Abstract
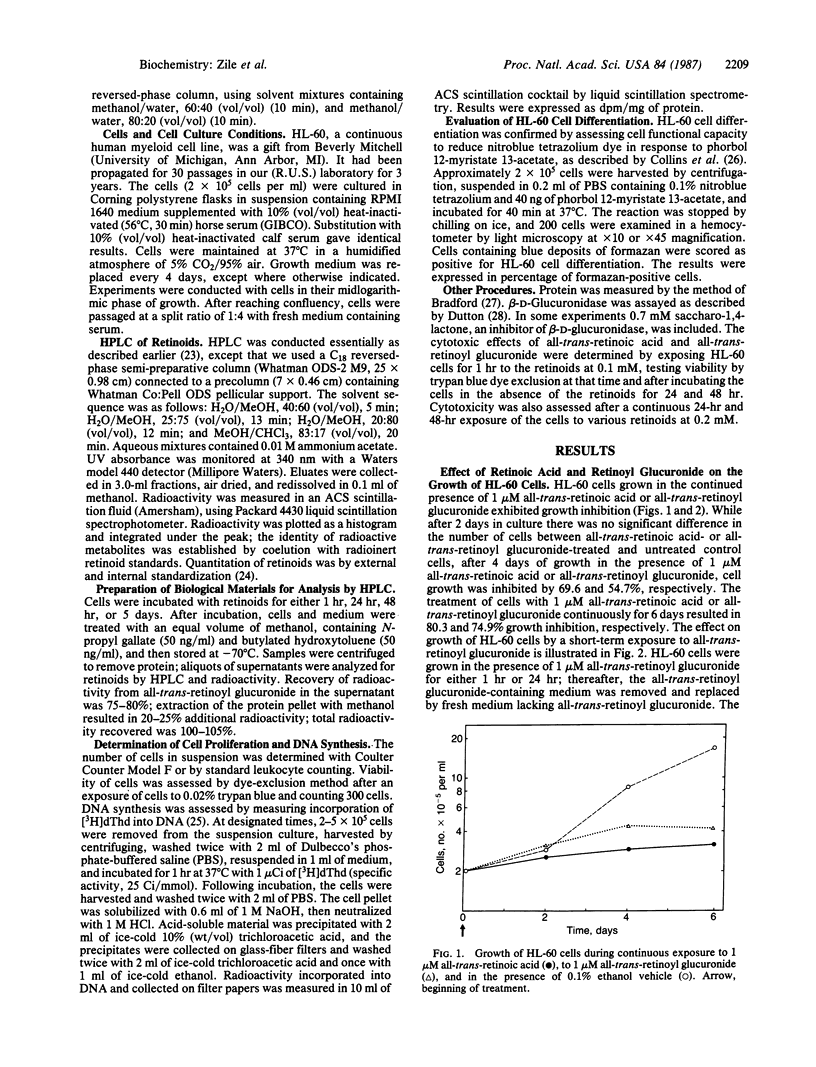
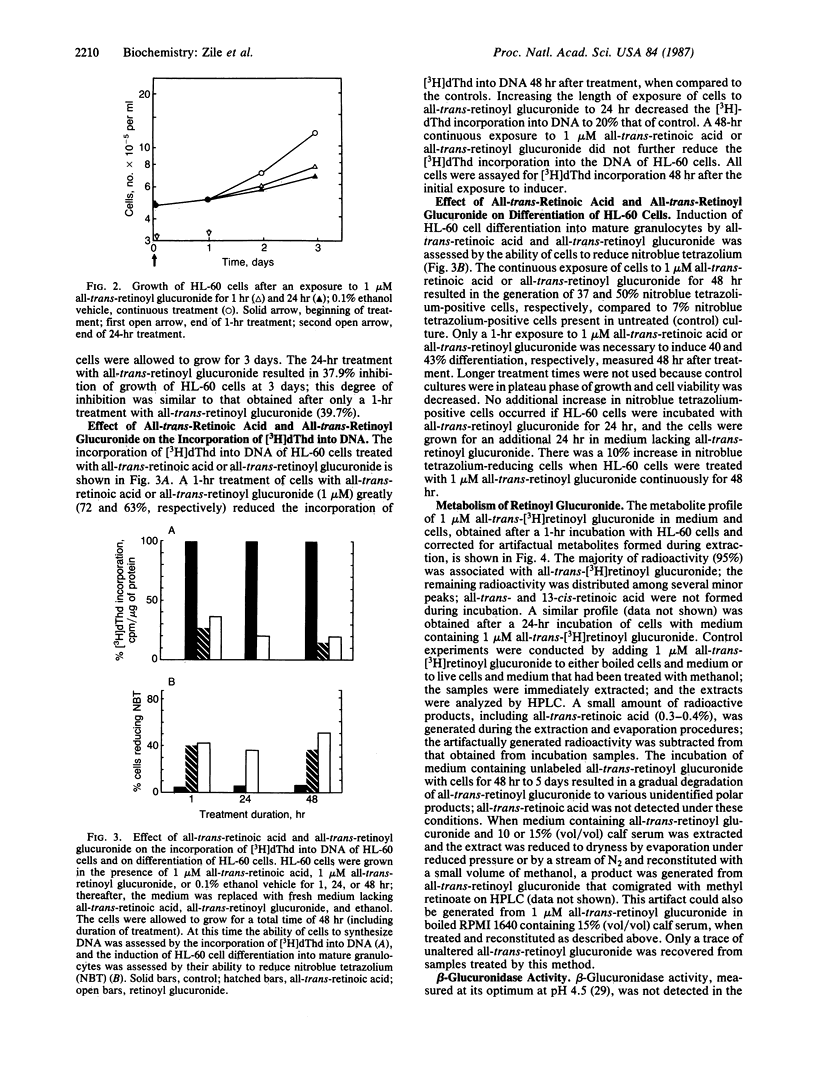
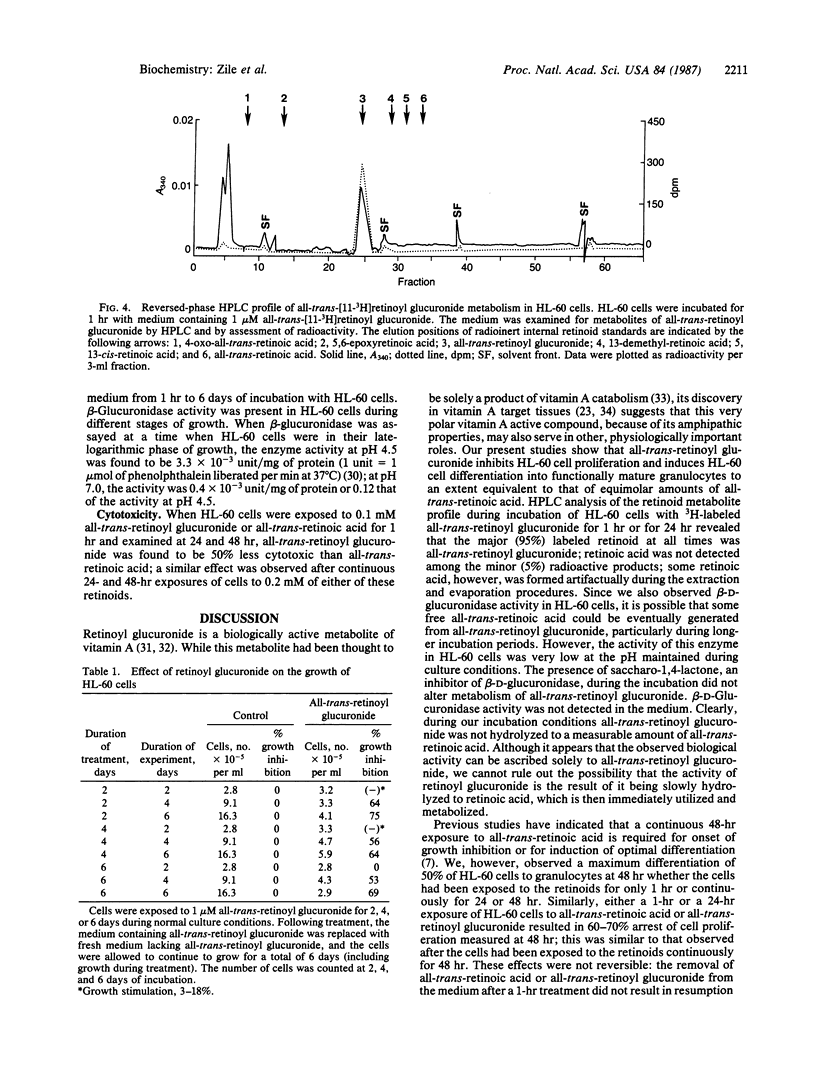
We examined the differentiation activity of retinoyl beta-D-glucuronide, a biologically active physiological metabolite of vitamin A, using the human promyelocytic leukemic cell line HL-60, which can be induced to differentiate with retinoic acid. Retinoyl beta-D-glucuronide (1 microM) inhibited HL-60 cell proliferation by 55-75%, inhibited tritiated thymidine incorporation into DNA by 63-80%, and induced 38-50% of the cells to differentiate into mature granulocytes. The potency of growth inhibition and induction of differentiation by retinoyl beta-D-glucuronide was similar to that of all-trans-retinoic acid. The continuous presence of either retinoyl beta-D-glucuronide or all-trans-retinoic acid was not required to obtain maximum growth arrest and differentiation: a 1-hr exposure of HL-60 cells to the retinoids gave the same response (measured after a total incubation time of 48 hr) as a 24-hr or 48-hr continuous treatment. Retinoyl beta-D-glucuronide (0.1-0.2 mM) was 50% less cytotoxic to HL-60 cells than all-trans-retinoic acid at an equimolar concentration. Retinoyl beta-D-glucuronide was not significantly metabolized to other retinoids; retinoic acid was not formed during incubation. We conclude that retinoyl beta-D-glucuronide can arrest HL-60 cell proliferation and induce their differentiation into mature granulocytes; it may act by itself or by being hydrolyzed to retinoic acid, which could be immediately utilized and metabolized. The therapeutic use of this retinoid as an antineoplastic agent is suggested.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barua A. B., Olson J. A. Chemical synthesis of all-trans retinoyl beta-glucuronide. J Lipid Res. 1985 Oct;26(10):1277–1282. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Collins S. J., Keene B. R. Terminal differentiation of human promyelocytic leukemic cells in primary culture in response to retinoic acid. Blood. 1981 Jun;57(6):1000–1004. [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979 Apr 1;149(4):969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullum M. E., Zile M. H. Metabolism of all-trans-retinoic acid and all-trans-retinyl acetate. Demonstration of common physiological metabolites in rat small intestinal mucosa and circulation. J Biol Chem. 1985 Sep 5;260(19):10590–10596. [PubMed] [Google Scholar]
- Cullum M. E., Zile M. H. Quantitation of biological retinoids by high-pressure liquid chromatography: primary internal standardization using tritiated retinoids. Anal Biochem. 1986 Feb 15;153(1):23–32. doi: 10.1016/0003-2697(86)90055-2. [DOI] [PubMed] [Google Scholar]
- Daenen S., Vellenga E., van Dobbenburgh O. A., Halie M. R. Retinoic acid as antileukemic therapy in a patient with acute promyelocytic leukemia and Aspergillus pneumonia. Blood. 1986 Feb;67(2):559–561. [PubMed] [Google Scholar]
- Douer D., Koeffler H. P. Retinoic acid. Inhibition of the clonal growth of human myeloid leukemia cells. J Clin Invest. 1982 Feb;69(2):277–283. doi: 10.1172/JCI110450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana J. A., Rogers J. S., 2nd, Durham J. P. The role of 13 cis-retinoic acid in the remission induction of a patient with acute promyelocytic leukemia. Cancer. 1986 Jan 15;57(2):209–217. doi: 10.1002/1097-0142(19860115)57:2<209::aid-cncr2820570204>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Amatruda T. T., 3rd The effect of retinoids on haemopoiesis--clinical and laboratory studies. Ciba Found Symp. 1985;113:252–267. doi: 10.1002/9780470720943.ch14. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P. Induction of differentiation of human acute myelogenous leukemia cells: therapeutic implications. Blood. 1983 Oct;62(4):709–721. [PubMed] [Google Scholar]
- LEVVY G. A. The preparation and properties of beta-glucuronidase. IV. Inhibition by sugar acids and their lactones. Biochem J. 1952 Nov;52(3):464–472. doi: 10.1042/bj0520464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippel K., Olson J. A. Origin of some derivatives of retinoic acid found in rat bile. J Lipid Res. 1968 Sep;9(5):580–586. [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Mahrle G. Retinoids in oncology. Curr Probl Dermatol. 1985;13:128–163. [PubMed] [Google Scholar]
- Meyskens F. L., Jr, Goodman G. E., Alberts D. S. 13-Cis-retinoic acid: pharmacology, toxicology, and clinical applications for the prevention and treatment of human cancer. Crit Rev Oncol Hematol. 1985;3(1):75–101. doi: 10.1016/s1040-8428(85)80040-8. [DOI] [PubMed] [Google Scholar]
- Murtaugh M. P., Dennison O., Stein J. P., Davies P. J. Retinoic acid-induced gene expression in normal and leukemic myeloid cells. J Exp Med. 1986 May 1;163(5):1325–1330. doi: 10.1084/jem.163.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sietsema W. K., DeLuca H. F. A new vaginal smear assay for vitamin A in rats. J Nutr. 1982 Aug;112(8):1481–1489. doi: 10.1093/jn/112.8.1481. [DOI] [PubMed] [Google Scholar]
- Stephens-Jarnagin A., Sietsema W. K., Miller D. A., DeLuca H. F. The biological activity of rat intestinal retinoic acid metabolites in the vaginal smear assay. Arch Biochem Biophys. 1983 Feb 1;220(2):502–508. doi: 10.1016/0003-9861(83)90441-1. [DOI] [PubMed] [Google Scholar]
- Strickland S., Breitman T. R., Frickel F., Nürrenbach A., Hädicke E., Sporn M. B. Structure-activity relationships of a new series of retinoidal benzoic acid derivatives as measured by induction of differentiation of murine F9 teratocarcinoma cells and human HL-60 promyelocytic leukemia cells. Cancer Res. 1983 Nov;43(11):5268–5272. [PubMed] [Google Scholar]
- Thomas G. A., Simpson R. U. High performance liquid chromatography analysis of 1,25-dihydroxyvitamin D3 receptor in malignant cells. Correlation of effects on cell proliferation and receptor concentration. Cancer Biochem Biophys. 1986 Jul;8(3):221–234. [PubMed] [Google Scholar]
- Vahlquist A. Clinical use of vitamin A and its derivatives--physiological and pharmacological aspects. Clin Exp Dermatol. 1985 Mar;10(2):133–143. doi: 10.1111/j.1365-2230.1985.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Wolf G. Multiple functions of vitamin A. Physiol Rev. 1984 Jul;64(3):873–937. doi: 10.1152/physrev.1984.64.3.873. [DOI] [PubMed] [Google Scholar]
- Yen A., Powers V., Fishbaugh J. Retinoic acid induced HL-60 myeloid differentiation: dependence of early and late events on isomeric structure. Leuk Res. 1986;10(6):619–629. doi: 10.1016/0145-2126(86)90264-x. [DOI] [PubMed] [Google Scholar]
- Zile M. H., Inhorn R. C., DeLuca H. F. Metabolism in vivo of all-trans-retinoic acid. Biosynthesis of 13-cis-retinoic acid and all-trans- and 13-cis-retinoyl glucuronides in the intestinal mucosa of the rat. J Biol Chem. 1982 Apr 10;257(7):3544–3550. [PubMed] [Google Scholar]


