Introduction
Gene therapy has become an important potential treatment modality for a variety of disorders and has shown great promise for treating genetic deficiencies and mutations, as well as for providing secreted therapeutic proteins and immune modulators. Gene therapy has also become important in preventing disease by use of DNA vaccines. Furthermore, in production animals, gene therapy has been used to enhance reproductive efficiency and production gains.
A variety of means for introducing genes into mammalian cells have been used that can be broadly categorized as viral, chemical, and physical methods. There is a general consensus that viral vectors are the most efficient means of gene transfection, but significant safety concerns, such as the potential to illicit an immune response and/or cause cellular transformation limit virus use in a variety of settings. Chemical methods can be effective in vitro, but their use in vivo needs further improvement because their transfection efficiency is generally lower than for viral and physical methods in most of cases. Physical transfection avoids many of the undesirable effects of chemical and viral methods, can be used repetitively, is relatively simple and cost-effective, and has essential no limitation on the coding length of the gene to be introduced. Of the physical methods of gene transfection, electroporation (EP) is the most commonly used method in a variety of animal and human trials.
Historically, gene therapy in large animals (defined as species of animals other than rats, mice, and small rodents) has been pursued using viral and non-viral vectors carrying genes to be transfected into host cells. Since in vivo EP has emerged as one of the few powerful non-viral vector delivery methods for efficiently and effectively delivering plasmid DNA and nucleic acids in vivo, this review will be limited to EP-based gene therapy. For the purpose of this review, in vivo EP refers to reversible EP for the purpose of gene delivery, not irreversible EP where the therapeutic intent is ablation of cells using EP technique alone. Several recent reviews examining the use of irreversible (1-7) and reversible (8-11) EP are published elsewhere. The focuses of this review are novel aspects of EP as applied to large animals, improvement of EP delivery technique, and development of EP-based vaccines.
The mechanism for EP-mediated DNA entry into cells is incompletely understood
During EP, a series of square-wave electric pulses are used to drive naked DNA into cells. A stable, non-dividing population of muscle cells is transfected when long-term expression of a gene is desired, for example in supplementing clotting factors to treat hemophilia (though immunogenicity often neutralizes the circulated clotting factor).(12,13) Alternatively, a tumor’s population of neoplastic cells, stromal cells, and attending inflammatory cells are transfected in tumor gene therapy, for example introducing interleukin 12 to trigger anti-tumor immunity.(14) Regardless of the target cells transfected, EP exposes tissue to a brief electric field which induces temporary and reversible breakdown of cell membranes and formation of pores. The electric field also takes advantage of the tendency of negatively-charged nucleic acid to migrate toward the positive pole in an electric field (an electrophoretic effect).
Within the cell membrane, pores form within 10 ns (15) and initially are less than 10 nm in diameter. (16,17) While the duration of pore formation may be short, reconstitution of the cell membrane may be prolonged by decreasing temperature. However with longer duration electric field application, pore number increases and pores begin to coalesce. When large enough pores form, the damage becomes irreversible and cells die (irreversible EP). Fortunately, the size of pores normally is not a limiting factor, both small oligonucleotides and nucleotides larger than 150 kb, which is larger than the pores, have been shown to readily enter the cell during EP. This fact suggests that the mechanism of gene transfer into cells may simply be based on diffusion;(18) or, as other researchers have suggested, that electric-pulse-induced membrane instability causes membrane bound vesicles containing DNA to form which are carried into the cell by endocytosis.(19) Despite numerous theories, the mechanism of nucleic acid entry into cells remains open to conjecture.(20) All models explaining nucleotide migration through the cell membrane must be based on several physical postulates including: the existence of long-lived electropores,(21-23) a preliminary binding step at the cell surface due to membrane plasmid DNA interaction and then DNA diffusion through electropores,(22) electrophoretic forces generated by the external field which push the plasmid DNA through the membrane,(24, 25) and adsorption by sphingosine/DNA interactions with insertion and passage of DNA through a hydrophilic percolated porous zone.(26) [Scott: this sentence is more or less repeat of ref 24 and 25 but it may not fit to the continuous process that you intend to develop. Please either remove the highlighted portion and merge the ref into the place of ref 22 or rewrite it] Interactions have been observed between DNA and model lipid bilayers which suggest that other mechanisms, including endocytosis, may also play a role membrane-DNA interaction. In fact, DNA-induced endocytosis has been observed in a number of studies, in the absence of any electric field.(27, 28)
It is generally accepted that when the cell membrane is not permeabilized, electric field lines or vectors follow the outer profile of the cell, and DNA flows in the direction of the field around the cell to the anode. When the membrane is permeabilized, electric field lines enter the cell membrane and DNA is trapped in the region of the cell membrane opposite the cathode where it is effectively pushed up against the membrane by electrophoretic force.(20) Interaction with the permeabilized membrane prevents DNA from flowing around the cell. Thus, Favard, et al., conclude that electrotransfection is a multistep process where negatively charged DNA migrates by electrophoresis towards the cell plasma membrane on the cathode side where it accumulates.(20) When electric fields exceed a certain threshold, the plasma membrane is permeabilized allowing accumulated plasmid DNA to enter. This translocation of plasmid DNA from the plasma membrane to the cytosol and subsequent passage to the nuclear envelope takes minutes to hours. Intracellular movement also occurs by an as yet undetermined mechanism which may involve simple diffusion, endocytosis, or electrophoretic movement. Upon entering the nucleus, gene transcription from plasmid DNA can take place.(20)
Progress in achieving high-level and long-term gene expression
In most cases, the DNA that enters the nucleus is transiently transcribed and rarely integrated into the host cell genome. Although this means that the frequency of genome disruption is lower than techniques involving integration of the gene in the host genome,(29) the optimal conditions for long-term levels of transgene expression without adverse side effects, remains a primary objective for researchers.(30) Despite the fact that transient expression of the transgene remains one of the major shortcomings of nonviral DNA delivery, this shortcoming can partially be alleviated by using post-mitotic, stable cells, such as myocytes. EP-mediated gene therapy in continually dividing cells often yields declining transgene expression, probably due to degradation, since extrachromosomal DNA is known to persist in post-mitotic tissues. Several recent studies, however, have shown that the integrase from bacteriophage ϕC31 confers genomic integration of plasmid DNA and long-term expression in mammalian cells in a variety of contexts. Used together, EP-mediated transfection and ϕC31 integrase could be a powerful combination for long-term, nonviral gene therapy.(31, 32)
Electron Avalanche Transfection and Electrosonoporation
Improvements in EP methods, equipment, and protocols are inevitable and there have been numerous alterations in EP technique and attempts to understand the mechanisms underlying EP in recent years. An interesting recent example of a technique based on EP that has been used for gene therapy in rabbit eyes, is electron avalanche transfection (EAT). In EAT, an electric field with high voltage amplitude is produced from microelectrodes adjacent to plasma bubbles or blebs (between the retina and choroid in this study). This forms a transient vapor cavity in the plasma space which is ionized, allowing conductance from the electrode through the vapor cavity to the tissue. At the same time, the cavitation bubble generates a propagating acoustic wave that exposes the tissue to mechanical stress synchronized with an electric field. In initial studies on chorioallantoic membrane, electron avalanche transfection was >10,000-fold more efficient and produced less tissue damage than conventional EP. Efficient plasmid DNA transfer to the rabbit retina after subretinal DNA injection and trans-scleral EAT was also demonstrated in this study. Electroretinograms and histology showed no evidence of damage from the procedure.(33)
EAT differs from conventional EP by using microelectrodes instead of large electrodes; by relying on ionization of the vapor cavity to deliver the electric field and mechanical stress; and by using short, biphasic electric pulses. Since arc production is considered detrimental in conventional EP, EAT delivers the electrical charge via an ionized vapor cavity which prevents arc generation, allowing use of much higher electric fields. Furthermore, short, biphasic pulses cause little or no muscle movement which is desirable for precision and patient comfort. Increased EP efficiency under the tensile stress created during EAT may occur because of increased lipid bilayer instability and resulting increased susceptibility to permeabilization.(33)
Another modification of EP, electrosonoporation (ES) is similar to EAT in that EP is combined with a physical method of inducing pore formation through interaction with cavitation bubbles. Sonoporation (SP) uses ultrasound to temporarily permeabilize cell membranes allowing uptake of compounds from the extracellular environment; since membrane alteration is transient, the compound is left trapped inside the cell after ultrasound exposure. Ultrasound produces microscopic cavitation bubbles within the extracellular milieu; the cavitation bubbles implode producing a shockwave while on or near the surface of a cell membrane; and the tiny shockwave produces pores in the cell membrane allowing compounds to diffuse into the cell.(34) Steps in ES include preparation of a DNA-microbubble preparation, injection, and tissue ultrasound exposure.(34)
Ultrasound offers good penetration through soft tissue, minimal damage to cells/tissues, and does not damage DNA; however, it is limited by breakdown of cell cytoskeleton which among other perturbations, alters DNA trafficking within cells.(35)
Combining EP with SP has been used in multiple studies for gene transfer and has been found to be effective.(36, 37) When used to transfect the luciferase reporter gene into muscle, ES was found to be twofold more effective than EP alone.(37)
Regardless of how EP is performed, a persistent, high-level of gene expression is unlikely. This is particularly true for secreted proteins because of their immunogenicity. Our results suggest that the secreted alkaline phosphatase (SEAP) reporter gene maintains long-term expression at a level of 5 ng/mL in blood, but expression is shut down rapidly after exceeding this level following intramuscular delivery of SEAP gene via EP (Figure 1). [Scott: please look at the legend]. This observation is most likely due to development of an immune response, since a titer of anti-SEAP antibody was detected in the animals expressing a high level of SEAP. Other mechanisms such as death of the cells with high reporter gene expression may also contribute.
Figure 1.
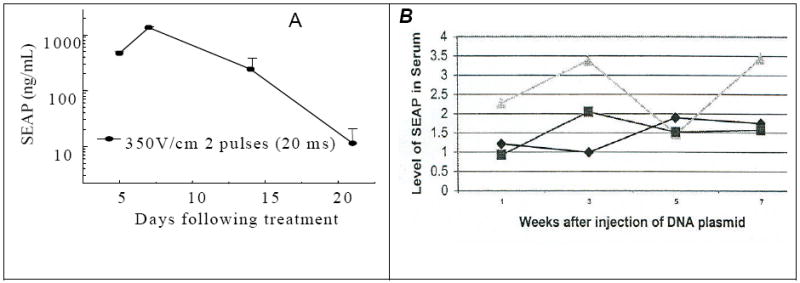
(A) (Left panel). SEAP expression decline sharply in serum on day 20 after the treatment when a high level SEAP protein was expressed after intramuscular administration a large dose (100 /μg) of SEAP-encoding DNA with electric pulses (n=5) under an electric field of 350V/cm. (B) (Right panel). Persistent level of SEAP expression (ng/mL serum) for 7 weeks when a low level of SEAP protein was expressed after intramuscular administration of a low dose (10 /μg) SEAP-encoding DNA under a low electric field (250 V/cm). Each curve represents the SEAP activity from an individual mouse.
EP formulations and novel concepts in setting EP parameters
Although EP is the determining factor that dictates gene transduction in vivo, the carrier solution in which genes are delivered by EP can affect the efficacy and damaging effects of EP. Physiologic saline is most commonly used, and when combined with EP, expression of luciferase is enhanced by 10,000 fold over direct injection in muscle.(38) In tumors, expression after EP of luciferase gene in saline was increased 1200 fold.(39) Alternatively, concentrated DNA formulation in phosphate-buffered saline (10 mg plasmid DNA per mL) yielded high levels of expression in skeletal muscle.(40) Salt concentrations influence transfection efficiency by altering the ionic atmosphere, ionic strength, and conductivity of the DNA formulation. Lee, et al., demonstrated a general trend toward increasing efficiency of luciferase reporter gene expression with decreasing vehicle cationic strength. However, overall transfection efficiency was diminished by tissue damage because of hypo-osmotic stress and electrical injury induced by low conductivity. Ultimately these authors found that the optimal saline concentration was 71 mM for EP in skeletal muscle.(41)
Ionic strength is not the only consideration when formulating a medium for EP of nucleic acids; a variety of polymers and adjuvants (in the case of vaccines) have also been added to EP formulations. Addition of a variety of polymers to DNA formulations have been shown to increase efficacy and decrease toxicity of EP. Poly-L-glutamate, polyacrylic acid, poly-L-aspartate, dextran sulfate, pectin, poloxamer 188, polyvinylpyrrolidone, and cationic liposomal formulations have been examined and found effective.(13, 42-49) Addition of 15-50 kDa poly-L-glutamate is one example of the increased efficacy, without toxic effect, that may be provided by adding polymers to the DNA formulation; 6 mg/ml of ploy-L-glutamate has consistently improved EP efficiency by 4-12 fold.(42, 46) In the case of poly-L-glutamate, these effects are thought to be a result of its ability to decrease DNA clearance and increase DNA stability in muscle.(42, 46) Poloxamer 188 provides an example of a polymer which has been added to decrease EP damage.(47) Perhaps most promising, are the cationic liposomal formulations that have been shown to increase transfection in a variety of mouse tumor systems.(49) Addition of adjuvants to the formulation can increase vaccine efficacy and will be discussed in the vaccine application section of this review.
The actual EP parameters and conditions are as important as or more important than the nucleic acid formulation for effective electrotransfection. Aside from the desirability of having adaptive constant-current EP discussed previously,(50) longer duration electric pulses with lower voltage have been shown to give the same EP effect as high voltage shorter duration pulses. Specifically, a pulse of 100 V/cm lasting 100 ms yields expression equivalent to 25 V/cm for 160 ms.(51) Thus, to minimize tissue injury, lower voltages can be used to decrease heat build-up and resultant necrosis. In other studies, Satukauskas, et al., have shown that a train of long identical pulses, or combinations of pore-creating high-voltage, short-duration electric pulses and electrophoretic low-voltage, long-duration electric pulses are necessary for efficient gene transfection.(52, 53) More recently, Andre’, et al., demonstrated high level gene expression in muscle by delivering a single 800 V/cm, 100 microsecond pulse, followed by four 80 V/cm, 100 millisecond pulses.(54, 55) Furthermore, when these investigators examined the effect of fast versus slow injection of transfection medium, they demonstrated that very fast injection of transfection medium into tissue (20 ul/2 sec) increases gene expression by 500-fold compared to the classic slow injection (20 ul/25 sec) technique.(56)
Application of EP in large animals, including humans
Until now, in vivo EP has been primarily conducted in murine models, but attempts and applications to large animals have gained momentum in recent years. Most applications in large animals use muscle as the target tissue. As alluded to previously, skeletal muscle is an ideal tissue for EP-mediated gene transfer. Muscle fibers are long-lived post-mitotic cells, and muscle is well vascularized, allowing efficient transport of gene products into the systemic circulation. Access to numerous muscle groups is also relatively easy in most species. Furthermore, gene expression in muscle after EP-mediated gene transfer has been reported to be as long as 9-19 months.(39, 57, 58) Thus, skeletal muscle-targeted EP has been used for introduction of numerous genes to supplement production of critical secretory molecules in deficient hosts or augment levels of gene product already present.
Perhaps the largest amount of work using EP-mediated gene therapy in large animals has been conducted optimizing EP parameters in pigs. Bureau, et al. demonstrated efficient EP-mediated transduction of growth hormone releasing hormone (GHRH) gene using electric pulses of low field intensity. They also found that internal needle electrodes give a 25-fold increase in expression levels compared with caliper electrodes in skeletal muscle in swine, and demonstrated that by optimizing the EP method, favorable physiological changes, such as enhanced weight gain and improved body composition, could be obtained at extremely low plasmid doses in a large mammal. Furthermore, they found that the degree of permeabilization of the muscle cells is dependent on the electric field intensity, length of pulses, shape and type of electrodes.(59) Somiari, et al. found that cell size was also an important parameter in determining degree of permeabilization.(60) Use of needle electrodes in large mammals, such as pigs or humans, is necessary because of the increased resistance of the skin, the thickness of the subcutaneous fat tissue, and the concern for tissue damage if the intensity of the electric field were to be proportionally increased using caliper or plate-type external electrodes.(40) Brown, et al. further optimized muscle EP-mediated gene transfer by determining that using constant current pulses, between 0.4 and 0.6 A applied 80 seconds after injection of 0.5 mg plasmid DNA expressing secreted embryonic alkaline phosphatase reporter gene in a total volume of 2 mL produced the highest level of expression in semimembranosis muscle in pigs. Increased injection volumes and increasing lag time between injection and EP did not improve transfection efficiency.(61)
Numerous other studies have applied EP-mediated gene transfer in pigs with excellent results. The bulk of applications thus far have been directed at regulating fat and muscle mass. Draghia-Akli, et al. note that EP-mediated gene transfer is particularly appropriate for modulating the intrinsic properties and mass of muscle and fat. Treatment conditions such as cachexia associated with chronic diseases, autoimmune diseases (e.g., myasthenia gravis), stimulation or suppression of appetite, and in vivo manipulation of glucose metabolism and fat deposition in patients with diabetes are some of the applications of EP-mediated gene therapy in muscle. Basic studies of muscle-specific transcription factors and their impact on development, also benefit from use of EP-mediated gene therapy. Additionally, it has recently been suggested that administration of the gene for leptin, a hormone predominantly produced by adipocytes and, functionally, a key regulator of body weight, may ameliorate obesity from a variety of causes.(62)
Young pigs that underwent muscle EP with GHRH plasmid had significantly greater weight gain, significantly increased lean body mass, and decreased fat mass when compared with controls. Additionally, pigs undergoing EP with GHRH plasmid were leaner at end of study than controls, and had a proportional increase in all internal organs and higher bone density.(40) Similarly, pregnant sows treated with GHRH gene intramuscular EP had offspring with optimal health and growth characteristics and significantly reduced morbidity and mortality. Treated pigs also expressed GHRH for at least one year, and beneficial effects on offspring occurred for three consecutive pregnancies.(63)
A study of GHRH gene intramuscular EP in thirty-two Holstein heifers yielded cows with improved immune function, health status, significantly increased body weights at 100 days of milk production, and improved body condition scores.(64)
Myogenic plasmid containing GHRH has also been delivered by muscle EP in severely debilitated dogs with naturally occurring tumors, and yielded significantly increased concentrations of IGF-1 and increased muscle mass.(65) Similar to the previously mentioned work by Andre’, et al., work in dogs demonstrated that a combination of 1 high voltage pulse (600 V/cm, 100 μs), followed by 4 low voltage pulses (80 V/cm, 100 ms, 1 Hz) yielded the same transfection efficiency as the standard trains of low voltage pulses, and was able to yield detectable systemic expression of human interleukin-12. Only mild and transitory local side effects, without clinically detectable systemic side effects, were seen, indicating that electrotransfection is a feasible, effective, and safe method for muscle targeted gene therapy in dogs, which could have potential for clinical applications in small animal veterinary practice.(66)
In other applications of muscle EP in dogs, Fewell et al. were able to produce measurable levels of factor IX in treatment of hemophilia B and described a method for producing high transfection efficiency with high levels of systemic factor IX following a single administration;(12, 13) Draghia-Akli, et al. were able to demonstrate effects of GHRH in young, healthy Beagles;(67) and Tone, et al. were able to demonstrate long-term gene expression in muscle EP in dogs.(58)
Electrotransfer of plasmid DNA into skeletal muscle has been successfully achieved in many different experimental animals including mice, rats and rabbits,(38, 68) cattle,(64, 69) goats,(69) sheep,(70) pigs,(71, 72) dogs,(13, 65) and monkeys.(73) As in the dog studies previously mentioned, it has been shown that a better transfection efficiency can be achieved using combination of one high voltage electric pulse followed by different numbers of low voltage electric pulses.(53) It has been hypothesized that the high voltage pulse first causes permeabilization of cell membrane, followed by electrophoresis of DNA across destabilized cell membrane during the low voltage pulses.(53, 59, 74) Large animal models and production use of EP-mediated gene therapy is therefore growing significantly; EP-mediated gene therapy in humans is not far behind.
Hellor’s group is now on the cusp of exciting developments in human gene therapy heralded by the completion of the first clinical phase 1 trial of EP-mediated gene therapy.(14) Daud, et al., reported safe and effective EP-mediated transfection of human melanoma patients with the gene for interleukin-12. Patients had regression of melanoma skin lesions and distant metastases, and the authors stated that, when compared to other forms of gene delivery, EP produced a greater magnitude of clinical benefit in metastatic melanoma.(14) Additionally, a phase 1 clinical trial using the gene for interleukin-2 for the treatment of melanoma is also underway with promising preliminary results.(75)
Application of EP for DNA vaccines
Vaccines (biological agents capable of triggering specific immunity against infectious diseases or cancer) can be delivered in a variety of ways, including EP. They can be categorized as inactivated/killed, attenuated/live, toxoid, component, and gene-based (DNA, RNA, oligonucleotides) vaccines that can be administered for a variety of purposes. These purposes were historically limited to infectious disease prevention, but now include tumor vaccine development and use in a variety of immune-mediated degenerative diseases. Of the different types of vaccine and their different targets, perhaps use of genetic vaccines as applied to both tumor vaccines and vaccination for infectious diseases shows the most promise when combined with EP.
Gene vaccines evolved from revolutions in molecular engineering and gene delivery, and their usage has become commonplace over the last few years. Gene vaccines use DNA to express immunogen and induce an immune response. Various gene delivery approaches are available to administer gene vaccines which, similar to other gene therapies, can be categorized as viral and non-viral. As stated previously, the use of viral vectors can be very effective in transfecting cells and inducing an immune response, but is limited by safety issues. Injection of naked DNA vaccine is safe, and in muscle yields long-term gene expression, but very little antigen response is produced.(76) DNA injection followed by EP is much more effective, and induces a similar level of immune response as protein immunization. (77) Despite some early experimental successes, developing safe and effective DNA vaccines requires optimization of several variables before widespread EP-mediated gene vaccine administration becomes commonplace. Ultimately, the simplicity and effectiveness of genetic vaccination using EP may allow widespread use of gene vaccination in large animals and humans in the near future.
Optimization of gene construction can markedly enhance transfection efficiency and the resulting immune response. To be an effective vector, plasmid DNA should contain a strong viral promoter and a strong polyadenylation transcription termination signal. Additionally, most vaccination vectors also contain an intron to increase expression. When the whole antigen is toxic or immunosuppressive, epitopes from the antigen may be utilized and can be expressed as mini-genes, which are inserted into unrelated but highly immunogenic sequences that successfully induce both cellular and humoral responses.
Optimization of EP parameters can also improve the outcome of genetic vaccine. As noted previously, to select optimal parameters for EP-mediated DNA delivery in vivo, specific needs for different tissues, vaccine formulations, and DNA dosages must be considered simultaneously. Also noted previously, muscle is the most commonly targeted tissue for EP-mediated gene delivery because of, among other reasons, the large quantity of tissue and its rich blood supply which allows systemic circulation of secreted proteins. For vaccination, EP-induced muscle cell damage may be beneficial because of the release of a variety of cytokines which may help initiate immune response by attracting antigen presenting cells (APCs) to the injection site.(70) Muscle selection is also important; aside from accessibility, muscle should be chosen based on EP efficiency difference in different muscles. For example, in mice the anterior tibialis muscle has been demonstrated to have the highest expression of muscles tested for secreted alkaline phosphatase.(50) Unfortunately, even when all the aforementioned factors are optimized, intramuscular administration of gene vaccine may produce a less than optimal immune response in some circumstances.(50)
Skin is a more traditional target tissue for vaccination because it is readily accessible and has a large population of unique antigen presenting cells. Keratinocytes are primarily responsible for transgene expression after intradermal (ID) administration.(78) Expression of immunogen by keratinocytes can induce an immune response through interaction with bone marrow-derived dermal Langerhan’s cells and dermal dendritic cells.(79) Similar to the findings of enhanced intramuscular expression of genes when delivered by EP in muscle, 100 to 1000-fold higher gene expression was induced after ID delivery of plasmid DNA when introduced by EP. Specifically, higher levels of prostate-specific antigen (PSA)-stimulated CD8+ T cells were induced after intradermal EP delivery of low-dose PSA DNA vaccine in a mouse model.(80) Thus, skin continues to be a common target of EP-mediated vaccine use now and in the future.
Conversely, there is some evidence that in certain settings, IM EP does produce better immunization than ID EP of gene vaccines.(68) The low levels of EP-mediated, gene vaccine-induced immunity in muscle alluded to before, may be a result of the lack of cytokines released by professional APCs. When expressed immunogen is secreted and taken up by large numbers of professional APCs, the APCs present antigen and cross-prime large numbers of cells.(81) In contrast, ID administration exposes a much smaller number of APCs to transfected cells and development of immunity.
As is the case for traditional vaccines, addition of adjuvant can significantly increase the magnitude and duration of vaccine-induced immune response in gene vaccines. Lipopolysaccharide (LPS), a component of gram negative bacterial cell walls and potent endotoxin, has been used to augment immune responses through toll-like receptor 4 (TLR4).(82) Because granulocyte macrophage-colony stimulating factor (GM-CSF) has a potent effect on DC differentiation and maturation, and also on expression of MHC and co-stimulatory molecules, it has been utilized as immune adjuvant for vaccine against numerous infectious diseases and cancer.(83-87) Oligonucleotides are also being investigated as adjuvant with promising initial results.(88-92) Incorporating adjuvant into the gene construct has been demonstrated in an elegant example of enhanced anti-tumor vaccine efficacy using dendritic cells electrotransfected with mRNA containing the gene for tumor associated antigens (TAA) linked to mRNA encoding ubiquitin. The resulting ubiquitinated TAA product was effectively targeted to the proteasome, enhancing degradation of TAA which resulted in more efficient priming of TAA-specific CD8+ T-cells.(86, 93)
EP has also been used in the development of “cell vaccines”. For example, dendritic cells have been EP transfected with the gene for tumor-associated antigens ex vivo and reintroduced to patients to enhance their anti-tumor immune response. EP transfection of DCs with mRNA results in higher protein expression in DCs than DNA, and carries no risk of integration into host genome. RNA instability can be minimized by modifying the mRNA with a 3’-poly(A) tail and a 5’ 7-methylguanosine cap. A number of studies have been reported using this approach to various antigens such as melanoma, carcinoembryonic antigen,(84) human telomerase reverse transcriptase, and HER-2/neu antigen.(94, 95) Using this approach for infectious disease vaccines has been much less common; nevertheless one recent publication reported improvement in hepatitis C prevention using mRNA-transfected DC-mediated vaccine.(96)
Although active research using EP-mediated gene vaccines has grown exponentially over the past few years, most of the research to date has been in small animals. In general, studies in large animals have demonstrated less efficacy than in small animals, but the number of studies using large animals pales in comparison to those in mice. Effective EP-mediated gene vaccination in primates has been demonstrated however; Zhao and Xu looked at numerous combinations of EP parameters for vaccination against hepatitis B virus (HBV) and found a great variation in efficiency depending on the EP parameters selected.(97) These authors also provided another example of using immune-modulating fusion genes (interleukin-2 and gamma interferon) as an adjuvant enhancing immune responses in EP-mediated gene vaccination.(97) Thus EP-mediated gene vaccination shows great promise for achieving high gene expression, efficient humoral and cellular responses, and specific protection against antigens, including in more clinically relevant species such as the Rhesus macaques used in this study. Furthermore, EP-mediated gene vaccination has proven safe, stable, easy to manipulate, and relatively inexpensive.
Innovative applications of EP gene therapy
Investigations into treatment of type I diabetes mellitus (T1D) have also used muscle-targeted gene via EP delivery. T1D is due to a loss of immune tolerance to islet antigen and thus, there is intense interest in developing therapies that can re-establish tolerance. Tolerance is maintained by complex mechanisms that include inhibitory molecules and several types of regulatory T cells (Treg). A major historical question is whether gene therapy can be employed to generate Treg cells. Recent studies indicate that gene transfer of immunoregulatory molecules can prevent T1D and other autoimmune diseases. In studies by Prud’homme, et al., in vivo EP-mediated gene transfer was thought to have the potential to be used to perform DNA vaccination against islet cell antigens. When combined with appropriate immune ligands, this would result in the generation of Treg cells and protection against T1D. In vivo, EP can also be applied for non-immune therapy of diabetes. It can be used to deliver protein drugs such as glucagon-like peptide 1 (GLP-1), leptin, or transforming growth factor beta (TGF-beta). These act in T1D or type II diabetes (T2D) by restoring glucose homeostasis, promoting islet cell survival and growth or improving wound healing and other complications of T1D.(98)
Bone marrow cells, splenocyte and T cells generally are difficult to achieve a high level of gene delivery in regardless of gene delivery methods employed. Studies by Tervo, et al. found that both EP and nucleofection resulted in high-level transgene expression (up to 60% transgene-positive T cells) from both small and large green fluorescent protein reporter constructs in activated rabbit T cells with moderate cytotoxicity. Both non-viral gene delivery methods were vastly superior to retroviral, lentiviral, or adenoviral transduction approaches. These studies also established conventional EP as an efficient and inexpensive procedure to render primary rabbit T cells accessible to rapid functional ex vivo analyses. Furthermore, the viability of electroporated rabbit T cells was remarkably high (47±7%); compared to analogous studies conducted in primary T cells from rats and mice.(99)
In an interesting study combining in vitro EP and surgically transplanted transfection product, hepatocytes were isolated from a surgically resected liver wedge, electroporated with an insulin expression plasmid ex vivo and reimplanted intraparenchymally under ultrasonic guidance into the liver in each of 10 streptozotocin-induced diabetic Yorkshire pigs. Based on positive results, authors concluded that autologous hepatocytes could be efficiently, simply and safely modified by EP of a plasmid DNA to express, process and secrete insulin. This strategy achieved significant and sustained therapeutic efficacy, and may have broader future applications for the treatment of other acquired and inherited diseases for which systemic reconstitution of a specific protein deficiency is desirable. Combining autologous hepatocytes with ex vivo gene transfer has several advantages. Using this technique, hepatocytes are likely to be of higher quality and can be used fresh (instead of preserved). This also allows use of high voltage EP for transfecting primary somatic cells which otherwise might cause tissue necrosis in vivo. Using autologous cells also overcome the problem of donor scarcity and avoid the need for chronic immunosuppressive therapy.(100)
Another ex vivo example of physical gene transfer has been described by Nakamura, et al., who vaccinated liver allografts with DNA before transplantation to provide protective immunity against infection and malignancy.(101) Although they used a gene gun, EP would have been another viable option for vaccination.
Small double strand RNAs, involved in gene silencing or RNA interference, or closely related micro RNAs derived from endogenous hairpin precursors can bind to RNA-induced silencing complexes and either degrade messenger RNA, block translation, or otherwise suppress gene expression. EP may help overcome the fact that routine therapy or studies with siRNAs is complicated by the fact that these highly charged molecules do not easily enter cells.(102)
In nonhuman primates, gene targeting can produce animal models for translational studies of human diseases. Gene targeting in fibroblasts followed by somatic cell nuclear transfer (SCNT) has been successful in numerous large animal species, including primates. In rhesus macaques gene targeting in a primary culture of adult rhesus macaque fibroblasts was accomplished by culture of adult male fibroblasts transfected by EP of S-phase synchronized cells with a construct containing a SV40 enhancer with human telomerase reverse transcriptase to overcome senescence and allow long term in vitro manipulations.(103) It is thought that these cell lines can be used for the production of null mutant rhesus macaque models of human genetic disease using SCNT technology.(104) Null mutant sheep, goats, pigs and cattle have been produced using an alternative approach: gene targeting in somatic cells followed by nuclear transfer to enucleated oocytes (SCNT; reproductive cloning) whose gene targeting efficiency could also potentially be improved using EP.(105-114)
Unsolved concerns pertaining to EP
One concern in EP-mediated gene transfer in vivo is the amount of tissue damage produced secondary to heat generated. Draghia-Akli, et al. and others have suggested that constant current EP (instead of constant voltage) may reduce tissue damage and contribute to overall success.(43, 64, 71) Unfortunately, exclusively focusing on using lowered voltage pulses in order to decrease cell death through necrosis/oncosis, may not prevent death through cell apoptosis which has been shown to take place even with low voltage EP.(115) Although most of the adverse effects of EP have been characterized in muscle, mild damage has also been reported with ID EP, but this damage was resolved within one week of EP.(79) Additional means of decreasing tissue damage include addition of polymers to the injected DNA formulation, alterations in ionic strength and composition, and augmentation of EP with other transfection techniques such as sonoporation.
Pain is another concern in cases of in vivo EP – especially if the technique is to be applied clinically to non-anesthetized patients. In humans, patients describe muscle contractions as being surprising, sometimes unpleasant, but not painful.(116) Pain from EP is proportional to the absolute applied voltage,(117) and one way of lowering the total voltage is by decreasing the gap between electrodes to 0.4 cm.(116) During EP of cutaneous masses, muscle contractions can also be palliated by elevating or tenting the skin to be electroporated well above the underlying musculature.(118) In certain settings, ex vivo EP may be practical, which would allow for the EP procedure to be conducted on cells harvested from the patient for EP, and subsequently reintroduced in situ, completely eliminating the chance of EP-induced pain.(100)
Vascular effects of EP have also been a concern for many investigators, but recent studies suggest that changes in afferent and efferent vessels during EP may be beneficial, particularly when applied to tumor gene therapy. High voltage pulses cause a brief reflex constriction of afferent arterioles in normal tissue, and in tumor tissue (which have more fragile and tortuous blood vessels) long-term hypoperfusion can occur after EP.(119) These vascular effects may be beneficial in electrochemotherapy because higher concentrations of drug may remain trapped in the tumor due to lack of “wash out” at the time of EP. Similarly, in gene therapy, transient hypoperfusion has been shown to enhance gene expression.(120-122)
One of the advantages of using EP and other non-viral vectors is that they are not hampered by vector immunogenicity if properly designed (by removal of CpG islands and residual bacterial sequences). If not properly designed, CpG-mediated nonspecific inflammatory effects (e.g. mediated through binding to TLR-9) can injure tissues and/or confuse the interpretation of immunological studies. Additionally, many viral promoters are turned off by inflammatory cytokines.(123-125) Another approach to minimize immunogenicity is to delete most vector elements producing “minicircles” containing the expression cassette.(126) Furthermore, if the gene being transfected is to be secreted, signal peptide sequences may also play an important role in functional expression. For example, studies involving nonhuman primates that received an erythropoietin encoding plasmid showed that changing the transgene leader sequence and optimizing the gene codon usage yielded higher levels of circulating transgene product and a more significant biological effect than the wild-type gene.(73) Thus, by altering the plasmid or transfected DNA design and sequence, one can minimize the dose necessary to attain physiological levels of the target hormone, enzyme, or peptide, and manipulate the expression of the newly produced transgene product.
Conclusion
In summary, in vivo EP-mediated gene therapy is gaining ground as one of the most important means for non-viral gene therapy. Further understanding of the mechanisms of target cell DNA entry, intracellular DNA transport, and nuclear processing will allow further optimization of the technique through optimization of gene formulations and electrical pulse parameters. Furthermore, the ability to augment EP with tension forces (in EAT and ES), use it in an ex vivo setting, and incorporate integrase enzyme to allow genomic integration of transfected genes may broaden the appeal of this technique. At this point, there appear to be few limitations, and potential uses continue to grow as these limitations are overcome.
References
- 1.Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat. 2007 Aug;6(4):295–300. doi: 10.1177/153303460700600405. [DOI] [PubMed] [Google Scholar]
- 2.Esser AT, Smith KC, Gowrishankar TR, Weaver JC. Towards solid tumor treatment by irreversible electroporation: intrinsic redistribution of fields and currents in tissue. Technol Cancer Res Treat. 2007 Aug;6(4):261–74. doi: 10.1177/153303460700600402. [DOI] [PubMed] [Google Scholar]
- 3.Rubinsky B. Irreversible electroporation in medicine. Technol Cancer Res Treat. 2007 Aug;6(4):255–60. doi: 10.1177/153303460700600401. [DOI] [PubMed] [Google Scholar]
- 4.Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality--clinical implications. Technol Cancer Res Treat. 2007 Feb;6(1):37–48. doi: 10.1177/153303460700600106. [DOI] [PubMed] [Google Scholar]
- 5.Miller L, Leor J, Rubinsky B. Cancer cells ablation with irreversible electroporation. Technol Cancer Res Treat. 2005 Dec;4(6):699–705. doi: 10.1177/153303460500400615. [DOI] [PubMed] [Google Scholar]
- 6.Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005 Feb;33(2):223–31. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 7.Al-Sakere B, Andre F, Bernat C, Connault E, Opolon P, Davalos RV, Rubinsky B, Mir LM. Tumor ablation with irreversible electroporation. PLoS ONE. 2007;2(11):e1135. doi: 10.1371/journal.pone.0001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mir LM. Application of electroporation gene therapy: past, current, and future. Methods Mol Biol. 2008;423:3–17. doi: 10.1007/978-1-59745-194-9_1. [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki M, Obata Y, Abe K, Furusu A, Koji T, Tabata Y, Kohno S. Gene Transfer Using Nonviral Delivery Systems. Perit Dial Int. 2006 November-December;26(6):633–40. [PubMed] [Google Scholar]
- 10.Teissie J, Golzio M, Rols MP. Mechanisms of cell membrane electropermeabilization: a minireview of our present (lack of ?) knowledge. Biochim Biophys Acta. 2005 Aug 5;1724(3):270–80. doi: 10.1016/j.bbagen.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003 Apr;177(4):437–47. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- 12.Fewell JG. Factor IX gene therapy for hemophilia. Methods Mol Biol. 2008;423:375–82. doi: 10.1007/978-1-59745-194-9_29. [DOI] [PubMed] [Google Scholar]
- 13.Fewell JG, MacLaughlin F, Mehta V, Gondo M, Nicol F, Wilson E, Smith LC. Gene therapy for the treatment of hemophilia B using PINC-formulated plasmid delivered to muscle with electroporation. Mol Ther. 2001 Apr;3(4):574–83. doi: 10.1006/mthe.2001.0295. [DOI] [PubMed] [Google Scholar]
- 14.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL, Heller R. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008 Dec 20;26(36):5896–903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benz R, Zimmermann U. Pulse-length dependence of the electrical breakdown in lipid bilayer membranes. Biochim Biophys Acta. 1980 Apr 24;597(3):637–42. doi: 10.1016/0005-2736(80)90236-9. [DOI] [PubMed] [Google Scholar]
- 16.Benz R, Zimmermann U. The resealing process of lipid bilayers after reversible electrical breakdown. Biochim Biophys Acta. 1981 Jan 8;640(1):169–78. doi: 10.1016/0005-2736(81)90542-3. [DOI] [PubMed] [Google Scholar]
- 17.Chang DC, Reese TS. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys J. 1990 Jul;58(1):1–12. doi: 10.1016/S0006-3495(90)82348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knutson JC, Yee D. Electroporation: parameters affecting transfer of DNA into mammalian cells. Anal Biochem. 1987 Jul;164(1):44–52. doi: 10.1016/0003-2697(87)90365-4. [DOI] [PubMed] [Google Scholar]
- 19.Xie TD, Sun L, Tsong TY. Study of mechanisms of electric field-induced DNA transfection. I. DNA entry by surface binding and diffusion through membrane pores. Biophys J. 1990 Jul;58(1):13–9. doi: 10.1016/S0006-3495(90)82349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favard C, Dean DS, Rols MP. Electrotransfer as a non viral method of gene delivery. Curr Gene Ther. 2007 Feb;7(1):67–77. doi: 10.2174/156652307779940207. [DOI] [PubMed] [Google Scholar]
- 21.Neumann E, Kakorin S, Toensing K. Fundamentals of electroporative delivery of drugs and genes. Bioelectrochem Bioenerg. 1999 Feb;48(1):3–16. doi: 10.1016/s0302-4598(99)00008-2. [DOI] [PubMed] [Google Scholar]
- 22.Xie TD, Tsong TY. Study of mechanisms of electric field-induced DNA transfection. V. Effects of DNA topology on surface binding, cell uptake, expression, and integration into host chromosomes of DNA in the mammalian cell. Biophys J. 1993 Oct;65(4):1684–9. doi: 10.1016/S0006-3495(93)81208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Gennes PG. Passive entry of a DNA molecule into a small pore. Proc Natl Acad Sci U S A. 1999 Jun 22;96(13):7262–4. doi: 10.1073/pnas.96.13.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klenchin VA, Sukharev SI, Serov SM, Chernomordik LV, Chizmadzhev Yu A. Electrically induced DNA uptake by cells is a fast process involving DNA electrophoresis. Biophys J. 1991 Oct;60(4):804–11. doi: 10.1016/S0006-3495(91)82115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sukharev SI, Klenchin VA, Serov SM, Chernomordik LV, Chizmadzhev Yu A. Electroporation and electrophoretic DNA transfer into cells. The effect of DNA interaction with electropores. Biophys J. 1992 Nov;63(5):1320–7. doi: 10.1016/S0006-3495(92)81709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hristova NI, Tsoneva I, Neumann E. Sphingosine-mediated electroporative DNA transfer through lipid bilayers. FEBS Lett. 1997 Sep 22;415(1):81–6. doi: 10.1016/s0014-5793(97)01097-1. [DOI] [PubMed] [Google Scholar]
- 27.Angelova MI, Hristova N, Tsoneva I. DNA-induced endocytosis upon local microinjection to giant unilamellar cationic vesicles. Eur Biophys J. 1999;28(2):142–50. doi: 10.1007/s002490050193. [DOI] [PubMed] [Google Scholar]
- 28.Angelova MI, Tsoneva I. Interactions of DNA with giant liposomes. Chem Phys Lipids. 1999 Aug;101(1):123–37. doi: 10.1016/s0009-3084(99)00060-2. [DOI] [PubMed] [Google Scholar]
- 29.Drinkwater NR, Klinedinst DK. Chemically induced mutagenesis in a shuttle vector with a low-background mutant frequency. Proc Natl Acad Sci U S A. 1986 May;83(10):3402–6. doi: 10.1073/pnas.83.10.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isaka Y, Imai E. Electroporation-mediated gene therapy. Expert Opin Drug Deliv. 2007 Sep;4(5):561–71. doi: 10.1517/17425247.4.5.561. [DOI] [PubMed] [Google Scholar]
- 31.Thyagarajan B, Olivares EC, Hollis RP, Ginsburg DS, Calos MP. Site-specific genomic integration in mammalian cells mediated by phage phiC31 integrase. Mol Cell Biol. 2001 Jun;21(12):3926–34. doi: 10.1128/MCB.21.12.3926-3934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollis RP, Nightingale SJ, Wang X, Pepper KA, Yu XJ, Barsky L, Crooks GM, Kohn DB. Stable gene transfer to human CD34(+) hematopoietic cells using the Sleeping Beauty transposon. Exp Hematol. 2006 Oct;34(10):1333–43. doi: 10.1016/j.exphem.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 33.Chalberg TW, Vankov A, Molnar FE, Butterwick AF, Huie P, Calos MP, Palanker DV. Gene transfer to rabbit retina with electron avalanche transfection. Invest Ophthalmol Vis Sci. 2006 Sep;47(9):4083–90. doi: 10.1167/iovs.06-0092. [DOI] [PubMed] [Google Scholar]
- 34.Ohta S, Suzuki K, Ogino Y, Miyagawa S, Murashima A, Matsumaru D, Yamada G. Gene transduction by sonoporation. Dev Growth Differ. 2008 Aug;50(6):517–20. doi: 10.1111/j.1440-169X.2008.01026.x. [DOI] [PubMed] [Google Scholar]
- 35.Skorpikova J, Dolnikova M, Hrazdira I, Janisch R. Changes in microtubules and microfilaments due to a combined effect of ultrasound and cytostatics in HeLa cells. Folia Biol (Praha) 2001;47(4):143–7. [PubMed] [Google Scholar]
- 36.Yamashita Y, Shimada M, Minagawa R, Tsujita E, Harimoto N, Tanaka S, Shirabe K, Miyazaki J, Maehara Y. Muscle-targeted interleukin-12 gene therapy of orthotopic hepatocellular carcinoma in mice using in vivo electrosonoporation. Mol Cancer Ther. 2004 Sep;3(9):1177–82. [PubMed] [Google Scholar]
- 37.Yamashita Y, Shimada M, Tachibana K, Harimoto N, Tsujita E, Shirabe K, Miyazaki J, Sugimachi K. In vivo gene transfer into muscle via electro-sonoporation. Hum Gene Ther. 2002 Nov 20;13(17):2079–84. doi: 10.1089/10430340260395929. [DOI] [PubMed] [Google Scholar]
- 38.Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud JM, Delaere P, Branellec D, Schwartz B, Scherman D. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci U S A. 1999 Apr 13;96(8):4262–7. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bettan M, Emmanuel F, Darteil R, Caillaud JM, Soubrier F, Delaere P, Branelec D, Mahfoudi A, Duverger N, Scherman D. High-level protein secretion into blood circulation after electric pulse-mediated gene transfer into skeletal muscle. Mol Ther. 2000 Sep;2(3):204–10. doi: 10.1006/mthe.2000.0117. [DOI] [PubMed] [Google Scholar]
- 40.Draghia-Akli R, Ellis KM, Hill LA, Malone PB, Fiorotto ML. High-efficiency growth hormone-releasing hormone plasmid vector administration into skeletal muscle mediated by electroporation in pigs. FASEB J. 2003 Mar;17(3):526–8. doi: 10.1096/fj.02-0671fje. [DOI] [PubMed] [Google Scholar]
- 41.Lee MJ, Cho SS, Jang HS, Lim YS, You JR, Park J, Suh H, Kim JA, Park JS, Kim DK. Optimal salt concentration of vehicle for plasmid DNA enhances gene transfer mediated by electroporation. Exp Mol Med. 2002 Sep 30;34(4):265–72. doi: 10.1038/emm.2002.37. [DOI] [PubMed] [Google Scholar]
- 42.Nicol F, Wong M, MacLaughlin FC, Perrard J, Wilson E, Nordstrom JL, Smith LC. Poly-L-glutamate, an anionic polymer, enhances transgene expression for plasmids delivered by intramuscular injection with in vivo electroporation. Gene Ther. 2002 Oct;9(20):1351–8. doi: 10.1038/sj.gt.3301806. [DOI] [PubMed] [Google Scholar]
- 43.Draghia-Akli R, Khan AS, Cummings KK, Parghi D, Carpenter RH, Brown PA. Electrical enhancement of formulated plasmid delivery in animals. Technol Cancer Res Treat. 2002 Oct;1(5):365–72. doi: 10.1177/153303460200100507. [DOI] [PubMed] [Google Scholar]
- 44.Quaglino E, Iezzi M, Mastini C, Amici A, Pericle F, Di Carlo E, Pupa SM, De Giovanni C, Spadaro M, Curcio C, Lollini PL, Musiani P, Forni G, Cavallo F. Electroporated DNA vaccine clears away multifocal mammary carcinomas in her-2/neu transgenic mice. Cancer Res. 2004 Apr 15;64(8):2858–64. doi: 10.1158/0008-5472.can-03-2962. [DOI] [PubMed] [Google Scholar]
- 45.Spadaro M, Ambrosino E, Iezzi M, Di Carlo E, Sacchetti P, Curcio C, Amici A, Wei WZ, Musiani P, Lollini PL, Cavallo F, Forni G. Cure of mammary carcinomas in Her-2 transgenic mice through sequential stimulation of innate (neoadjuvant interleukin-12) and adaptive (DNA vaccine electroporation) immunity. Clin Cancer Res. 2005 Mar 1;11(5):1941–52. doi: 10.1158/1078-0432.CCR-04-1873. [DOI] [PubMed] [Google Scholar]
- 46.Maurer PH. Antigenicity of polypeptides (poly-alpha-amino acids). XVII. Immunologic studies in humans with polymers containing L or D and L-alpha-amino acids. J Immunol. 1965 Dec;95(6):1095–9. [PubMed] [Google Scholar]
- 47.Hartikka J, Sukhu L, Buchner C, Hazard D, Bozoukova V, Margalith M, Nishioka WK, Wheeler CJ, Manthorp M, Sawdey M. Electroporation-facilitated delivery of plasmid DNA in skeletal muscle: plasmid dependence of muscle damage and effect of poloxamer 188. Mol Ther. 2001 Nov;4(5):407–15. doi: 10.1006/mthe.2001.0483. [DOI] [PubMed] [Google Scholar]
- 48.Mendiratta SK, Thai G, Eslahi NK, Thull NM, Matar M, Bronte V, Pericle F. Therapeutic tumor immunity induced by polyimmunization with melanoma antigens gp100 and TRP-2. Cancer Res. 2001 Feb 1;61(3):859–63. [PubMed] [Google Scholar]
- 49.Cemazar M, Sersa G, Wilson J, Tozer GM, Hart SL, Grosel A, Dachs GU. Effective gene transfer to solid tumors using different nonviral gene delivery techniques: electroporation, liposomes, and integrin-targeted vector. Cancer Gene Ther. 2002 Apr;9(4):399–406. doi: 10.1038/sj.cgt.7700454. [DOI] [PubMed] [Google Scholar]
- 50.Draghia-Akli R, Khan AS, Brown PA, Pope MA, Wu L, Hirao L, Weiner DB. Parameters for DNA vaccination using adaptive constant-current electroporation in mouse and pig models. Vaccine. 2008 Sep 19;26(40):5230–7. doi: 10.1016/j.vaccine.2008.03.071. [DOI] [PubMed] [Google Scholar]
- 51.Muramatsu T, Nakamura A, Park HM. In vivo electroporation: a powerful and convenient means of nonviral gene transfer to tissues of living animals (Review) Int J Mol Med. 1998 Jan;1(1):55–62. doi: 10.3892/ijmm.1.1.55. [DOI] [PubMed] [Google Scholar]
- 52.Satkauskas S, Andre F, Bureau MF, Scherman D, Miklavcic D, Mir LM. Electrophoretic component of electric pulses determines the efficacy of in vivo DNA electrotransfer. Hum Gene Ther. 2005 Oct;16(10):1194–201. doi: 10.1089/hum.2005.16.1194. [DOI] [PubMed] [Google Scholar]
- 53.Satkauskas S, Bureau MF, Puc M, Mahfoudi A, Scherman D, Miklavcic D, Mir LM. Mechanisms of in vivo DNA electrotransfer: respective contributions of cell electropermeabilization and DNA electrophoresis. Mol Ther. 2002 Feb;5(2):133–40. doi: 10.1006/mthe.2002.0526. [DOI] [PubMed] [Google Scholar]
- 54.Hojman P, Gissel H, Andre F, Cournil-Henrionnet C, Eriksen J, Gehl J, Mir LM. Physiological Effects of High and Low Voltage Pulse Combinations for Gene Electrotransfer in Muscle. Hum Gene Ther. 2008 Aug 22; doi: 10.1089/hum.2008.059. [DOI] [PubMed] [Google Scholar]
- 55.Andre F, Gehl J, Sersa G, Preat V, Hojman P, Eriksen J, Golzio M, Cemazar M, Pavselj N, Rols MP, Miklavcic D, Neumann E, Teissie J, Mir LM. Efficiency of High and Low Voltage Pulse Combinations for Gene Electrotransfer in Muscle, Liver, Tumor and Skin. Hum Gene Ther. 2008 Aug 21; doi: 10.1089/hum.2008.060. [DOI] [PubMed] [Google Scholar]
- 56.Andre FM, Cournil-Henrionnet C, Vernerey D, Opolon P, Mir LM. Variability of naked DNA expression after direct local injection: the influence of the injection speed. Gene Ther. 2006 Dec;13(23):1619–27. doi: 10.1038/sj.gt.3302827. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto T, Komori K, Shoji T, Kuma S, Kume M, Yamaoka T, Mori E, Furuyama T, Yonemitsu Y, Sugimachi K. Successful and optimized in vivo gene transfer to rabbit carotid artery mediated by electronic pulse. Gene Ther. 2001 Aug;8(15):1174–9. doi: 10.1038/sj.gt.3301502. [DOI] [PubMed] [Google Scholar]
- 58.Tone CM, Cardoza DM, Carpenter RH, Draghia-Akli R. Long-term effects of plasmid-mediated growth hormone releasing hormone in dogs. Cancer Gene Ther. 2004 May;11(5):389–96. doi: 10.1038/sj.cgt.7700717. [DOI] [PubMed] [Google Scholar]
- 59.Bureau MF, Gehl J, Deleuze V, Mir LM, Scherman D. Importance of association between permeabilization and electrophoretic forces for intramuscular DNA electrotransfer. Biochim Biophys Acta. 2000 May 1;1474(3):353–9. doi: 10.1016/s0304-4165(00)00028-3. [DOI] [PubMed] [Google Scholar]
- 60.Somiari S, Glasspool-Malone J, Drabick JJ, Gilbert RA, Heller R, Jaroszeski MJ, Malone RW. Theory and in vivo application of electroporative gene delivery. Mol Ther. 2000 Sep;2(3):178–87. doi: 10.1006/mthe.2000.0124. [DOI] [PubMed] [Google Scholar]
- 61.Brown PA, Khan AS, Draghia-Akli R. Delivery of DNA into skeletal muscle in large animals. Methods Mol Biol. 2008;423:215–24. doi: 10.1007/978-1-59745-194-9_15. [DOI] [PubMed] [Google Scholar]
- 62.Draghia-Akli R, Khan AS. Muscle and fat mass modulation in different clinical models. Methods Mol Biol. 2008;423:449–60. doi: 10.1007/978-1-59745-194-9_35. [DOI] [PubMed] [Google Scholar]
- 63.Draghia-Akli R, Fiorotto ML. A new plasmid-mediated approach to supplement somatotropin production in pigs. J Anim Sci. 2004;82(E-Suppl):E264–9. doi: 10.2527/2004.8213_supplE264x. [DOI] [PubMed] [Google Scholar]
- 64.Brown PA, Davis WC, Draghia-Akli R. Immune-enhancing effects of growth hormone-releasing hormone delivered by plasmid injection and electroporation. Mol Ther. 2004 Oct;10(4):644–51. doi: 10.1016/j.ymthe.2004.06.1015. [DOI] [PubMed] [Google Scholar]
- 65.Draghia-Akli R, Malone PB, Hill LA, Ellis KM, Schwartz RJ, Nordstrom JL. Enhanced animal growth via ligand-regulated GHRH myogenic-injectable vectors. FASEB J. 2002 Mar;16(3):426–8. doi: 10.1096/fj.01-0702fje. [DOI] [PubMed] [Google Scholar]
- 66.Pavlin D, Tozon N, Sersa G, Pogacnik A, Cemazar M. Efficient electrotransfection into canine muscle. Technol Cancer Res Treat. 2008 Feb;7(1):45–54. doi: 10.1177/153303460800700106. [DOI] [PubMed] [Google Scholar]
- 67.Draghia-Akli R, Cummings KK, Khan AS, Brown PA, Carpenter RH. Effects of plasmid-mediated growth hormone releasing hormone supplementation in young, healthy Beagle dogs. J Anim Sci. 2003 Sep;81(9):2301–10. doi: 10.2527/2003.8192301x. [DOI] [PubMed] [Google Scholar]
- 68.Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998 Sep;16(9):867–70. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 69.Tollefsen S, Vordermeier M, Olsen I, Storset AK, Reitan LJ, Clifford D, Lowrie DB, Wiker HG, Huygen K, Hewinson G, Mathiesen I, Tjelle TE. DNA injection in combination with electroporation: a novel method for vaccination of farmed ruminants. Scand J Immunol. 2003 Mar;57(3):229–38. doi: 10.1046/j.1365-3083.2003.01218.x. [DOI] [PubMed] [Google Scholar]
- 70.Scheerlinck JP, Karlis J, Tjelle TE, Presidente PJ, Mathiesen I, Newton SE. In vivo electroporation improves immune responses to DNA vaccination in sheep. Vaccine. 2004 Apr 16;22(13-14):1820–5. doi: 10.1016/j.vaccine.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 71.Khan AS, Smith LC, Abruzzese RV, Cummings KK, Pope MA, Brown PA, Draghia-Akli R. Optimization of electroporation parameters for the intramuscular delivery of plasmids in pigs. DNA Cell Biol. 2003 Dec;22(12):807–14. doi: 10.1089/104454903322625019. [DOI] [PubMed] [Google Scholar]
- 72.Babiuk S, Baca-Estrada ME, Foldvari M, Middleton DM, Rabussay D, Widera G, Babiuk LA. Increased gene expression and inflammatory cell infiltration caused by electroporation are both important for improving the efficacy of DNA vaccines. J Biotechnol. 2004 May 13;110(1):1–10. doi: 10.1016/j.jbiotec.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 73.Fattori E, Cappelletti M, Zampaglione I, Mennuni C, Calvaruso F, Arcuri M, Rizzuto G, Costa P, Perretta G, Ciliberto G, La Monica N. Gene electro-transfer of an improved erythropoietin plasmid in mice and non-human primates. J Gene Med. 2005 Feb;7(2):228–36. doi: 10.1002/jgm.652. [DOI] [PubMed] [Google Scholar]
- 74.Andre F, Mir LM. DNA electrotransfer: its principles and an updated review of its therapeutic applications. Gene Ther. 2004 Oct;11(Suppl 1):S33–42. doi: 10.1038/sj.gt.3302367. [DOI] [PubMed] [Google Scholar]
- 75.Horton HM, Lalor PA, Rolland AP. IL-2 plasmid electroporation: from preclinical studies to phase I clinical trial. Methods Mol Biol. 2008;423:361–72. doi: 10.1007/978-1-59745-194-9_28. [DOI] [PubMed] [Google Scholar]
- 76.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–8. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 77.Wu CJ, Lee SC, Huang HW, Tao MH. In vivo electroporation of skeletal muscles increases the efficacy of Japanese encephalitis virus DNA vaccine. Vaccine. 2004 Mar 29;22(11-12):1457–64. doi: 10.1016/j.vaccine.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 78.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998 Sep 21;188(6):1075–82. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Medi BM, Singh J. Skin targeted DNA vaccine delivery using electroporation in rabbits II. Safety Int J Pharm. 2006 Feb 3;308(1-2):61–8. doi: 10.1016/j.ijpharm.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 80.Roos AK, Moreno S, Leder C, Pavlenko M, King A, Pisa P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol Ther. 2006 Feb;13(2):320–7. doi: 10.1016/j.ymthe.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 81.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996 Oct;2(10):1122–8. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 82.Ueda Y, Itoh T, Fuji N, Harada S, Fujiki H, Shimizu K, Shiozaki A, Iwamoto A, Shimizu T, Mazda O, Kimura T, Sonoda Y, Taniwaki M, Yamagishi H. Successful induction of clinically competent dendritic cells from granulocyte colony-stimulating factor-mobilized monocytes for cancer vaccine therapy. Cancer Immunol Immunother. 2007 Mar;56(3):381–9. doi: 10.1007/s00262-006-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yen HH, Scheerlinck JP. Co-delivery of plasmid-encoded cytokines modulates the immune response to a DNA vaccine delivered by in vivo electroporation. Vaccine. 2007 Mar 30;25(14):2575–82. doi: 10.1016/j.vaccine.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 84.Park MY, Kim HS, Woo SJ, Kim CH, Park JS, Sohn HJ, Kim HJ, Oh ST, Kim TG. Efficient antitumor immunity in a murine colorectal cancer model induced by CEA RNA-electroporated B cells. Eur J Immunol. 2008 Aug;38(8):2106–17. doi: 10.1002/eji.200737960. [DOI] [PubMed] [Google Scholar]
- 85.Onodera S, Ohshima S, Tohyama H, Yasuda K, Nishihira J, Iwakura Y, Matsuda I, Minami A, Koyama Y. A novel DNA vaccine targeting macrophage migration inhibitory factor protects joints from inflammation and destruction in murine models of arthritis. Arthritis Rheum. 2007 Feb;56(2):521–30. doi: 10.1002/art.22407. [DOI] [PubMed] [Google Scholar]
- 86.Hosoi A, Takeda Y, Sakuta K, Ueha S, Kurachi M, Kimura K, Maekawa R, Kakimi K. Dendritic cell vaccine with mRNA targeted to the proteasome by polyubiquitination. Biochem Biophys Res Commun. 2008 Jun 27;371(2):242–6. doi: 10.1016/j.bbrc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 87.Zhang X, Divangahi M, Ngai P, Santosuosso M, Millar J, Zganiacz A, Wang J, Bramson J, Xing Z. Intramuscular immunization with a monogenic plasmid DNA tuberculosis vaccine: Enhanced immunogenicity by electroporation and co-expression of GM-CSF transgene. Vaccine. 2007 Jan 26;25(7):1342–52. doi: 10.1016/j.vaccine.2006.09.089. [DOI] [PubMed] [Google Scholar]
- 88.Smooker PM, Rainczuk A, Kennedy N, Spithill TW. DNA vaccines and their application against parasites--promise, limitations and potential solutions. Biotechnol Annu Rev. 2004;10:189–236. doi: 10.1016/S1387-2656(04)10007-0. [DOI] [PubMed] [Google Scholar]
- 89.Roman M, Martin-Orozco E, Goodman JS, Nguyen MD, Sato Y, Ronaghy A, Kornbluth RS, Richman DD, Carson DA, Raz E. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997 Aug;3(8):849–54. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 90.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997 Nov 17;186(10):1623–31. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Erhardt M, Gorschluter M, Sager J, Ziske C, Strehl J, Lilienfeld-Toal MV, Schmidt-Wolf IG. Transfection of human monocyte-derived dendritic cells with CpG oligonucleotides. Immunol Cell Biol. 2005 Jun;83(3):278–85. doi: 10.1111/j.1440-1711.2005.01328.x. [DOI] [PubMed] [Google Scholar]
- 92.Kim CH, Yoon JS, Sohn HJ, Kim CK, Paik SY, Hong YK, Kim TG. Direct vaccination with pseudotype baculovirus expressing murine telomerase induces anti-tumor immunity comparable with RNA-electroporated dendritic cells in a murine glioma model. Cancer Lett. 2007 Jun 8;250(2):276–83. doi: 10.1016/j.canlet.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 93.Sasawatari S, Tadaki T, Isogai M, Takahara M, Nieda M, Kakimi K. Efficient priming and expansion of antigen-specific CD8+ T cells by a novel cell-based artificial APC. Immunol Cell Biol. 2006 Dec;84(6):512–21. doi: 10.1111/j.1440-1711.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- 94.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004 Jun;199:251–63. doi: 10.1111/j.0105-2896.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 95.Grunebach F, Muller MR, Brossart P. New developments in dendritic cell-based vaccinations: RNA translated into clinics. Cancer Immunol Immunother. 2005 Jun;54(6):517–25. doi: 10.1007/s00262-004-0605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu H, Babiuk LA, van Drunen Littel-van den Hurk S. Immunity and protection by adoptive transfer of dendritic cells transfected with hepatitis C NS3/4A mRNA. Vaccine. 2007 Feb 26;25(10):1701–11. doi: 10.1016/j.vaccine.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 97.Zhao YG, Xu Y. Electroporation-mediated HBV DNA vaccination in primate models. Methods Mol Biol. 2008;423:487–95. doi: 10.1007/978-1-59745-194-9_38. [DOI] [PubMed] [Google Scholar]
- 98.Prud’homme GJ, Draghia-Akli R, Wang Q. Plasmid-based gene therapy of diabetes mellitus. Gene Ther. 2007 Apr;14(7):553–64. doi: 10.1038/sj.gt.3302907. [DOI] [PubMed] [Google Scholar]
- 99.Tervo HM, Allespach I, Keppler OT. High-level transfection of primary rabbit T lymphocytes. J Immunol Methods. 2008 Jul 20;336(1):85–9. doi: 10.1016/j.jim.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 100.Chen NK, Wong JS, Kee IH, Lai SH, Thng CH, Ng WH, Ng RT, Tan SY, Lee SY, Tan ME, Sivalingam J, Chow PK, Kon OL. Nonvirally modified autologous primary hepatocytes correct diabetes and prevent target organ injury in a large preclinical model. PLoS ONE. 2008;3(3):e1734. doi: 10.1371/journal.pone.0001734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakamura M, Wang J, Murakami T, Ajiki T, Hakamata Y, Kaneko T, Takahashi M, Okamoto H, Mayumi M, Kobayashi E. DNA immunization of the grafted liver by particle-mediated gene gun. Transplantation. 2003 Nov 15;76(9):1369–75. doi: 10.1097/01.TP.0000091118.22413.E1. [DOI] [PubMed] [Google Scholar]
- 102.Prud’homme GJ, Glinka Y, Khan AS, Draghia-Akli R. Electroporation-enhanced nonviral gene transfer for the prevention or treatment of immunological, endocrine and neoplastic diseases. Curr Gene Ther. 2006 Apr;6(2):243–73. doi: 10.2174/156652306776359504. [DOI] [PubMed] [Google Scholar]
- 103.Meehan DT, Zink MA, Mahlen M, Nelson M, Sanger WG, Mitalipov SM, Wolf DP, Ouellette MM, Norgren RB., Jr Gene targeting in adult rhesus macaque fibroblasts. BMC Biotechnol. 2008;8:31. doi: 10.1186/1472-6750-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Norgren RB., Jr Creation of non-human primate neurogenetic disease models by gene targeting and nuclear transfer. Reprod Biol Endocrinol. 2004 Jun 16;2:40. doi: 10.1186/1477-7827-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003 Jan 17;299(5605):411–4. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002 Feb 8;295(5557):1089–92. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 107.Dai Y, Vaught TD, Boone J, Chen SH, Phelps CJ, Ball S, Monahan JA, Jobst PM, McCreath KJ, Lamborn AE, Cowell-Lucero JL, Wells KD, Colman A, Polejaeva IA, Ayares DL. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002 Mar;20(3):251–5. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 108.Denning C, Burl S, Ainslie A, Bracken J, Dinnyes A, Fletcher J, King T, Ritchie M, Ritchie WA, Rollo M, de Sousa P, Travers A, Wilmut I, Clark AJ. Deletion of the alpha(1,3)galactosyl transferase (GGTA1) gene and the prion protein (PrP) gene in sheep. Nat Biotechnol. 2001 Jun;19(6):559–62. doi: 10.1038/89313. [DOI] [PubMed] [Google Scholar]
- 109.Piedrahita JA. Targeted modification of the domestic animal genome. Theriogenology. 2000 Jan; doi: 10.1016/s0093-691x(99)00244-7. [DOI] [PubMed] [Google Scholar]
- 110.McCreath KJ, Howcroft J, Campbell KH, Colman A, Schnieke AE, Kind AJ. Production of gene-targeted sheep by nuclear transfer from cultured somatic cells. Nature. 2000 Jun 29;405(6790):1066–9. doi: 10.1038/35016604. [DOI] [PubMed] [Google Scholar]
- 111.Yu G, Chen J, Yu H, Liu S, Xu X, Sha H, Zhang X, Wu G, Xu S, Cheng G. Functional disruption of the prion protein gene in cloned goats. J Gen Virol. 2006 Apr;87(Pt 4):1019–27. doi: 10.1099/vir.0.81384-0. [DOI] [PubMed] [Google Scholar]
- 112.Shen SN, Xu Z, Qian XP, Ding YT, Yu LX, Liu BR. RNA-electroporated CD40-activated B cells induce functional T-cell responses against HepG2 cells. Eur J Cancer Care (Engl) 2008 Jul;17(4):404–11. doi: 10.1111/j.1365-2354.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- 113.Kuroiwa Y, Kasinathan P, Matsushita H, Sathiyaselan J, Sullivan EJ, Kakitani M, Tomizuka K, Ishida I, Robl JM. Sequential targeting of the genes encoding immunoglobulin-mu and prion protein in cattle. Nat Genet. 2004 Jul;36(7):775–80. doi: 10.1038/ng1373. [DOI] [PubMed] [Google Scholar]
- 114.Richt JA, Kasinathan P, Hamir AN, Castilla J, Sathiyaseelan T, Vargas F, Sathiyaseelan J, Wu H, Matsushita H, Koster J, Kato S, Ishida I, Soto C, Robl JM, Kuroiwa Y. Production of cattle lacking prion protein. Nat Biotechnol. 2007 Jan;25(1):132–8. doi: 10.1038/nbt1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Matsuki N, Ishikawa T, Imai Y, Yamaguchi T. Low voltage pulses can induce apoptosis. Cancer Lett. 2008 Sep 28;269(1):93–100. doi: 10.1016/j.canlet.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 116.Gehl J, Geertsen PF. Efficient palliation of haemorrhaging malignant melanoma skin metastases by electrochemotherapy. Melanoma Res. 2000 Dec;10(6):585–9. doi: 10.1097/00008390-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 117.Heller R, Jaroszeski M, Atkin A, Moradpour D, Gilbert R, Wands J, Nicolau C. In vivo gene electroinjection and expression in rat liver. FEBS Lett. 1996 Jul 8;389(3):225–8. doi: 10.1016/0014-5793(96)00590-x. [DOI] [PubMed] [Google Scholar]
- 118.Gehl J. Electroporation for drug and gene delivery in the clinic: doctors go electric. Methods Mol Biol. 2008;423:351–9. doi: 10.1007/978-1-59745-194-9_27. [DOI] [PubMed] [Google Scholar]
- 119.Sersa G, Cemazar M, Parkins CS, Chaplin DJ. Tumour blood flow changes induced by application of electric pulses. EurJ Cancer. 1999 Apr;35(4):672–7. doi: 10.1016/s0959-8049(98)00426-2. [DOI] [PubMed] [Google Scholar]
- 120.Takeshita S, Isshiki T, Sato T. Increased expression of direct gene transfer into skeletal muscles observed after acute ischemic injury in rats. Lab Invest. 1996 Jun;74(6):1061–5. [PubMed] [Google Scholar]
- 121.Tsurumi Y, Takeshita S, Chen D, Kearney M, Rossow ST, Passeri J, Horowitz JR, Symes JF, Isner JM. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation. 1996 Dec 15;94(12):3281–90. doi: 10.1161/01.cir.94.12.3281. [DOI] [PubMed] [Google Scholar]
- 122.Gehl J, Skovsgaard T, Mir LM. Vascular reactions to in vivo electroporation: characterization and consequences for drug and gene delivery. Biochim Biophys Acta. 2002 Jan 15;1569(1-3):51–8. doi: 10.1016/s0304-4165(01)00233-1. [DOI] [PubMed] [Google Scholar]
- 123.Bromberg JS, Debruyne LA, Qin L. Interactions between the immune system and gene therapy vectors: bidirectional regulation of response and expression. Adv Immunol. 1998;69:353–409. [PubMed] [Google Scholar]
- 124.Chen D, Ding Y, Zhang N, Schroppel B, Fu S, Zang W, Zhang H, Hancock WW, Bromberg JS. Viral IL-10 gene transfer inhibits the expression of multiple chemokine and chemokine receptor genes induced by inflammatory or adaptive immune stimuli. Am J Transplant. 2003 Dec;3(12):1538–49. doi: 10.1046/j.1600-6135.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- 125.Qin J, Peng Z, McLeod MV. In vitro mutagenesis to define functional domains. Methods Mol Biol. 2004;241:189–94. doi: 10.1385/1-59259-646-0:189. [DOI] [PubMed] [Google Scholar]
- 126.Darquet AM, Rangara R, Kreiss P, Schwartz B, Naimi S, Delaere P, Crouzet J, Scherman D. Minicircle: an improved DNA molecule for in vitro and in vivo gene transfer. Gene Ther. 1999 Feb;6(2):209–18. doi: 10.1038/sj.gt.3300816. [DOI] [PubMed] [Google Scholar]


