridomas. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1678–1682. doi: 10.1073/pnas.82.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L. Expression of human adenosine deaminase using a transmissable murine retrovirus vector system. Proc Natl Acad Sci U S A. 1985 Feb;82(3):703–707. doi: 10.1073/pnas.82.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N., Ashwell J. D., Lechler R. I., Margulies D. H., Nickerson K. M., Suzuki G., Tou J. Y. "Exon-shuffling" maps control of antibody- and T-cell-recognition sites to the NH2-terminal domain of the class II major histocompatibility polypeptide A beta. Proc Natl Acad Sci U S A. 1985 May;82(9):2940–2944. doi: 10.1073/pnas.82.9.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N., Malissen B. Analysis of the expression and function of class-II major histocompatibility complex-encoded molecules by DNA-mediated gene transfer. Annu Rev Immunol. 1986;4:281–315. doi: 10.1146/annurev.iy.04.040186.001433. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Bernstein A. Retrovirus transduction: segregation of the viral transforming function and the herpes simplex virus tk gene in infectious Friend spleen focus-forming virus thymidine kinase vectors. Mol Cell Biol. 1983 Dec;3(12):2191–2202. doi: 10.1128/mcb.3.12.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Kaufman J. F., Auffray C., Korman A. J., Shackelford D. A., Strominger J. The class II molecules of the human and murine major histocompatibility complex. Cell. 1984 Jan;36(1):1–13. doi: 10.1016/0092-8674(84)90068-0. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Korman A. J., Boss J. M., Spies T., Sorrentino R., Okada K., Strominger J. L. Genetic complexity and expression of human class II histocompatibility antigens. Immunol Rev. 1985 Jul;85:45–86. doi: 10.1111/j.1600-065x.1985.tb01130.x. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Knudsen P. J., Kaufman J. F., Strominger J. L. cDNA clones for the heavy chain of HLA-DR antigens obtained after immunopurification of polysomes by monoclonal antibody. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1844–1848. doi: 10.1073/pnas.79.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman A. J., Ploegh H. L., Kaufman J. F., Owen M. J., Strominger J. L. Cell-free synthesis and processing of the heavy and light chains of HLA-DR antigens. J Exp Med. 1980 Aug 1;152(2 Pt 2):65s–82s. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long E. O., Wake C. T., Gorski J., Mach B. Complete sequence of an HLA-dR beta chain deduced from a cDNA clone and identification of multiple non-allelic DR beta chain genes. EMBO J. 1983;2(3):389–394. doi: 10.1002/j.1460-2075.1983.tb01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Wake C. T., Strubin M., Gross N., Accolla R. S., Carrel S., Mach B. Isolation of distinct cDNA clones encoding HLA-DR beta chains by use of an expression assay. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7465–7469. doi: 10.1073/pnas.79.23.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Law M. F., Verma I. M. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985 Mar;5(3):431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H., Kaplan D. R., Whitman M., Roberts T. M. Retrovirus shuttle vector for study of kinase activities of pp60c-src synthesized in vitro and overproduced in vivo. Mol Cell Biol. 1986 Jun;6(6):2033–2040. doi: 10.1128/mcb.6.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Miller J. S., Kimelman D., Cepko C. L., Lemischka I. R., Mulligan R. C. Individual adenovirus type 5 early region 1A gene products elicit distinct alterations of cellular morphology and gene expression. J Virol. 1985 Nov;56(2):404–413. doi: 10.1128/jvi.56.2.404-413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J. L., Nicolas J. F., Jacob F. Construction of a retrovirus capable of transducing and expressing genes in multipotential embryonic cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7137–7140. doi: 10.1073/pnas.81.22.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamboeck A., Korman A. J., Kamb A., Strominger J. L. Organization of the transcriptional unit of a human class II histocompatibility antigen: HLA-DR heavy chain. Nucleic Acids Res. 1983 Dec 20;11(24):8663–8675. doi: 10.1093/nar/11.24.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastri N., Malissen B., Hood L. Ia-transfected L-cell fibroblasts present a lysozyme peptide but not the native protein to lysozyme-specific T cells. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5885–5889. doi: 10.1073/pnas.82.17.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Temin H. M. Loss of intervening sequences in genomic mouse alpha-globin DNA inserted in an infectious retrovirus vector. Nature. 1982 Sep 16;299(5880):265–268. doi: 10.1038/299265a0. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Straus D., Raines R., Kawashima E., Knowles J. R., Gilbert W. Active site of triosephosphate isomerase: in vitro mutagenesis and characterization of an altered enzyme. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2272–2276. doi: 10.1073/pnas.82.8.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F., Vanek M., Vennström B. Transfer of genes into embryonal carcinoma cells by retrovirus infection: efficient expression from an internal promoter. EMBO J. 1985 Mar;4(3):663–666. doi: 10.1002/j.1460-2075.1985.tb03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake C. T., Long E. O., Strubin M., Gross N., Accolla R., Carrel S., Mach B. Isolation of cDNA clones encoding HLA-DR alpha chains. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6979–6983. doi: 10.1073/pnas.79.22.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. F., von Rüden T., Kantoff P. W., Garber C., Seiberg M., Rüther U., Anderson W. F., Wagner E. F., Gilboa E. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3194–3198. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]