Abstract
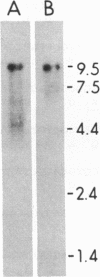
Four overlapping cDNA clones encoding a partial sequence of the cation-independent 215-kDa mannose 6-phosphate receptor have been identified by screening a fetal calf liver cDNA library with oligonucleotide probes. RNA hybridization analysis showed that the length of the mRNA is approximately 9.5 kilobases. Sequence analysis demonstrated that the clones consist of 4647 contiguous nucleotides and contain an open reading frame coding for a polypeptide of 1461 amino acids, which we estimate represents greater than 75% of the primary structure of the receptor. The deduced amino acid sequence indicates that the receptor has a carboxyl-terminal cytoplasmic domain of 163 amino acids that is rich in acidic residues, a 23-amino acid transmembrane segment, and an extracellular domain containing at least eight homologous repeats of approximately 145 amino acids. One of the repeats contains an additional 43-residue segment that is similar to the type II repeat of fibronectin. Each repeat contains a highly conserved 13-amino acid unit bordered by cysteine residues that may be functionally important.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvares K., Balasubramanian A. S. A binding protein for lysosomal enzymes isolated from brain by phosphomannan-sepharose chromatography. Biochem Biophys Res Commun. 1983 Apr 29;112(2):398–406. doi: 10.1016/0006-291x(83)91477-8. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Dixit V. M., Hennessy S. W., Grant G. A., Rotwein P., Frazier W. A. Characterization of a cDNA encoding the heparin and collagen binding domains of human thrombospondin. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5449–5453. doi: 10.1073/pnas.83.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K., Dordal M. S., Reynolds L. Mannose-binding proteins isolated from rat liver contain carbohydrate-recognition domains linked to collagenous tails. Complete primary structures and homology with pulmonary surfactant apoprotein. J Biol Chem. 1986 May 25;261(15):6878–6887. [PubMed] [Google Scholar]
- Drickamer K., Mamon J. F., Binns G., Leung J. O. Primary structure of the rat liver asialoglycoprotein receptor. Structural evidence for multiple polypeptide species. J Biol Chem. 1984 Jan 25;259(2):770–778. [PubMed] [Google Scholar]
- Esch F. S., Ling N. C., Böhlen P., Ying S. Y., Guillemin R. Primary structure of PDC-109, a major protein constituent of bovine seminal plasma. Biochem Biophys Res Commun. 1983 Jun 29;113(3):861–867. doi: 10.1016/0006-291x(83)91078-1. [DOI] [PubMed] [Google Scholar]
- Fischer H. D., Creek K. E., Sly W. S. Binding of phosphorylated oligosaccharides to immobilized phosphomannosyl receptors. J Biol Chem. 1982 Sep 10;257(17):9938–9943. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Grant G. A., Sacchettini J. C., Welgus H. G. A collagenolytic serine protease with trypsin-like specificity from the fiddler crab Uca pugilator. Biochemistry. 1983 Jan 18;22(2):354–358. doi: 10.1021/bi00271a019. [DOI] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Hoflack B., Fujimoto K., Kornfeld S. The interaction of phosphorylated oligosaccharides and lysosomal enzymes with bovine liver cation-dependent mannose 6-phosphate receptor. J Biol Chem. 1987 Jan 5;262(1):123–129. [PubMed] [Google Scholar]
- Hoflack B., Kornfeld S. Purification and characterization of a cation-dependent mannose 6-phosphate receptor from murine P388D1 macrophages and bovine liver. J Biol Chem. 1985 Oct 5;260(22):12008–12014. [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Maler T., Rosenblum B. B., Jourdian G. W. Properties of the Syrian hamster phosphomannosyl receptor: an aggregate of low molecular weight proteins. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8379–8383. doi: 10.1073/pnas.82.24.8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen B. A., Fujikawa K. Amino acid sequence of the heavy chain of human alpha-factor XIIa (activated Hageman factor). J Biol Chem. 1985 May 10;260(9):5328–5341. [PubMed] [Google Scholar]
- Mizusawa S., Nishimura S., Seela F. Improvement of the dideoxy chain termination method of DNA sequencing by use of deoxy-7-deazaguanosine triphosphate in place of dGTP. Nucleic Acids Res. 1986 Feb 11;14(3):1319–1324. doi: 10.1093/nar/14.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahagian G. G., Distler J., Jourdian G. W. Characterization of a membrane-associated receptor from bovine liver that binds phosphomannosyl residues of bovine testicular beta-galactosidase. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4289–4293. doi: 10.1073/pnas.78.7.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahagian G. G., Neufeld E. F. Biosynthesis and turnover of the mannose 6-phosphate receptor in cultured Chinese hamster ovary cells. J Biol Chem. 1983 Jun 10;258(11):7121–7128. [PubMed] [Google Scholar]
- Sahagian G. G., Steer C. J. Transmembrane orientation of the mannose 6-phosphate receptor in isolated clathrin-coated vesicles. J Biol Chem. 1985 Aug 15;260(17):9838–9842. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. W., Rome L. H. Assay and purification of a solubilized membrane receptor that binds the lysosomal enzyme alpha-L-iduronidase. Arch Biochem Biophys. 1982 Apr 1;214(2):681–687. doi: 10.1016/0003-9861(82)90074-1. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Berman C. H., Dull T. J., Gray A., Lee J. M. Isolation of the human insulin-like growth factor I gene using a single synthetic DNA probe. EMBO J. 1984 Feb;3(2):361–364. doi: 10.1002/j.1460-2075.1984.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Snider M. D., Porter M., Lodish H. F. Mutants of vesicular stomatitis virus blocked at different stages in maturation of the viral glycoprotein. Cell. 1980 Sep;21(2):417–427. doi: 10.1016/0092-8674(80)90478-x. [DOI] [PubMed] [Google Scholar]
- von Figura K., Gieselmann V., Hasilik A. Mannose 6-phosphate-specific receptor is a transmembrane protein with a C-terminal extension oriented towards the cytosol. Biochem J. 1985 Jan 15;225(2):543–547. doi: 10.1042/bj2250543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]