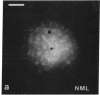
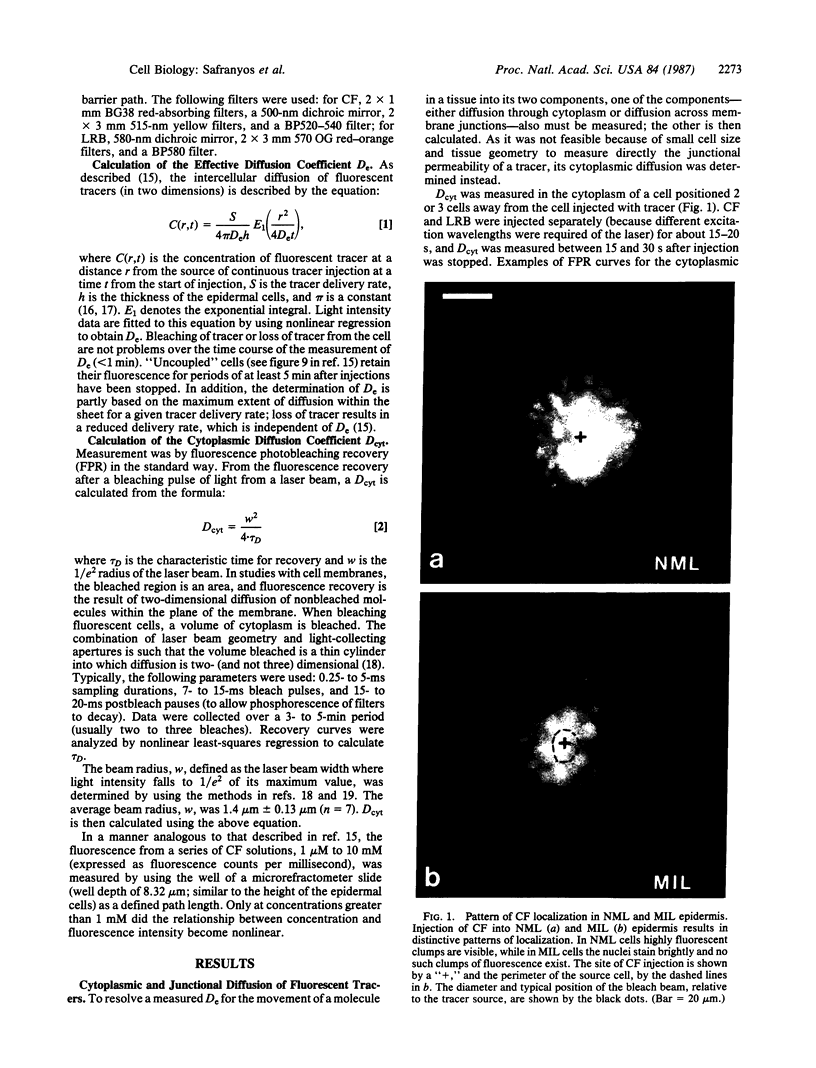
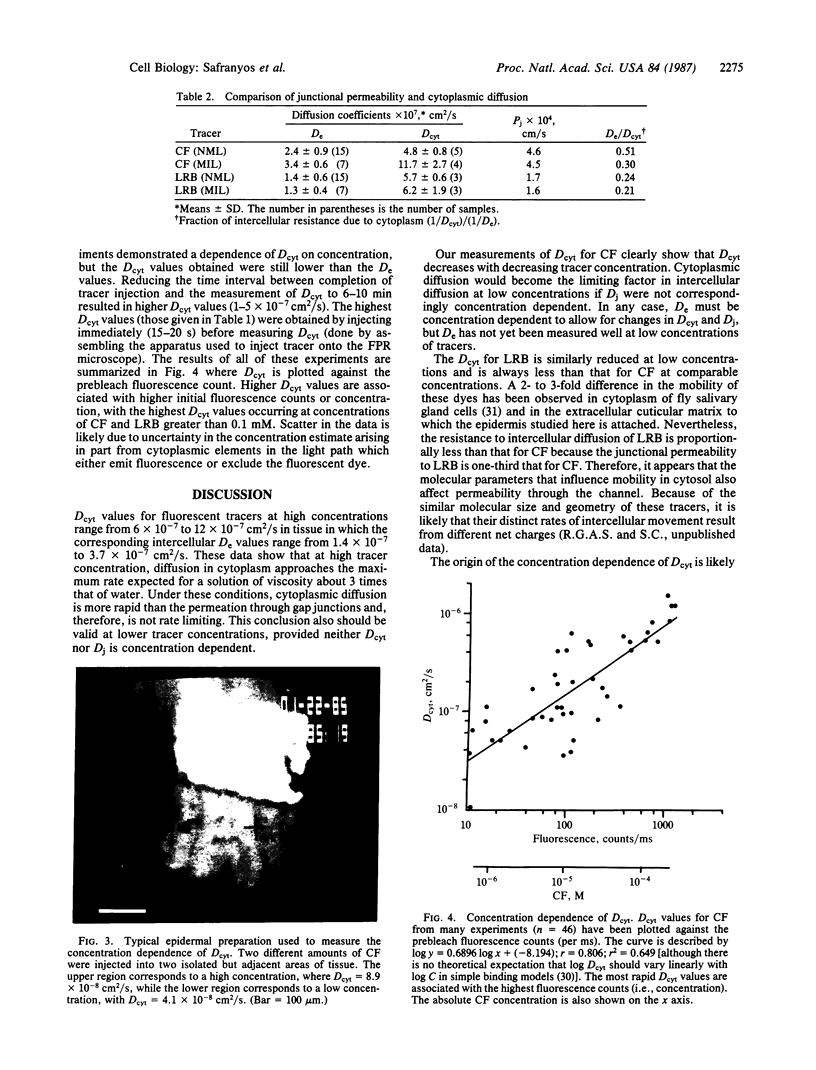
Abstract
Intercellular (tissue) diffusion of molecules requires cytoplasmic diffusion and diffusion through gap junctional (or cell-to-cell) channels. The rates of tissue and cytoplasmic diffusion of fluorescent tracers, expressed as an effective diffusion coefficient, De, and a cytoplasmic diffusion coefficient, Dcyt, have been measured among the developing epidermal cells of a larval beetle, Tenebrio molitor L., to determine the contribution of the junctional channels to intercellular diffusion. Tracer diffusion was measured by injecting fluorescent tracers into cells and quantitating the rate of subsequent spread into adjacent cells. Cytoplasmic diffusion was determined by fluorescence photobleaching. These experiments show that gap junctional channels constitute approximately 70-80% of the total cell-to-cell resistance to the diffusion of organic tracers at high concentrations in this tissue. At low concentrations, however, the binding of tracer to cytoplasm slows down the cytoplasmic diffusion, which may limit intercellular diffusion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brink P. R., Dewey M. M. Nexal membrane permeability to anions. J Gen Physiol. 1978 Jul;72(1):67–86. doi: 10.1085/jgp.72.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink P. R., Ramanan S. V. A model for the diffusion of fluorescent probes in the septate giant axon of earthworm. Axoplasmic diffusion and junctional membrane permeability. Biophys J. 1985 Aug;48(2):299–309. doi: 10.1016/S0006-3495(85)83783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillé J. P., Hinke J. A. The volume available to diffusion in the muscle fiber. Can J Physiol Pharmacol. 1974 Aug;52(4):814–828. doi: 10.1139/y74-107. [DOI] [PubMed] [Google Scholar]
- Cole W. C., Garfield R. E., Kirkaldy J. S. Gap junctions and direct intercellular communication between rat uterine smooth muscle cells. Am J Physiol. 1985 Jul;249(1 Pt 1):C20–C31. doi: 10.1152/ajpcell.1985.249.1.C20. [DOI] [PubMed] [Google Scholar]
- Crick F. Diffusion in embryogenesis. Nature. 1970 Jan 31;225(5231):420–422. doi: 10.1038/225420a0. [DOI] [PubMed] [Google Scholar]
- De Mello W. C., Gonzalez Castillo M., van Loon P. Intercellular diffusion of Lucifer yellow CH in mammalian cardiac fibers. J Mol Cell Cardiol. 1983 Sep;15(9):637–643. doi: 10.1016/0022-2828(83)90273-0. [DOI] [PubMed] [Google Scholar]
- Elson E. L., Reidler J. A. Analysis of cell surface interactions by measurements of lateral mobility. J Supramol Struct. 1979;12(4):481–489. doi: 10.1002/jss.400120408. [DOI] [PubMed] [Google Scholar]
- Gershon N. D., Porter K. R., Trus B. L. The cytoplasmic matrix: its volume and surface area and the diffusion of molecules through it. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5030–5034. doi: 10.1073/pnas.82.15.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B., Perelson A. S. The hemolytic plaque assay: theory for finite layers. Biophys Chem. 1977 Jun;7(1):15–32. doi: 10.1016/0301-4622(77)87011-7. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaga I. Cell-to-cell diffusion of procion yellow in sheep and calf Purkinje fibers. J Membr Biol. 1974;16(4):381–388. doi: 10.1007/BF01872425. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wojcieszyn J. The translational mobility of substances within the cytoplasmic matrix. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6747–6751. doi: 10.1073/pnas.81.21.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K., Taylor D. L., Lanni F. Probing the structure of cytoplasm. J Cell Biol. 1986 Jun;102(6):2015–2022. doi: 10.1083/jcb.102.6.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastro A. M., Babich M. A., Taylor W. D., Keith A. D. Diffusion of a small molecule in the cytoplasm of mammalian cells. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3414–3418. doi: 10.1073/pnas.81.11.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastro A. M., Keith A. D. Diffusion in the aqueous compartment. J Cell Biol. 1984 Jul;99(1 Pt 2):180s–187s. doi: 10.1083/jcb.99.1.180s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safranyos R. G., Caveney S. Rates of diffusion of fluorescent molecules via cell-to-cell membrane channels in a developing tissue. J Cell Biol. 1985 Mar;100(3):736–747. doi: 10.1083/jcb.100.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Weingart R. Inotropic effect of cyclic AMP in calf ventricular muscle studied by a cut end method. J Physiol. 1976 Aug;260(1):117–141. doi: 10.1113/jphysiol.1976.sp011507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L., Lanni F., McNeil P. L., Ware B. R., Taylor D. L. Mobility of cytoplasmic and membrane-associated actin in living cells. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4660–4664. doi: 10.1073/pnas.79.15.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S. The diffusion of radiopotassium across intercalated disks of mammalian cardiac muscle. J Physiol. 1966 Nov;187(2):323–342. doi: 10.1113/jphysiol.1966.sp008092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingart R. The permeability to tetraethylammonium ions of the surface membrane and the intercalated disks of sheep and calf myocardium. J Physiol. 1974 Aug;240(3):741–762. doi: 10.1113/jphysiol.1974.sp010632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcieszyn J. W., Schlegel R. A., Jacobson K. A. Measurements of the diffusion of macromolecules injected into the cytoplasm of living cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):39–44. doi: 10.1101/sqb.1982.046.01.007. [DOI] [PubMed] [Google Scholar]
- Wojcieszyn J. W., Schlegel R. A., Wu E. S., Jacobson K. A. Diffusion of injected macromolecules within the cytoplasm of living cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4407–4410. doi: 10.1073/pnas.78.7.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Microtrabecular lattice of the cytoplasmic ground substance. Artifact or reality. J Cell Biol. 1979 Jul;82(1):114–139. doi: 10.1083/jcb.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. L., Rose B. Permeability properties of cell-to-cell channels: kinetics of fluorescent tracer diffusion through a cell junction. J Membr Biol. 1985;84(3):269–283. doi: 10.1007/BF01871390. [DOI] [PubMed] [Google Scholar]