Abstract
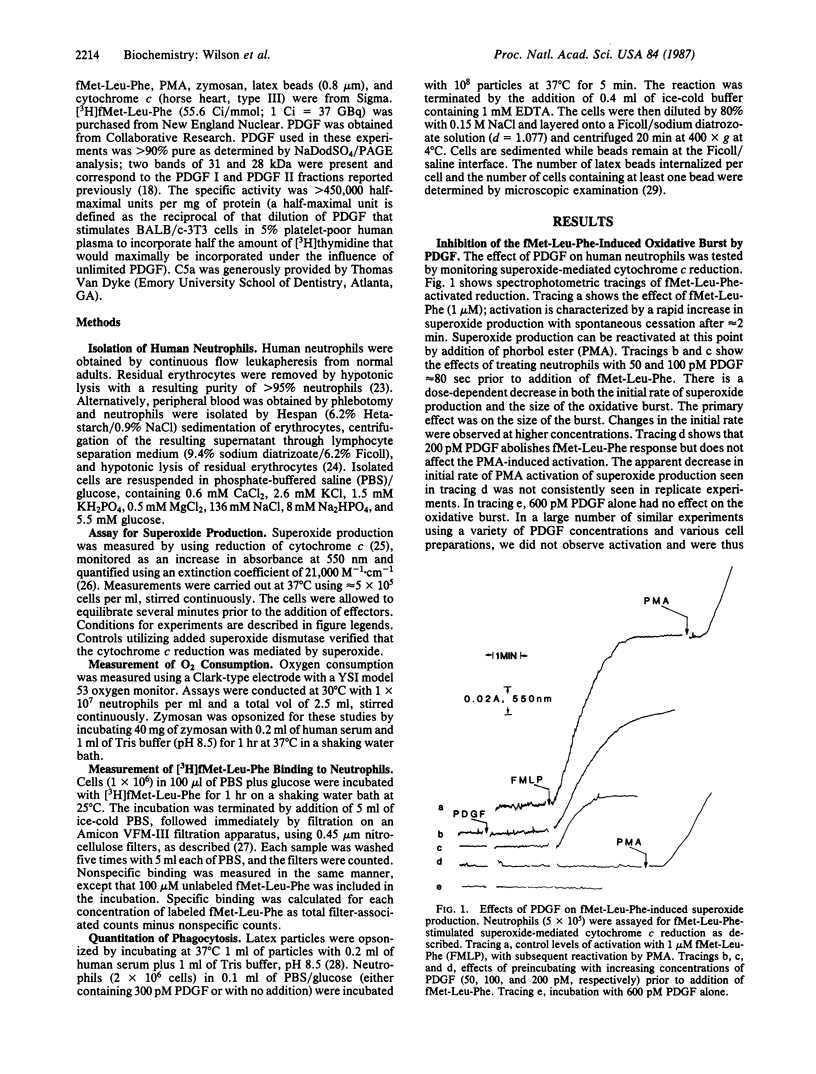
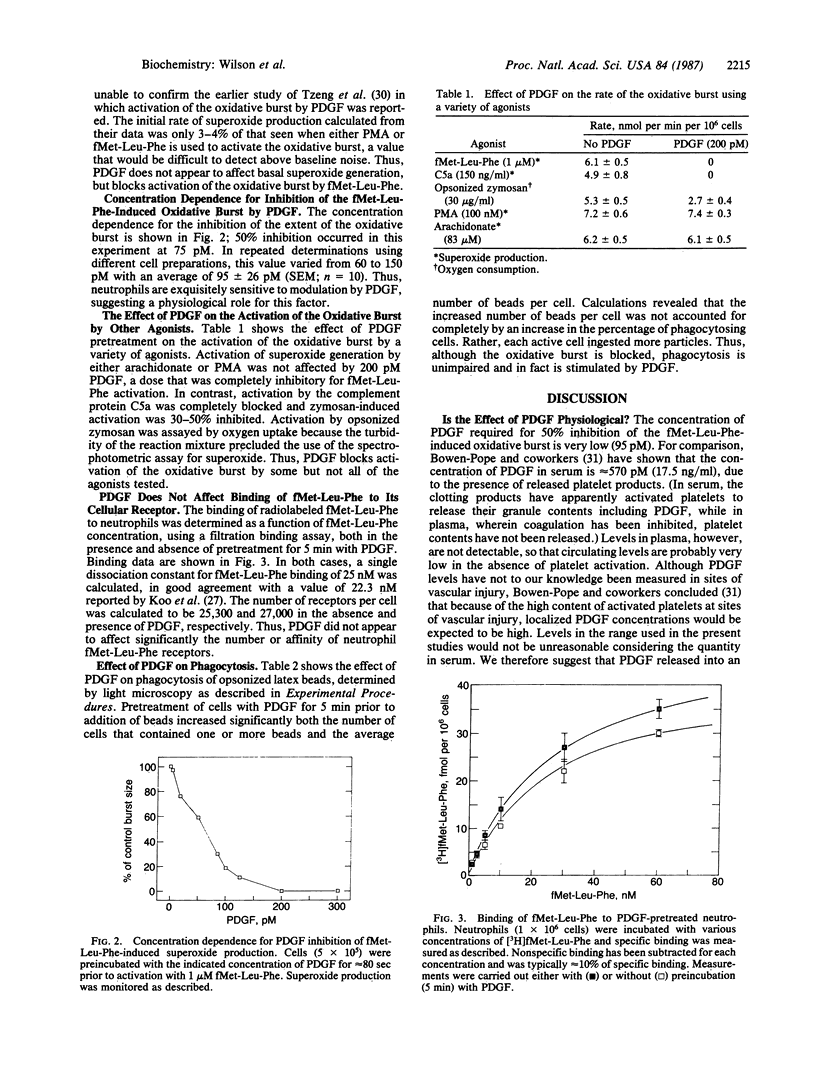
The effect of platelet-derived growth factor (PDGF) on agonist-induced activation of the superoxide-generating oxidative burst in human neutrophils was tested. PDGF had no effect on the resting level of superoxide generation but inhibited both the rate and the extent of fMet-Leu-Phe-stimulated superoxide production in a dose-dependent manner. The concentration required to inhibit the response by 50% was 95 +/- 26 pM (n = 10). PDGF also blocked activation by other receptor-mediated agonists such as the complement protein C5a and opsonized zymosan, but not by phorbol myristate acetate or arachidonate, both of which may act at postreceptor sites. The growth factor, however, had no effect on the binding of fMet-Leu-Phe to its receptor. PDGF in concentrations that blocked the oxidative burst stimulated phagocytosis of opsonized latex particles. Thus, PDGF functions as a heterologous "down-regulator" of receptor-mediated activation of the neutrophil oxidative burst and an activator of phagocytosis. A model for a feedback regulatory loop between platelets and neutrophils is proposed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N. Human platelet-derived growth factor (PDGF): purification of PDGF-I and PDGF-II and separation of their reduced subunits. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7314–7317. doi: 10.1073/pnas.78.12.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswanikumar S., Corcoran B., Schiffmann E., Day A. R., Freer R. J., Showell H. J., Becker E. L. Demonstration of a receptor on rabbit neutrophils for chemotactic peptides. Biochem Biophys Res Commun. 1977 Jan 24;74(2):810–817. doi: 10.1016/0006-291x(77)90375-8. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., Kipnes B. S. Pyridine nucleotide-dependent superoxide production by a cell-free system from human granulocytes. J Clin Invest. 1975 Oct;56(4):1035–1042. doi: 10.1172/JCI108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Becker E. L. A multifunctional receptor on the neutrophil for synthetic chemotactic oligopeptides. J Reticuloendothel Soc. 1979 Dec;26(Suppl):701–709. [PubMed] [Google Scholar]
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol trisphosphate formation and calcium mobilization in Swiss 3T3 cells in response to platelet-derived growth factor. Biochem J. 1984 Aug 15;222(1):195–201. doi: 10.1042/bj2220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Malpass T. W., Foster D. M., Ross R. Platelet-derived growth factor in vivo: levels, activity, and rate of clearance. Blood. 1984 Aug;64(2):458–469. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chignard M., Selak M. A., Smith J. B. Direct evidence for the existence of a neutrophil-derived platelet activator (neutrophilin). Proc Natl Acad Sci U S A. 1986 Nov;83(22):8609–8613. doi: 10.1073/pnas.83.22.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T. Activation of human neutrophil nicotinamide adenine dinucleotide phosphate, reduced (triphosphopyridine nucleotide, reduced) oxidase by arachidonic acid in a cell-free system. J Clin Invest. 1985 May;75(5):1740–1743. doi: 10.1172/JCI111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J., Czech M. P. Platelet-derived growth factor mimics phorbol diester action on epidermal growth factor receptor phosphorylation at threonine-654. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4080–4084. doi: 10.1073/pnas.82.12.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demopoulos C. A., Pinckard R. N., Hanahan D. J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem. 1979 Oct 10;254(19):9355–9358. [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M. Neutrophil degranulation. Contemp Top Immunobiol. 1984;14:189–219. doi: 10.1007/978-1-4757-4862-8_7. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Seppä H. E., Kleinman H. K., Martin G. R. Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3669–3672. doi: 10.1073/pnas.78.6.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Hanahan D. J. Platelet activating factor: a biologically active phosphoglyceride. Annu Rev Biochem. 1986;55:483–509. doi: 10.1146/annurev.bi.55.070186.002411. [DOI] [PubMed] [Google Scholar]
- Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol. 1985;32:115–154. doi: 10.1016/s0079-6603(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Chao F. C., Stiles C. D., Antoniades H. N., Scher C. D. Platelet alpha granules contain a growth factor for fibroblasts. Blood. 1979 Jun;53(6):1043–1052. [PubMed] [Google Scholar]
- Koo C., Lefkowitz R. J., Snyderman R. The oligopeptide chemotactic factor receptor on human polymorphonuclear leukocyte membranes exists in two affinity states. Biochem Biophys Res Commun. 1982 May 31;106(2):442–449. doi: 10.1016/0006-291x(82)91130-5. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Southwick F. S., Stossel T. P., Whitin J. C., Simons E., Cohen H. J. A variant of chronic granulomatous disease: deficient oxidative metabolism due to a low-affinity NADPH oxidase. N Engl J Med. 1981 Nov 26;305(22):1329–1333. doi: 10.1056/NEJM198111263052207. [DOI] [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Shirley P. S., Clayton C. C., Snyderman R. Activation of the respiratory burst enzyme from human neutrophils in a cell-free system. Evidence for a soluble cofactor. J Clin Invest. 1985 May;75(5):1735–1739. doi: 10.1172/JCI111884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Miller C. H., Lewis J. C., Wykle R. L., Bass D. A., McCall C. E., Waite M., DeChatelet L. R. Neutrophil responses to platelet-activating factor. Inflammation. 1981 Sep;5(3):193–201. doi: 10.1007/BF00914443. [DOI] [PubMed] [Google Scholar]
- Olashaw N. E., O'Keefe E. J., Pledger W. J. Platelet-derived growth factor modulates epidermal growth factor receptors by a mechanism distinct from that of phorbol esters. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3834–3838. doi: 10.1073/pnas.83.11.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Moncalvo S., Rossi F., Romeo D. Enzymatic basis of metabolic stimulation in leucocytes during phagocytosis: the role of activated NADPH oxidase. Arch Biochem Biophys. 1971 Jul;145(1):255–262. doi: 10.1016/0003-9861(71)90034-8. [DOI] [PubMed] [Google Scholar]
- Pember S. O., Barnes K. C., Brandt S. J., Kinkade J. M., Jr Density heterogeneity of neutrophilic polymorphonuclear leukocytes: gradient fractionation and relationship to chemotactic stimulation. Blood. 1983 Jun;61(6):1105–1115. [PubMed] [Google Scholar]
- Pember S. O., Shapira R., Kinkade J. M., Jr Multiple forms of myeloperoxidase from human neutrophilic granulocytes: evidence for differences in compartmentalization, enzymatic activity, and subunit structure. Arch Biochem Biophys. 1983 Mar;221(2):391–403. doi: 10.1016/0003-9861(83)90158-3. [DOI] [PubMed] [Google Scholar]
- Ross R. The fibroblast and wound repair. Biol Rev Camb Philos Soc. 1968 Feb;43(1):51–96. doi: 10.1111/j.1469-185x.1968.tb01109.x. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Segal A. W., Coade S. B. Kinetics of oxygen consumption by phagocytosing human neutrophils. Biochem Biophys Res Commun. 1978 Oct 16;84(3):611–617. doi: 10.1016/0006-291x(78)90749-0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Dorling J., Coade S. Kinetics of fusion of the cytoplasmic granules with phagocytic vacuoles in human polymorphonuclear leukocytes. Biochemical and morphological studies. J Cell Biol. 1980 Apr;85(1):42–59. doi: 10.1083/jcb.85.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G. R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982 Feb;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. S., Snyderman R., Friedman E., Mellors A., Mayer M. M. Chemotactic and anaphylatoxic fragment cleaved from the fifth component of guinea pig complement. Science. 1968 Oct 18;162(3851):361–363. doi: 10.1126/science.162.3851.361. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P., Osler A. G. Destruction of rabbit platelets in the allergic response of sensitized leukocytes. I. Demonstration of a fluid phase intermediate. J Immunol. 1971 May;106(5):1244–1251. [PubMed] [Google Scholar]
- Smith C. D., Lane B. C., Kusaka I., Verghese M. W., Snyderman R. Chemoattractant receptor-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate in human polymorphonuclear leukocyte membranes. Requirement for a guanine nucleotide regulatory protein. J Biol Chem. 1985 May 25;260(10):5875–5878. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Peptide growth factors and inflammation, tissue repair, and cancer. J Clin Invest. 1986 Aug;78(2):329–332. doi: 10.1172/JCI112580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng D. Y., Deuel T. F., Huang J. S., Senior R. M., Boxer L. A., Baehner R. L. Platelet-derived growth factor promotes polymorphonuclear leukocyte activation. Blood. 1984 Nov;64(5):1123–1128. [PubMed] [Google Scholar]
- Unkeless J. C., Wright S. D. Structure and modulation of Fc and complement receptors. Contemp Top Immunobiol. 1984;14:171–187. doi: 10.1007/978-1-4757-4862-8_6. [DOI] [PubMed] [Google Scholar]
- Volpi M., Yassin R., Naccache P. H., Sha'afi R. I. Chemotactic factor causes rapid decreases in phosphatidylinositol,4,5-bisphosphate and phosphatidylinositol 4-monophosphate in rabbit neutrophils. Biochem Biophys Res Commun. 1983 May 16;112(3):957–964. doi: 10.1016/0006-291x(83)91711-4. [DOI] [PubMed] [Google Scholar]
- Wharton W., Leof E., Pledger W. J., O'Keefe E. J. Modulation of the epidermal growth factor receptor by platelet-derived growth factor and choleragen: effects on mitogenesis. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5567–5571. doi: 10.1073/pnas.79.18.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E., Olcott M. C., Bell R. M., Merrill A. H., Jr, Lambeth J. D. Inhibition of the oxidative burst in human neutrophils by sphingoid long-chain bases. Role of protein kinase C in activation of the burst. J Biol Chem. 1986 Sep 25;261(27):12616–12623. [PubMed] [Google Scholar]
- Wrann M., Fox C. F., Ross R. Modulation of epidermal growth factor receptors on 3T3 cells by platelet-derived growth factor. Science. 1980 Dec 19;210(4476):1363–1365. doi: 10.1126/science.6254158. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Sullivan S. J. Sensory adaptation of leukocytes to chemotactic peptides. J Cell Biol. 1979 Aug;82(2):517–527. doi: 10.1083/jcb.82.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]


