Abstract
Evidence shows that the serine/threonine protein phosphatase 1 (PP1) plays a critical role in synaptic plasticity and memory. Little is known about the contribution of the serine/threonine protein phosphatase 2A (PP2A) to synaptic plasticity. Both protein phosphatases can target the transcription factor cAMP response element binding protein (CREB), whose phosphorylation at Ser133 we previously found to be down-regulated during long-term depression (LTD) of glutamatergic transmission in area CA1 of the adult hippocampus in vivo. Other work from our group showed that the activity of PP2A, as well as that of PP1, is increased after LTD induction in area CA1 in vivo. We therefore investigated here whether both protein phosphatases are necessary for LTD in area CA1, and whether they both are involved in the LTD-associated modification of CREB. We found that inhibition of either PP1 or PP2A interferes with the establishment of LTD. Furthermore, inhibition of either enzyme alone abrogated the LTD-associated dephosphorylation of CREB. Interestingly, inhibition of PP1 disrupted CREB dephosphosphorylation rapidly after LTD-inducing stimulation, whereas inhibition of PP2A did not blunt the CREB modification until a later time point. Thus, both PP1 and PP2A regulate CREB during LTD in area CA1, although possibly through different signaling pathways. Our results demonstrate that PP2A, similar to PP1, plays an essential role in the molecular events that underlie LTD at glutamatergic synapses in hippocampal area CA1 in vivo. We propose that one of the mechanisms through which these protein phosphatases may contribute to the prolonged maintenance of LTD is through the regulation of CREB.
Keywords: synaptic plasticity, signaling pathways, NMDA receptor, transcriptional regulation, rat
Changes in synaptic communication that are long-lasting but triggered by relatively brief periods of heightened synaptic activation are thought to be part of the neural events that underlie information storage in the brain (Bliss & Collingridge, 1993; Braunewell & Manahan-Vaughn, 2001; Malenka & Bear, 2004). The molecular mechanisms underlying these long-lasting changes in synaptic communication involve, among others, alterations in protein phosphorylation. Increased protein phosphorylation, typically attributed to increases in the activity of protein kinases, has been implicated in long-term synaptic potentiation (LTP), and decreased protein phosphorylation, typically attributed to increases in the activity of protein phosphatases, has been implicated in long-term synaptic depression (LTD) (Roberson et al., 1996; Winder & Sweatt, 2001; Lisman et al., 2002; Blitzer et al., 2005). Considerable evidence has accumulated demonstrating a role for protein phosphatase 1 (PP1) and protein phosphatase 2B (PP2B, aka calcineurin) in LTD of glutamatergic synapses in hippocampus, a structure well-known to mediate certain types of long-term memory (Isaac 2001; Winder & Sweatt, 2001; Colbran, 2004; Mansuy & Shenolikar, 2006; Squire, 2009). Less is known about a possible role of protein phosphatase 2A in synaptic plasticity.
The catalytic subunit of PP2A is structurally and pharmacologically similar to that of PP1, and commonly used inhibitors of PP1, such as okadaic acid, microcystin LR, calyculin A, or tautomycin, also potently inhibit PP2A (MacKintosh & Klumpp, 1990; MacKintosh et al., 1990; Holmes & Boland 1993). Effects of these agents on synaptic plasticity could thus be attributable, at least in part, to their inhibition of PP2A. Observations that PP2A activity, similar to PP1 activity, is increased after LTD-inducing stimulation in area CA1 of adult hippocampus in vivo (Thiels et al., 1998) suggest a role for PP2A in LTD, as do recent findings that overexpression of SV40 small t antigen protein, a protein that interferes with the proper assembly of PP2A, impairs induction of LTD in area CA1 of young hippocampus in vitro (Nicolls et al., 2008). We therefore sought to determine whether PP2A, as well as PP1, contribute to LTD in adult area CA1 in vivo using specific pharmacologic inhibitors of the respective enzymes.
PP1’s role in LTD has been ascribed primarily to its interactions with synaptic targets, including amino acid receptors and synaptic signaling enzymes (Isaac, 2001; Dell’Aqua et al., 2006; Lee, 2006). Another well-known plasticity-related target of PP1 is the transcription factor cAMP response element binding protein (CREB) (Hagiwara et al., 1992; Alberts et al., 1994; Bito et al., 1996), and a number of studies suggest that negative regulation of CREB by PP1 contributes to long-term changes in synaptic function as well as memory (Deisseroth et al., 1996; Sala et al., 2000; Genoux et al., 2002; Peters et al., 2009). Of potential relevance to this possibility are findings that CREB phosphorylation is reduced immediately after LTD induction in adult area CA1 in vivo (Thiels et al., 2002a). The dephosphorylation of CREB, however, may be mediated not only by PP1 but also by PP2A. Similar to PP1, PP2A can bind to and dephosphorylate CREB (Wadzinski et al., 1993; Wheat et al., 1994). Additionally, PP2A can dephosphorylate extracellular signal-regulated kinase (ERK) (Alessi et al., 1995; Silverstein et al., 2002; Ho et al., 2007), a critical element of a signaling pathway that regulates CREB phosphorylation during LTP (Impey et al., 1998; Davis et al., 1998). A second goal of the present work therefore was to determine whether both PP1 and PP2A contribute to the LTD-associated dephosphorylation of CREB. Our findings demonstrate that PP2A, in addition to PP1, is necessary for the development of LTD in area CA1 of the adult hippocampus in vivo. They furthermore show that both protein phosphatases play a critical role in the regulation of CREB during LTD. However, PP2A’s effect on CREB is temporally delayed relative to that of PP1.
Materials and methods
Electrophysiological recordings
We used electrophysiological methods as described previously (Thiels et al., 1994, 2002a) to obtain recordings from hippocampal area CA1 evoked by stimulation of the contralateral hippocampal area CA3 in anesthetized adult male rats (Sprague Dawley, 270–360 g; Hilltop, Scottsdale, PA). All procedures were in compliance with and approved by the Institutional Animal Care and Use Committee, University of Pittsburgh. Briefly, animals were anesthetized with chloral hydrate (400 mg/kg, ip) and small holes were drilled bilaterally into the skull overlaying the cortex above the hippocampus. Bipolar metal stimulating electrodes (insulated except for 150–200 µm at the tip) were lowered into dorsal area CA3 of the left hippocampus and a glass recording pipette (0.8–1.2 MΩ) was lowered into either stratum pyramidale or stratum radiatum of dorsal area CA1 of the right hippocampus. Series of 10 test pulses (30–80 µA, 100-µsec duration; stimulation frequency: 0.1 Hz) were delivered at 5-min intervals before and after LTD-inducing paired-pulse stimulation (PPS). After stable responding to test pulses was established (≥15 min of recording), PPS that consisted of 200 pairs of pulses (inter-stimulus interval within a pair: 25 ms; inter-pair interval: 2 sec) was delivered to the dorsal commissural pathway. PPS was delivered using a stimulation intensity that evoked an area CA1 population spike with an amplitude ~70% of the maximum amplitude, as determined based on an input-output function produced in str. pyramidale after drug infusion was well underway (see below). For recordings in str. pyramidale, test pulses were delivered using a stimulation intensity that evoked an area CA1 population spike with an amplitude ~40% of the maximum amplitude, as determined based on the same input-output function as above; for recordings in str. radiatum, test pulses were delivered using a stimulation intensity that evoked an area CA1 population excitatory postsynaptic potential (pEPSP) with an initial slope ~30% of the maximum slope, as determined based on an input-output function produced in str. radiatum after drug infusion was well underway and the recording electrode had been lowered to its final position. Responding to test pulses was monitored for up to 37 min after PPS onset. The animals were maintained under anesthesia with supplemental injections of chloral hydrate (60 mg/kg) via a tail vein cannula. Their body temperature was maintained at 37°C with the aid of a heating pad.
Intra-hippocampal drug infusions were delivered via a glass pipette (ID at the tip: 25 to 35 µm) placed ~300 µm lateral and ~200 µm ventral relative to the tip of the recording electrode. Drugs were infused with the aid of a positive pressure pump (Harvard Apparatus, Holliston, MA) beginning 1 hr before determining the first input-output function, which was about 1.5 to 2 hr before PPS. The infusion rate was set to 5 to 8 nL/min for the first 30 min and then reduced to 2 to 3 nL/min for the remainder of the experiment, i.e., until the end of the final series of test pulse stimulation. The drugs infused included okadaic acid (10 µM; dissolved in 1% dimethylsulfoxide [DMSO] and 99% physiological saline); Nipp-1, a specific PP1 inhibitor (50 nM; dissolved in 1% DMSO, 5% supplier-provided formulation, and 94% of physiological saline); and fostriecin, a specific inhibitor of PP2A-type phosphatases (1 – 3 µM; dissolved in 1% DMSO and 99% physiological saline). All drugs were purchased from Calbiochem/EMD Chemicals (San Diego, CA). Recorded waveforms were amplified, filtered (0.1–10 kHz), digitized (10 kHz), and stored electronically for later analysis of the amplitude of the evoked CA1 population spike or the initial slope (1.0 msec after onset) of the evoked CA1 pEPSP. Statistical comparisons of response measures between a given drug group and its corresponding vehicle control group were conducted using two-way repeated measures ANOVAs (between-subject factor: drug; within-subject factor: timer before/after PPS) followed by post-hoc comparisons using the Bonferroni method. An α-level of ≤ 0.05 was applied to determine statistical significance.
For purposes of biochemical analyses, animals were killed either immediately before or at various times after PPS, and their right hippocampus was removed rapidly from the brain in the presence of ice cold artificial CSF (in mM: 124 NaCl, 5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1.5 MgCl2 and 2.5 CaCl2). A block of area CA1 (~1 mm3) was excised from the dorsal and the ventral portion of the hippocampus, and each tissue block was placed in individual, coded vials on dry ice, which then were stored at −80° C until biochemical analysis.
Western blot analyses
We used procedures to isolate nuclear extracts and conduct Western blot analyses as described previously (Thiels et al., 2002a). Briefly, the microdissected dorsal and ventral area CA1 tissue samples were placed in ice-cold buffer A (10 mM HEPES-OH, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 1 mM DTT, 1 mM NaF, 1 mM sodium orthovanadate, 2 mM sodium pyrophosphate, 1 mM PMSF, 10 uM benzamidine, 1 µg/ml leupeptin, 1 µg/ml aprotinin, and 1 µg/ml pepstatin) and incubated on ice for 20 min. Cells were disrupted with a dounce homogenizer (~20–40 strokes) until nuclei were free of cytoskeletal attachments as detected microscopically with phase-contrast examination and then centrifuged at 13,200 g at 4° C for 2 min. The supernatant was decanted, and the nuclear pellet was resuspended in 30 µl of ice-cold buffer B (10 mM HEPES, pH 7.0, 450 mM NaCl, 5 mM EDTA, 0.05% SDS, 1% Triton X-100, 2 mM DTT, 1 mM NaF, 1 mM sodium orthovanadate, 2 mM sodium pyrophosphate, 1 mM PMSF, 10 µM benzamidine, 1 µg/ml leupeptin, 1 µg/ml aprotinin, and 1 µg/ml pepstatin) and incubated for 45 min on ice with gentle rocking, followed by centrifugation at 13,200 g at 4° C for 10 min. The resulting supernatant was decanted, saved, and used as nuclear extract.
Protein concentrations in the nuclear extracts were determined according to the method of the BCA assay (Pierce Biotechnology, Rockford, IL) using bovine serum albumin (BSA) as standard. Equivalent amounts of protein for each sample were resolved by 10% SDS-PAGE, blotted electrophoretically onto PVDF Immobilon membranes (Millipore, Bedford, MA), and blocked for 60 min with 5% milk. The membrane then was cut at the level of 50kD, and the lower portion containing proteins <50 kD was incubated in 5% BSA with an antibody that recognizes Ser133-phosphorylated CREB (1:1000; EMD Biosciences, San Diego, CA; or 1:1000, Upstate-Millipore, Billeria, MA) and the upper portion containing proteins >50 kD was incubated in an antibody that recognizes the neuronal nuclear marker NeuN (1:5000; Chemicon-Millipore, Billaria, MA). After incubation with the primary antibody overnight at 4° C, the membranes were washed three times with TTBS buffer (50 mM Tris-HCL, pH 7.5–8.0, 150 mM NaCl, 0.1% Tween 20), exposed to either an anti-rabbit IgG peroxidase-linked antibody (for pCREB; 1:5000 dilution; Cell Signaling Technology, Danvers, MA) or an anti-mouse IgG peroxidase-linked antibody (for NeuN; 1:5000; Cell Signaling Technologies), washed again three times with TTBS, and developed using an enhanced chemiluminescence reagent (LumiGLO, Cell Signaling Technologies). Blot images were captured digitally with a CCD camera (Hamamatsu Photonics, Hamamatsu, Japan) and an image analysis station (UVP Bioimaging systems, Upland, CA) and analyzed densitometrically using LabWorks image analysis software (UVP Bioimaging systems, Upland, CA). The lower membrane then was stripped in a heated (50° C) bath containing 50 mL activated stripping buffer (7.5 g Tris, 100 mL SDS 20%, 900 mL dH2O, and 315 µL mercaptoethanol), washed three times with TTBS, blocked for 30 min with 5% milk, and reprobed in 5% BSA with an antibody raised against total CREB (1:1000; EMD Biosciences, San Diego, CA; or 1:1000, Upstate-Millipore, Billeria, MA). Subsequent probing with the secondary antibody and densitometric analysis of the chemiluminescent signals involved the same steps as described above. Similar to the approach we applied previously to assess changes in protein phosphorylation after induction of LTD in dorsal area CA1 (Thiels et al., 2000, 2002a), we determined CREB phosphorylation (pCREB immunoreactivity/tCREB immunoreactivity) for each sample of dorsal area CA1 (experimental tissue sample) and ventral area CA1 from the same animal (control tissue sample). Samples from the same animal always were run next to one another on the same membrane. Occasionally, the tCREB immunoreactivity signal was very poor and difficult to analyze with confidence. In those instances (29% of observations), we used the NeuN immunoreactivity signal for both the dorsal and the ventral sample from the same animal to normalize the respective pCREB signals. Statistical evaluation of an effect of PPS on CREB phosphorylation in the various conditions was conducted by comparing the average ratio of CREB phosphorylation in dorsal to ventral area CA1 against the hypothetical ratio of 1.0 (no difference in phosphorylation status between experimental and control tissue) using two-tailed one-sample Student’s t-tests and applying an α-level of ≤ 0.05 to determine statistical significance.
Results
Long-term depression in area CA1 of the adult hippocampus in vivo fails to develop when either protein phosphatase 1 or protein phosphatase 2A is inhibited
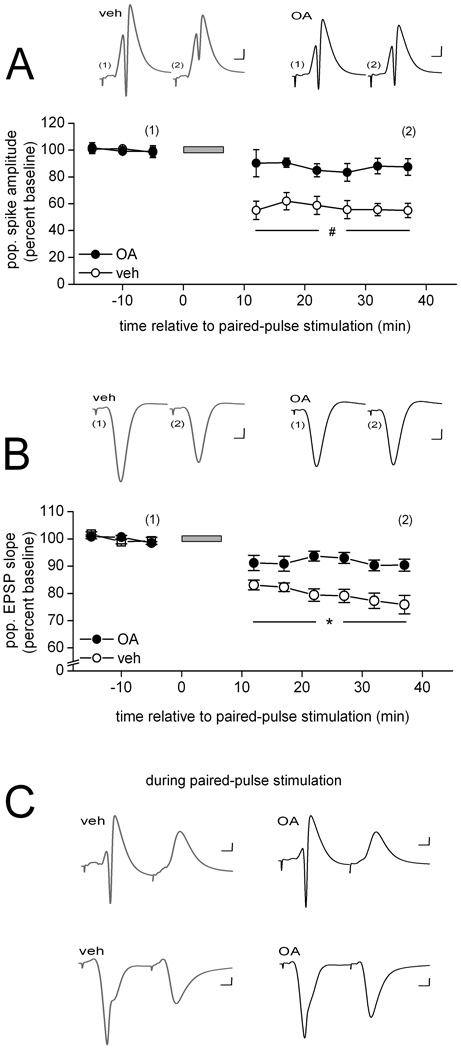
As a first step toward determining the role of PP1 and PP2A in LTD in area CA1 of the adult in vivo hippocampus, we examined the effect of intra-hippocampal application of okadaic acid, a selective inhibitor of both PP1 and PP2A, on LTD. Figure 1 shows that infusion of okadaic acid (10 µM in the drug pipette) near the recording site interfered with the development of a prolonged depression of the evoked population spike (Fig. 1A) as well as the evoked pEPSP after one train of PPS (Fig. 1B). A two-way ANOVA on the population spike data revealed a significant drug effect [F(1,9) = 16.00, p < 0.01], a significant within-subject time effect [F(8, 72) = 19.09, p < 0.01), and a significant drug × time interaction [F(8, 72) = 5.81, p < 0.01]. Post-hoc comparisons showed that the evoked population spike amplitude did not differ between the two drug conditions before PPS, but beginning with the first measurement after PPS the evoked response of vehicle-infused animals was significantly lower of that of okadaic acid-infused animals (all ps < 0.02) (Fig. 1A). Post-hoc tests also revealed that, whereas the evoked population spike amplitude recorded in vehicle-infused animals 37 min after the onset of PPS was significantly lower than that observed 5 min before PPS (p < 0.01), no lasting depression of the response developed in the presence of okadaic acid (p > 0.5). A similar pattern emerged for the pEPSP data, with both main effects and the interaction being significant [drug: F(1, 11) = 15.87; time: F(8, 88) = 50.05; and drug × time F(8, 88) = 9.75, all ps < 0.01], and post-hoc tests revealing no difference between the groups before PPS or immediately after PPS, but beginning with second series of measurements after PPS, the initial slope of the evoked pEPSP was significantly lower in vehicle- compared to okadaic acid-infused animals (all ps < 0.05) (Fig. 1B). Moreover, whereas PPS caused a significant depression of the pEPSP in the presence of vehicle solution (5 min before PPS vs. 37 min after PPS, p < 0.01), it failed to do so in the presence of okadaic acid (p > 0.2). The failure of LTD development in the presence of okadaic acid, combined with our previous demonstration that the induction of LTD by PPS is dependent on NMDA receptor activation (Thiels et al., 1998), is consistent with previous observations of an effect of this inhibitor on NMDA receptor-dependent LTD in area CA1 or the entorhinal cortex (Xiao et al., 1995; Jouvenceau et al., 2003; Kourrich et al., 2008). Furthermore, it suggests that either one or both of the okadaic acid-sensitive protein phosphatases are necessary for the development of NMDA receptor-dependent LTD.
Figure 1.
The PP1 and PP2A inhibitor okadaic acid interferes with PPS-induced LTD in area CA1 of the adult hippocampus in vivo. (A) Means ± SEMs of the amplitude of the population spike evoked by commissural stimulation before and after paired-pulse stimulation (PPS; grey horizontal bar) delivered to the same fibers. PPS was delivered in the presence of either vehicle solution (veh; open circles; n = 5) or okadaic acid (10 µM in the drug pipette; OA; filled cirles; n = 6). Representative waveforms (average of 10) collected from an animal in each of the two drug conditions at the times indicated in the line graph are shown above. Scale: horizontal, 4 msec; vertical, 1 mV. Post-hoc between-drug comparisons for the indicated time points: #, p < 0.02. (B) Similar data for the initial slope of the population EPSP evoked by commissural stimulation. As above, PPS was delivered in the presence of either vehicle solution (open circles; n = 6) or okadaic acid (filled circles; n = 7). Representative waveforms (average of 10) collected from an animal in each of the two drug conditions at the times indicated in the line graph are shown above. Scale: horizontal, 4 msec; vertical, 1 mV. Post-hoc between-drug comparisons for the indicated time points: *, p < 0.05. (C) Representative waveforms evoked by a pair of pulses during PPS, averaged across the 200 pairs of the train and collected in either str. radiatum (top traces) or str. radiatum (bottom traces) in the presence of either vehicle solution (grey traces) or okadaic acid (black traces). Scale: horizontal, 4 msec; vertical, 1 mV.
Because the disruptive effect was evident relatively quickly after PPS, one might argue that LTD failed to develop because of altered cellular activation during PPS. One of the critical elements for the induction of LTD by PPS is that the first pulse of a pair of pulses triggers feed-forward and/or feedback inhibition that coincides with the arrival of the excitatory input evoked by the second pulse, preventing the second pulse from triggering postsynaptic cell firing. In the absence of this GABA receptor-mediated paired-pulse inhibition, LTD fails to develop (Thiels etal., 1994). Thus, the interference of LTD we observed here may stem from an effect of the PP inhibitor on paired-pulse inhibition. Alternatively, the protein phosphatase inhibitor might have reduced current flow during PPS, thereby altering the conditions required for LTD induction. To address these possibilities, we assessed evoked firing and total evoked current flow during PPS. We found that neither the average amplitude of the population spike evoked by either the first pulse [vehicle: 7.8 ± 1.0 mV, okadaic acid: 6.4 ± 1.2 mV; Student’s t(9) < 1.0] or the second pulse during PPS [vehicle: 0.0 ± 0.0 mV, okadaic acid: 0.0 ± 0.0 mV; t(9) < 1.0] was affected by okadaic acid, nor was the average integral of the pEPSP evoked during PPS significantly altered in the presence of the inhibitor [vehicle: 49.5 ± 4.6 mV/msec, okadaic acid: 45.8 ± 3.4 mV/msec; t(11) < 1.0]. Figure 1C depicts examples of the average evoked waveform during PPS as recorded in str. pyramidale (top traces) or str. radiatum (bottom traces) for each of the two drug conditions. Our observations do not support an explanation of the impairment of LTD in terms of differences in cell firing, paired-pulse inhibition, or total synaptic input during the stimulation train. We therefore favor the conclusion that LTD failed to develop in the presence of okadaic acid because of a critical contribution by one or both of the okadaic acid-sensitive protein phosphatase(s) to a process necessary for the establishment of LTD.
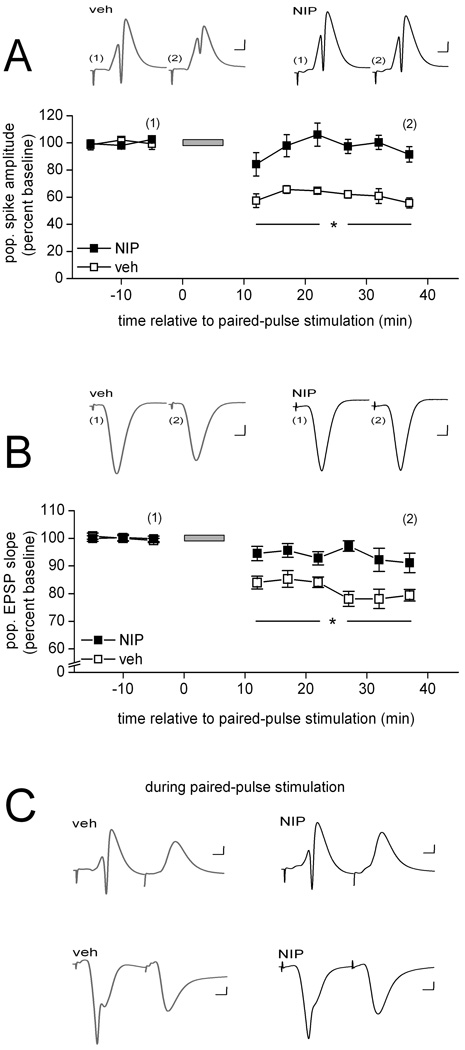
Previous work showed that PP1 plays a critical role in the development of LTD induced by prolonged low-frequency stimulation in area CA1 in vitro (Mulkey et al., 1994; Morishita et al., 2001; Jouvenceau et al, 2006). The results observed here therefore might be attributable exclusively to the inhibition of PP1. To determine whether only one or both of the okadaic acid-sensitive protein phosphatases play a role in LTD, we infused into area CA1 near the recording site either the specific PP1 inhibitor Nipp1 (50 nM in the drug pipette) or the specific PP2A-type phosphatase inhibitor fostriecin (1 to 3 µM in the drug pipette). Consistent with previous work, LTD failed to develop in the presence of Nipp-1 (Fig. 2). Analysis of the population spike data depicted in Figure 2A indicated a significant drug effect [F(1, 12) = 26.17, p < 0.01], a significant within-subject time effect [F(8, 96) = 13.38, p < 0.01), and a significant drug × time interaction [F(8, 96) = 8.32, p < 0.01]. The significant interaction could be attributed to no difference in the response between the two drug conditions before PPS but a significantly lower evoked population spike amplitude in vehicle- compared to Nipp1-infused animals beginning with the first measurement after PPS (all ps < 0.05) (Fig. 2A). As was the case above, PPS caused a significant reduction of the response when vehicle solution was infused (p < 0.01), but this effect failed to emerge in the presence of the PP1 inhibitor (p > 0.5). The results for the initial slope of the evoked pEPSP are shown in Figure 2B. Both main effects and the interaction were significant [drug: F(1, 9) = 18.74; time: F(8, 72) = 17.93; and drug × time F(8, 72) = 5.39, all ps < 0.01], as a result of no difference between the groups before PPS, but a significantly lower response in vehicle- compared to Nipp1-infused animals beginning with first series of measurements after PPS (all ps < 0.05) (Fig. 2B). PPS produced a significant depression of the dendritic response when the stimulation train was delivered in the presence of vehicle solution (p < 0.02), whereas no lasting depression of the pEPSP developed in the presence of Nipp1 (p > 0.5).
Figure 2.
Selective inhibition of PP1 is sufficient to block the development of PPS-induced LTD in area CA1 in vivo. (A) Means ± SEMs of the amplitude of the population spike evoked by commissural stimulation before and after paired-pulse stimulation (PPS; grey horizontal bar) delivered to the same fibers. PPS was delivered in the presence of either vehicle solution (veh; open squares; n = 6) or Nipp-1(50 nM in the drug pipette; NIP; filled squares; n = 8). Representative waveforms (average of 10) collected from an animal in each of the two drug conditions at the times indicated in the line graph are shown above. Scale: horizontal, 4 msec; vertical, 1 mV. Post-hoc between-drug comparisons for the indicated time points: *, p < 0.05. (B) Similar data for the initial slope of the population EPSP evoked by commissural stimulation. As above, PPS was delivered in the presence of either vehicle solution (open squares; n = 5) or Nipp-1 (filled squares; n = 6). Representative waveforms (average of 10) collected from an animal in each of the two drug conditions at the times indicated in the line graph are shown above. Scale: horizontal, 4 msec; vertical, 1 mV. Post-hoc between-drug comparisons for the indicated time points: *, p < 0.05. (C) Representative waveforms evoked by a pair of pulses during PPS, averaged across the 200 pairs of the train and collected in either str. radiatum (top traces) or str. radiatum (bottom traces) in the presence of either vehicle solution (grey traces) or Nipp-1 (black traces). Scale: horizontal, 4 msec; vertical, 1 mV.
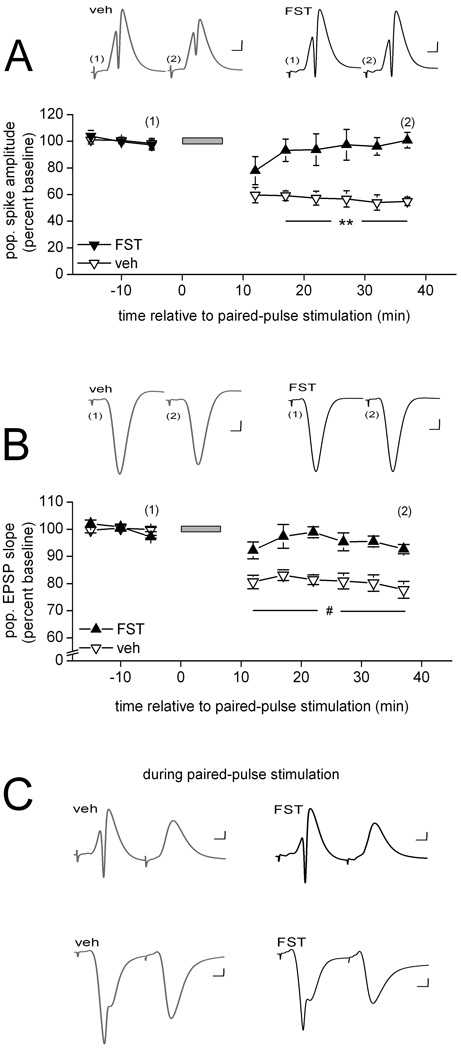
Figure 3 shows that infusion of the PP2A inhibitor fostriecin near the recording site was as effective in interfering with the development of LTD as was infusion of a combined PP1 and PP2A inhibitor (Fig. 1). Analysis of the population spike data depicted in Figure 3A revealed a significant drug effect [F(1,8) = 17.79, p < 0.01], a significant within-subject time effect [F(8, 64) = 11.45, p < 0.01), and a significant drug × time interaction [F(8, 64) = 6.82, p < 0.01]. Post-hoc comparisons confirmed that the two drug conditions did not differ before PPS, but that the response was significantly lower in vehicle- than in fostriecin-infused animals beginning with the second measurement after PPS (all ps < 0.01) (Fig. 3A). Post-hoc comparisons furthermore confirmed that the evoked response recorded in vehicle-infused animals decreased significantly after PPS, compared to pre-PPS levels (p < 0.01), whereas such depression did not develop in the presence of fostriecin (p > 0.5). The results for the initial slope of the evoked pEPSP were similar in that both main effects and the interaction were significant [drug: F(1, 9) = 22.53; time: F(8, 72) = 21.51; and drug × time F(8, 72) = 7.97, all ps < 0.01], as a result of no difference between the groups before PPS, but a significantly lower response in vehicle- compared to fostriecin-infused animals beginning immediately after PPS (all ps < 0.02) (Fig. 3B). A robust depression of the evoked pEPSP emerged upon PPS among vehicle-infused animals (p < 0.01), but failed to do so in the presence of fostriecin (p > 0.5). Our findings of a disruption of LTD by fostriecin are consistent with a recent report on mice that overexpress SV40 small t antigen protein, a protein that interferes with the proper assembly of PP2A. LTD induced by prolonged low-frequency stimulation was found to be impaired in area CA1 of hippocampal slices obtained from these mice (Nicholls et al., 2008). Taken together with our previous observations of increased PP2A activity after LTD-inducing PPS (Thiels et al., 1998), our present findings ascribe a pivotal role to PP2A in the signaling events that underlie the establishment of LTD in hippocampal area CA1.
Figure 3.
Inhibition of PP2A also is sufficient to interfere with PPS-induced LTD in area CA1 in vivo. (A) Means ± SEMs of the amplitude of the population spike evoked by commissural stimulation before and after paired-pulse stimulation (PPS; grey horizontal bar) delivered to the same fibers. PPS was delivered in the presence of either vehicle solution (veh; open triangles; n = 5) or fostriecin (1–3 µM in the drug pipette; FST; filled triangles; n = 5). Representative waveforms (average of 10) collected from an animal in each of the two drug conditions at the times indicated in the line graph are shown above. Scale: horizontal, 4 msec; vertical, 1 mV. Post-hoc between-drug comparisons for the indicated time points: **, p < 0.01. (B) Similar data for the initial slope of the population EPSP evoked by commissural stimulation. As above, PPS was delivered in the presence of either vehicle solution (open symbols; n = 6) or fostriecin (filled triangles; n = 5). Representative waveforms (average of 10) collected from an animal in each of the two drug conditions at the times indicated in the line graph are shown above. Scale: horizontal, 4 msec; vertical, 1 mV. Post-hoc between-drug comparisons for the indicated time points: #, p < 0.02. (C) Representative waveforms evoked by a pair of pulses during PPS, averaged across the 200 pairs of the train and collected in either str. radiatum (top traces) or str. radiatum (bottom traces) in the presence of either vehicle solution (grey traces) or fostriecin (black traces). Scale: horizontal, 4 msec; vertical, 1 mV.
In contrast to the differences in response measures between vehicle- and inhibitor-infused animals after PPS, we found no effect of either Nipp1 or fostriecin on responding during PPS. Examples of the average evoked waveform during PPS in the presence of either vehicle solution or Nipp1 are shown in Figure 2C (str. pyramidale recordings – top traces; str. radiatum recordings – bottom traces), and in the presence of either vehicle solution or fostriecin are depicted in Figure 3C (ibid). Analysis of the waveforms recorded during PPS revealed that the average amplitude of the population spike evoked by either the first pulse or the second pulse did not differ either between vehicle-infused and Nipp1-infused animals (first pulse- vehicle: 8.7 ± 0.5 mV and Nipp1: 8.0 ± 0.6 mV; second pulse- vehicle: 0.0 ± 0.0 mV and Nipp1: 0.0 ± 0.0 mV; both ts(12) < 1.0) or between vehicle-infused and fostriecin-infused animals [first pulse-vehicle: 7.2 ± 1.1 mV and fostriecin: 6.9 ± 0.8 mV; second pulse- vehicle: 0.0 ± 0.0 mV and fostriecin: 0.0 ± 0.0 mV; both ts(9) < 1.0]. Similarly, the average integral of the pEPSP evoked during PPS did not differ either between vehicle-infused and Nipp1-infused animals [vehicle: 54.6 ± 1.8 mV/msec and Nipp1: 47.4 ± 3.1 mV/msec; t(9) = 1.9, p > 0.08] or between vehicle-infused and fostriecin-infused animals [vehicle: 46.7 ± 6.9 mV/msec and fostriecin: 48.1 ± 4.7 mV/msec; t(9) < 1.0]. These findings suggest that the interference of LTD development in the presence of either of the two specific inhibitors did not result from altered activation during the patterned stimulation phase. Rather, the impairment of LTD appears to arise from the interference of processes mediated by PP1 and PP2A that are required for the expression and possibly also the maintenance of persistent depression of the CA1 response evoked by CA3 input.
CREB phosphorylation during long-term depression is sensitive to inhibition of PP2A following a different time course than it sensitivity to inhibition of PP1
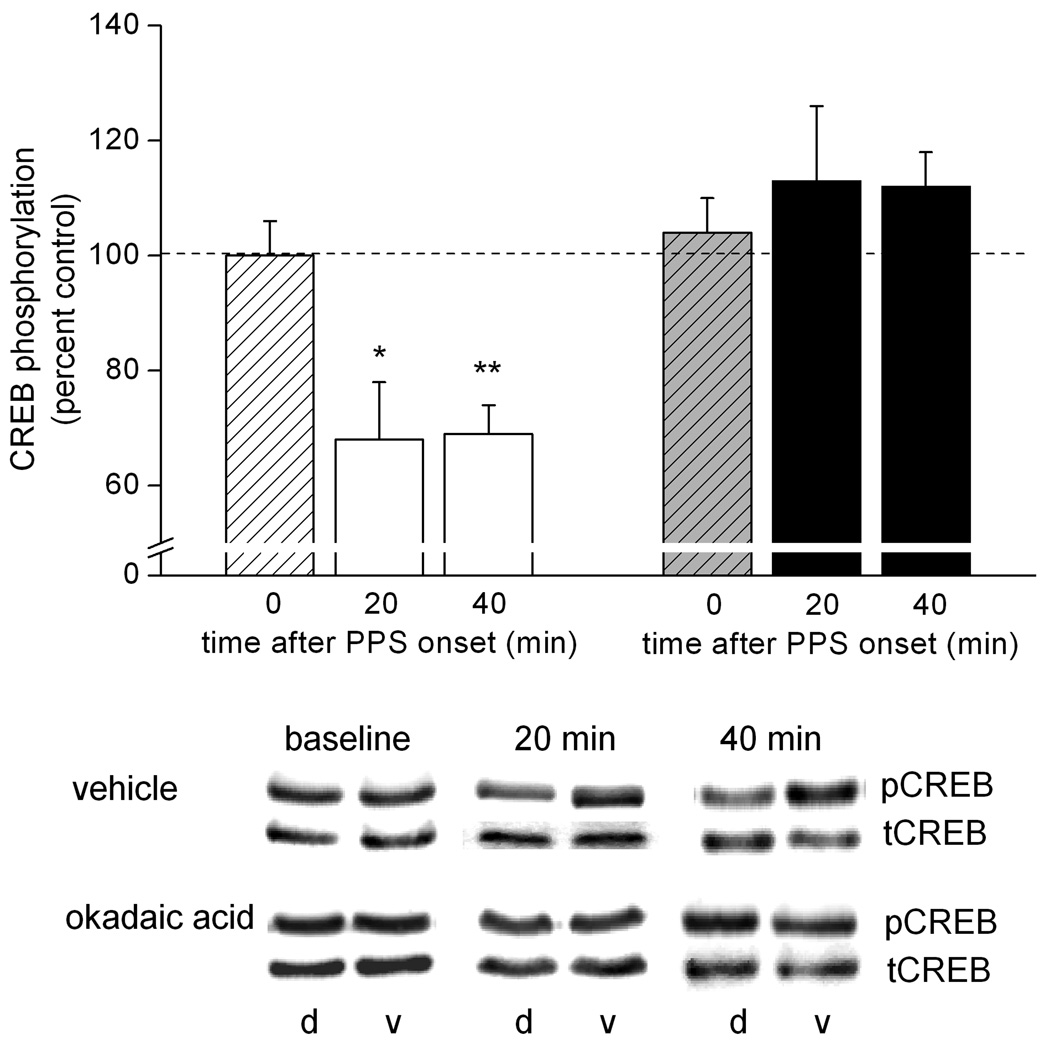
One mechanism through which PP1 and/or PP2A might contribute to LTD is by influencing transcriptional regulation. We previously reported that CREB phosphorylation is reduced markedly after the induction of LTD by PPS in area CA1 in vivo (Thiels et al., 2002a). In light of our observations of an increase in PP1 and PP2A activity after LTD induction by PPS (Thiels et al., 1998), combined with reports of targeting of CREB by PP1 as well as PP2A (Wadzinski et al., 1993; Alberts et al., 1994; Wheat et al., 1994), we hypothesized that the LTD-associated dephosphorylation of CREB in area CA1 could be mediated by PP1 and/or PP2A. As a first test of this possibility, we determined whether the reduction in CREB phosphorylation is sensitive to okadaic acid. CREB phosphorylation in area CA1 that underwent LTD was determined by applying Western blot analysis using an antibody that recognizes Ser133- phosphorylated CREB on tissue excised from dorsal area CA1 near the recording site (experimental sample) and on a similar-sized tissue sample excised from ventral area CA1 (within-subject control sample), a region not innervated by the dorsal commissural fibers stimulated in these experiments (Laurberg, 1979; Ishizuka et al., 1990). The results from animals that received continuous infusion of vehicle solution near the recording site, depicted in Figure 4, show that prior to PPS, i.e., under baseline conditions, CREB phosphorylation (pCREB immunoreactivity/tCREB immunoreactivity) in dorsal area CA1 (experimental tissue) was comparable to that in ventral area CA1 (control tissue) [t(5) < 1.0]. After PPS, however, CREB phosphorylation was reduced significantly in tissue samples that contained synapses that underwent LTD, relative to CREB phosphorylation in control tissue. This effect developed within 20 min or less after PPS and lasted at least 40 min after the LTD-inducing stimulation [20-min time point: t(4) = 3.0, p < 0.05; 40-min time point: t(10) = 6.4, p < 0.01]. The PPS-induced reduction in CREB phosphorylation was abolished completely when PPS was delivered in the presence of okadaic acid (10 µM in the drug pipette); neither 20 min nor 40 min after PPS did CREB phosphorylation in experimental samples differ significantly from the phosphorylation level detected in control samples in okadaic acid-infused animals [20 min: t(5) <1.0; 40 min: t(12) = 2.0, p > 0.05]. Basal CREB phosphorylation was unaffected by continuous infusion okadaic acid for 2 hr to 2.5 hr in that the phosphorylation level was comparable in experimental and control tissue [t(4) < 1.0], similar to what was observed after prolonged infusion of vehicle solution. This outcome indicates that the blockade of the PPS-induced dephosphorylation of CREB was not due to hyperphosphorylation of the transcription factor in the presence of the phosphatase inhibitor. Moreover, the lack of an effect of the inhibitor on basal CREB phosphorylation suggests that the turn-over of phosphorylation at the Ser-133 site is limited in the absence of patterned synaptic input. Taken together, these results indicate that PP1 and/or PP2A are responsible, either directly or indirectly, for the reduction in CREB phosphorylation after LTD-inducing stimulation.
Figure 4.
Okadaic acid abolishes completely the LTD-associated dephosphorylation of CREB. Means ± SEMs of pCREB immunoreactivity relative to tCREB immunoreactivity in dorsal area CA1 tissue samples, expressed as a percent of pCREB-to-tCREB immunoreactivity in ventral (control) area CA1 tissue samples, collected either after baseline stimulation only (0 min) or either 20 min or 40 min after the onset of paired-pulse stimulation (PPS). PPS was delivered in the presence of either vehicle solution (striped bar, 0 min; n = 6; open bars, 20 min: n = 5, 40 min: n = 11) or okadaic acid (10 µM in the drug pipette; striped bar, 0 min; n = 5; filled bars, 20 min: n = 6, 40 min: n = 13). Representative immunoblots of pCREB and tCREB for samples from animals of each of the two drug conditions and each of the three time points are shown below. d = dorsal area CA1, v = ventral area CA1 from the same animal. Dorsal/ventral comparison, *, p < 0.05; **, p < 0.01.
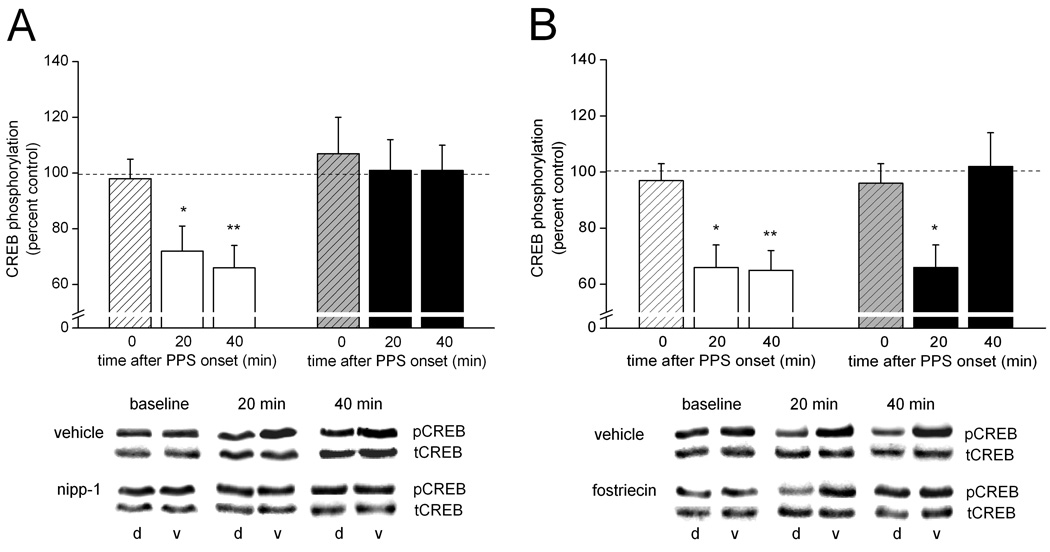
To determine which of the two candidate enzymes might be responsible for the dephosphorylation effect, we assessed the change in CREB phosphorylation after PPS when delivered in the presence of continuous infusion of either the PP1 inhibitor Nipp-1 or the PP2A inhibitor fostriecin. Figures 5 illustrates that both protein phosphatases regulate CREB after PPS; the time course of their CREB regulation, however, appears to differ between them. The left side of Figure 5A shows that in the presence of vehicle solution used for the Nipp1 experiments CREB phosphorylation was comparable in experimental and control tissue samples before PPS [t(4) < 1.0], but it was significantly decreased in experimental relative to control tissue samples both 20 min and 40 min after PPS [t(5) = 2.91, p < 0.05; and t(9) = 4.4, p < 0.01, respectively]. In contrast, in the presence of Nipp-1 (50 nM in the drug pipette), the PPS-induced decrease in CREB phosphorylation was blocked, with CREB phosphorylation levels in experimental tissue samples remaining near control levels at both time points after PPS (right side of Fig. 5A) [20 min: t(5) <1.0; 40 min: t(13) < 1.0]. As was the case with okadaic acid, the lack of a PPS effect on CREB in the presence of Nipp-1 was not the result of hyperphosphorylation of CREB by the inhibitor, as under baseline conditions prolonged (2 to 2.5 hr) infusion of Nipp-1 was not associated with an increase in CREB phosphorylation [baseline: t(4) < 1.0]. Figure 5B shows on the left side the now familiar and robust PPS-induced decrease in CREB phosphorylation in the presence of vehicle solution [baseline: t(4) < 1.0; 20 min: t(4) = 4.0, p < 0.02; 40 min: t(10) = 5.3, p < 0.01. The right side of Figure 5B shows that, different from Nipp-1, fostriecin (1 – 3 µM in the drug pipette) failed to block the early phase of this PPS-induced decrease in CREB phosphorylation. That is, similar to what we observed in the presence of vehicle solution, CREB phosphorylation in experimental tissue samples from animals that received continuous infusion of fostriecin was lower than control levels 20 min after PPS [t(6) = 3.1, p < 0.05]. The later phase of the PPS-induced reduction in CREB phosphorylation, however, was blocked by fostriecin, as indicated by a comparable level of CREB phosphorylation in experimental and control tissue samples 40 min after PPS [t(9) < 1.0]. This difference in fostriecin effect between the two time points after PPS emerged even though the total amount and duration of drug infusion was comparable between these two conditions (20-min group: 414 ± 32 nL, 128 ± 7 min; 40-min group: 490 ± 38 nL, 141 ± 5 min; both ps > 0.1). As was the case with the other inhibitors, prolonged infusion of fostriecin had no effect on basal CREB phosphorylation [t(5) < 1.0]. Taken together, these results implicate both PP1 and PP2A in the regulation of CREB phosphorylation in area CA1 after LTD-inducing stimulation. The time course of action of the two enzymes vis-à-vis CREB, however, appears to differ in that PP1 seems to target CREB or a CREB-regulating enzyme immediately after LTD-inducing stimulation, whereas PP2A does not seem to impact CREB’s phosphorylation state until tens of minutes since the induction event have passed, and synaptic communication has attained a new stable level.
Figure 5.
Inhibition of PP1 blocks the dephosphorylation of CREB beginning immediately after LTD-inducing synaptic stimulation, whereas inhibition PP2A does not interfere with the CREB dephosphorylation until a later time during LTD. (A) Means ± SEMs of pCREB immunoreactivity relative to tCREB immunoreactivity in dorsal area CA1 tissue samples, expressed as a percent of pCREB-to-tCREB immunoreactivity in ventral (control) area CA1 tissue samples, collected either after baseline stimulation only (0 min) or either 20 min or 40 min after the onset of paired-pulse stimulation (PPS). PPS was delivered in the presence of either vehicle solution (striped bar, 0 min; n = 5; open bars, 20 min: n = 6, 40 min: n = 10) or nipp-1 (50 nM in the drug pipette; striped bar, 0 min; n = 5; filled bars, 20 min: n = 6, 40 min: n = 14). Representative immunoblots of pCREB and tCREB for samples from animals of each of the two drug conditions and each of the three time points are shown below. d = dorsal area CA1, v = ventral area CA1 from the same animal. Dorsal/ventral comparison, *, p < 0.05; **, p < 0.01. (B) Similar data for animals that received PPS in the presence of either vehicle solution (striped bar, 0 min; n = 5; open bars, 20 min: n = 5, 40 min: n = 11) or fostriecin (1–3 µM in the drug pipette; striped bar, 0 min; n = 6; filled bars, 20 min: n = 7, 40 min: n = 10). Representative immunoblots of pCREB and tCREB for samples from animals of each of the two drug conditions and each of the three time points are shown below. d = dorsal area CA1, v = ventral area CA1 from the same animal. d = dorsal area CA1, v = ventral area CA1 from the same animal. Dorsal/ventral comparison, *, p < 0.05; **, p < 0.01.
Discussion
A number of studies showed that the PP1 and PP2A inhibitor okadaic acid disrupts NMDA receptor-dependent LTD at glutamatergic synapses in hippocampus as well as elsewhere in brain (Mulkey et al., 1993; Kirkwood & Bear, 1994; Morishita et al., 2001; Jouvenceau et al., 2003; Belmeguenai & Hansel, 2005; Kourrich et al., 2008), implicating one or both of these protein phosphatases in activity-dependent modification of synaptic strength. Here we sought to determine the role of each of these two protein phosphatases individually to NMDA receptor-dependent LTD in area CA1 in vivo (Thiels et al., 1998), taking advantage of specific inhibitors of PP1 and PP2A, respectively. In addition, we wanted to determine whether only one or both protein phosphatases play a role in the regulation of CREB phosphorylation after induction of LTD. Our findings show that inhibition of either protein phosphatase disrupts LTD in area CA1, and, furthermore, that inhibition of either protein phosphatase abolishes the PPS-associated decrease in CREB phosphorylation. Interestingly, inhibition of PP2A did not prevent the decrease in CREB phosphorylation observed early after LTD induction, whereas inhibition of PP1 prevented the decrease in CREB phosphorylation beginning at the earliest time point during LTD assayed here.
Our findings of an effect of the phosphatase inhibitors on PPS-induced LTD immediately after termination of stimulation suggest that PP1 and PP2A contribute to LTD, at least during the early phase of LTD in area CA1, by acting on synaptic targets, such as glutamate receptors or proteins involved in glutamate receptor regulation or trafficking (Wang et al., 1994; Roche et al., 1996; Lee et al., 1998, 2000; Lin et al., 2000; Tomita et al., 2005; Hu et al., 2007; c.f., Lee, 2006). We previously found that induction of LTD by PPS in area CA1 in vivo is associated with a reduction in phosphorylation of Ser831 and Ser845 in the C-terminus of the GluR1 subunit of the AMPA receptor (Thiels et al., 2002b). This LTD-associated reduction of GluR1 phosphorylation was blocked when PPS was delivered in the presence of okadaic acid, which suggests that PP1 and PP2A may act on these GluR1 sites directly (Thiels et al., 2002b). Alternatively, the LTD-associated reduction of GluR1 phosphorylation may have been blocked because the inhibitor interfered with protein phosphatase-mediated dephosphorylation of protein kinases that regulate these GluR1 phosphorylation sites. For instance, the activity of PKC, one of the protein kinases that targets Ser831 on GluR1 (Roche et al., 1996), was shown to be regulated negatively by okadaic acid-sensitive protein phosphatases, including during LTD in area CA1 in vivo (Bornancin & Parker, 1996; Lee et al., 1996; Thiels et al., 2000). Others found that induction of LTD in area CA1 by bath-application of NMDA is associated with a reduction in the phosphorylation state of Thr840, another phospho-site in the C-terminus of GluR1, and that this dephosphorylation effect is blocked in the presence of a PP1 and PP2A inhibitor (Delgado et al., 2007). Ser880 in the C-terminus of GluR2, a site implicated in AMPA receptor trafficking, also has received attention in the context of hippocampal LTD (Kim et al 2001; Seidenman et al., 2003). Although the relation between phosphorylation of this site and GluR2 synaptic surface expression remains controversial, it is noteworthy that phosphorylation of this site in hippocampal neurons was shown to be regulated negatively by PP1 (States et al., 2008). The idea that protein phosphatases contribute to the early phase of LTD by acting directly on mechanisms at or near the synapse also is consistent with our observation that fostriecin interferes with LTD at a time point when the phosphorylation status of CREB is not yet subject to regulation by PP2A.
PP1 and PP2A may contribute to the prolonged maintenance of LTD through regulation of transcriptional and/or translational events. Both PP1 and PP2A were found to target and dephosphorylate CREB and thereby reduce CREB-mediated gene expression (Wadzinski et al., 1993; Alberts et al., 1994; Chang & Berg, 2001; Genoux et al., 2002). Our findings demonstrate that the decrease in nuclear CREB phosphorylation during LTD is abolished by inhibition of either PP1 or PP2A, consistent with findings that each protein phosphatase is capable of regulating CREB. Our data do not allow us to distinguish between direct targeting of nuclear CREB by the enzymes, or indirect regulation of the transcription factor through targeting of a CREB kinase by one or both of the protein phosphatases. For instance, PP2A was shown to regulate CREB indirectly through targeting of calcium/calmodulin-dependent protein kinase IV (CaMKIV) (Westphal et al., 1998; Anderson et al., 2004), a protein kinase responsive to variations in synaptic input patterns and catalytically active against CREB (Bito et al., 1996; Ho et al., 2000; Wu et al., 2001). Thus, it is possible that increased PP2A activity evoked by PPS leads to dephosphorylation of CaMKIV, which, in turn, leads to a loss in phosphorylation of CREB. Another signaling molecule implicated in the regulation of CREB in response to synaptic input and subject to negative regulation by PP2A is ERK (Alessi et al., 1995; Impey et al., 1996; Davis et al., 1998; Silverstein et al., 2002; Ho et al., 2007). ERK does not phosphorylate CREB directly but acts on a member of the p90 ribosomal S6 kinases, which, in turn, phosphorylates the Ser133 residue of CREB. We consider it unlikely that the LTD-associated reduction in CREB phosphorylation we observed here is secondary to a protein phosphatase-mediated down-regulation of ERK because we previously found that the induction of LTD by PPS is associated with a pronounced increase in ERK phosphorylation and activity (Thiels et al., 2002a). Furthermore, we observed no difference in ERK phosphorylation after PPS between samples from okadaic acid- or fostriecin-treated animals and vehicle-treated animals in a few cases in which we probed samples for pERK as well as pCREB (Mauna et al., unpublished observations). Our findings that inhibition of PP1 abolishes the decrease in CREB phosphorylation immediately after LTD induction and inhibition of PP2A does not interfere with CREB dephosphorylation until a later time suggest the intriguing possibility that PP1 targets CREB directly, whereas PP2A impacts CREB through an indirect mechanism that is associated with several temporal steps. One might argue that the delay in effect of the PP2A inhibitor on CREB merely reflects slower accumulation of an effective concentration of fostriecin compared to Nipp-1 in the nuclear compartment. We consider this explanation unlikely because (1) both drugs were infused near the recording site for about 1.5 to 2 hr before the onset of PPS, and (2) the amount and duration of fostriecin infusion did not differ significantly between a time point after PPS when the inhibitor did block (40 min post PPS) versus when the inhibitor had no effect on the LTD-associated CREB dephosphorylation (20 min post PPS). Nevertheless, we cannot rule out a contribution by differential drug diffusion characteristics to the apparent difference in the time course of nuclear CREB regulation between PP1 and PP2A. Future studies that include assessment of the effect of PPS on PP1 and PP2A activity in the nuclear compartment, as well as assessment of the effect of PPS on the phosphorylation state of CaMKIV will help shed light on the signaling mechanisms through which PP1 and PP2A may regulate CREB during PPS-induced LTD.
Whether the LTD-associated reduction in nuclear CREB phosphorylation has transcriptional consequences that contribute to prolonged depression of synaptic transmission remains to be determined. PPS-induced LTD in area CA1 was shown to last for days in freely moving animals (Doyère et al., 1996), which suggests that PPS-LTD involves molecular events that bring about more permanent changes in function. Findings that blockade of okadaic acid- or microcystin-sensitive protein phosphatases results in increased CREB/CRE-dependent gene expression (Wadzinski et al., 1993; Alberts et al., 1994; Wheat et al., 1994; Cannettieri et al 2003; Choe et al., 2004; Gao et al., 2009) ascribe an active regulatory role in CREB transcriptional activity to these protein phosphatases. The idea that regulation of CREB by protein phosphatases can have plasticity-relevant consequences is supported by observations that genetic inhibition of PP1 is associated with enhanced learning-induced CREB-mediated gene expression as well as memory function (Genoux et al., 2002). Of potential relevance to this idea are also our recent findings that the expression of the immediate early gene Arc/Arg3.1, whose promoter region includes binding sites for CREB (Kawashima et al., 2009), is reduced after the induction of PPS-LTD (Yilmaz-Rastoder et al., submitted). It therefore is tempting to speculate that the PP1- and PP2A-mediated modification of CREB described here plays an active role in the prolonged maintenance of PPS-induced LTD.
Fostriecin generally is used as a selective inhibitor of PP2A. Whereas its inhibitory potency against PP1 is low, fostriecin inhibits PP2A and protein phosphatase 4 (PP4), which shares considerable sequence homology with PP2A, with similar potency (Hastie & Cohen, 1998; Lewy et al., 2002). The expression of PP4 is widespread and includes hippocampal pyramidal cells (Kloecker et al., 1997), which thus raises the possibility that our drug manipulations affected PP4 as well as PP2A. Although little is known about the cellular function of PP4, especially as it relates to glutamatergic synaptic transmission and plasticity, we cannot rule out the possibility that the effects of fostriecin described here resulted from inhibition of not only PP2A but also other members of the PP2A-subfamily, such as PP4.
In summary, our findings demonstrate that inhibition of either PP1 or PP2A abrogates PPS-induced LTD in area CA1 of the adult hippocampus, which suggests that both protein phosphatases play a critical role in LTD. Furthermore, our findings show that inhibition of either PP1 or PP2A prevents the dephosphorylation of CREB normally observed during LTD. Inhibition of PP1 resulted in blockade of CREB dephosphorylation beginning immediately after LTD induction, whereas inhibition of PP2A did not interfere with CREB dephosphorylation until the early phase of LTD had been established. These findings implicate both protein phosphatases as critical CREB regulators during PPS-induced LTD in area CA1. The differential time course of effect on CREB between the PP1 and the PP2A inhibitors suggests that PP1 and PP2A may regulate CREB through different mechanisms, such as direct targeting of CREB in the case of PP1 and an indirect signaling mechanism that may also involve a CREB kinase in the case of PP2A. It remains to be determined in future studies whether the regulation of CREB by PP1 and PP2A affects the transcriptional activity of CREB after PPS, and if so, whether alteration in CREB-mediated transcription is a requirement for the prolonged maintenance of PPS-induced LTD.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke Grant RO1-046423 to E.T. The authors thank Dr. D.B. DeFranco for helpful comments on the manuscript.
References
- Alberts AS, Montminy M, Shenolikar S, Feramisco JR. Expression of a peptide inhibitor of protein phosphatase 1 increases phosphorylation and activity of CREB in NIH 3T3 fibroblasts. Molec Cell Biol. 1994;14:4398–4407. doi: 10.1128/mcb.14.7.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Gomez N, Moorhead G, Lewis T, Keyse SM, Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatse, but not CL100, in various cell lines. Curr Biol. 1995;5:283–295. doi: 10.1016/s0960-9822(95)00059-5. [DOI] [PubMed] [Google Scholar]
- Belmeguenai A, Hansel C. A role for protein phosphatases 1, 2A, and 2B in cerebellar long-term potentiation. J Neurosci. 2005;25:10768–10772. doi: 10.1523/JNEUROSCI.2876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: A Ca2+ - and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Iyengar R, Landau EM. Postsynaptic signaling networks: cellular cogwheels underlying long-term plasticity. Biol Psychiatry. 2005;57:113–119. doi: 10.1016/j.biopsych.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Bornancin F, Parker PJ. Phosphorylation of threonine 638 critically controls the dephosphorylation and inactivation of protein kinase Cα. Curr Biol. 1996;6:1114–1123. doi: 10.1016/s0960-9822(02)70678-7. [DOI] [PubMed] [Google Scholar]
- Braunewell KH, Manahan-Vaughan D. Long-term depression: a cellular basis for learning? Rev Neurosci. 2001;12:121–140. doi: 10.1515/revneuro.2001.12.2.121. [DOI] [PubMed] [Google Scholar]
- Canettieri G, Morantte I, Guzman E, Asahara H, Herzig S, Anderson SD, Yates JR, III, Montminy M. Attenuation of a phosphorylation-dependent activator by an HDAC-PP1 complex. Nat Struct Biol. 2003;10:175–181. doi: 10.1038/nsb895. [DOI] [PubMed] [Google Scholar]
- Chang KT, Berg DK. Voltage-gated channels block nicotinic regulation of CREB phosphorylation and gene expression in neurons. Neuron. 2001;32:855–865. doi: 10.1016/s0896-6273(01)00516-5. [DOI] [PubMed] [Google Scholar]
- Choe ES, Parelkar NK, Kim JY, Cho HW, Kang HS, Mao L, Wang JQ. The protein phosphatase 1/2A inhibitor okadaic acid increases CREB and Elk-1 phosphorylation and c-fos expression in the rat striatum in vivo. J Neurochem. 2004;89:383–390. doi: 10.1111/j.1471-4159.2003.02334.x. [DOI] [PubMed] [Google Scholar]
- Colbran RJ. Protein phosphatases and calcium/calmodulin-dependent protein kinase II-dependent synaptic plasticity. J Neurosci. 2004;24:8404–8409. doi: 10.1523/JNEUROSCI.3602-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pagès C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: Postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Delgado JY, Coba M, Anderson CN, Thompson KR, Gray EE, Heusner CL, Martin KC, Grant SG, O'Dell TJ. NMDA receptor activation dephosphorylates AMPA receptor glutamate receptor 1 subunits at threonine 840. J Neurosci. 2007;27:13210–13221. doi: 10.1523/JNEUROSCI.3056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur J Cell Biol. 2006;85:627–633. doi: 10.1016/j.ejcb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Doyère V, Errington ML, Laroche S, Bliss TVP. Low-frequency trains of paired stimuli induce long-term depression in area CA1 but not in the dentate gyrus of the intact rat. Hippocampus. 1996;6:52–57. doi: 10.1002/(SICI)1098-1063(1996)6:1<52::AID-HIPO9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Gao J, Siddoway B, Huang Q, Xia H. Inactivation of CREB mediated gene transcription by HDAC8 bound protein phosphatase. Biochem Biophys Res Commun. 2009;379:1–5. doi: 10.1016/j.bbrc.2008.11.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm DR, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Hagiwara M, Alberts A, Brindle P, Meinkoth J, Feramisco J, Deng T, Karin M, Shenolikar S, Montminy MR. Transcriptional attenuation following cAMP induction requires PP-1- mediated dephosphorylation of CREB. Cell. 1992;70:105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Hastie CJ, Cohen PT. Purification of protein phosphatase 4 catalytic subunit: inhibition by the antitumour drug fostriecin and other tumour suppressors and promoters. FEBS Lett. 1998;431:357–361. doi: 10.1016/s0014-5793(98)00775-3. [DOI] [PubMed] [Google Scholar]
- Ho N, Liauw JA, Blaeser F, Wei F, Hanissian S, Muglia LM, Wozniak DF, Nardi A, Arvin KL, Holtzman DM, Linden DJ, Zhuo M, Muglia LJ, Chatila TA. Impaired synaptic plasticity and cAMP response element-binding protein activation in Ca2+/calmodulin-dependent protein kinase type IV/Gr-deficient mice. J Neurosci. 2000;20:6459–6472. doi: 10.1523/JNEUROSCI.20-17-06459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y, Logue E, Callaway CW, DeFranco DB. Different mechanisms account for extracellular-signal regulated kinase activation in distinct brain regions following global ischemia and reperfusion. Neuroscience. 2007;145:248–255. doi: 10.1016/j.neuroscience.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CFB, Boland MP. Inhibitors of protein phosphatase-1 and -2A; two of the major serine/threonine protein phosphatases involved in cellular regulation. Curr Op Struct Biol. 1993;3:934–943. [Google Scholar]
- Hu XD, Huang Q, Yang X, Xia H. Differential regulation of AMPA receptor trafficking by neurabin-targeted synaptic protein phosphatase-1 in synaptic transmission and long-term depression in hippocampus. J Neurosci. 2007;27:4674–4686. doi: 10.1523/JNEUROSCI.5365-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Isaac J. Protein phosphatase 1 and LTD: synapses are the architects of depression. neur. 2001:963–966. doi: 10.1016/s0896-6273(01)00545-1. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jouvenceau A, Billard J-M, Haditsch U, Mansuy IM, Dutar P. Different phosphatase-dependent mechanisms mediate long-term depression and depotentiation of long-term potentiation in mouse hippocampal CA1 area. Eur J Neurosci. 2003;18:1279–1285. doi: 10.1046/j.1460-9568.2003.02831.x. [DOI] [PubMed] [Google Scholar]
- Jouvenceau A, Hedou G, Potier B, Kollen M, Dutar P, Mansuy IM. Partial inhibition of PP1 alters bidirectional synaptic plasticity in the hippocampus. Eur J Neurosci. 2006;24:564–572. doi: 10.1111/j.1460-9568.2006.04938.x. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Okuno H, Nonaka M, chi-Morishima A, Kyo N, Okamura M, Takemoto-Kimura S, Worley PF, Bito H. Synaptic activity-responsive element in the Arc/Arg3.1 promoter essential for synapse-to-nucleus signaling in activated neurons. Proc Natl Acad Sci U S A. 2009;106:316–321. doi: 10.1073/pnas.0806518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Chung HJ, Lee HK, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci U S A. 2001;98:11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Homosynaptic long-term depression in the visual cortex. J Neurosci. 1994;14:3404–3412. doi: 10.1523/JNEUROSCI.14-05-03404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloeker S, Bryant JC, Strack S, Colbran RJ, Wadzinski BE. Carboxymethylation of nuclear protein serine/threonine phosphatase X. Biochem J. 1997;327(Pt 2):481–486. doi: 10.1042/bj3270481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Glasgow SD, Caruana DA, Chapman CA. Postsynaptic signals mediating induction of long-term synaptic depression in the entorhinal cortex. Neural Plast. 2008:840374. doi: 10.1155/2008/840374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurberg S. Commissural and intrinsic connections of the rat hippocampus. J Comp Neurol. 1979;184:685–708. doi: 10.1002/cne.901840405. [DOI] [PubMed] [Google Scholar]
- Lee HK, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21:1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Ther. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Hannun YA, Obeid LM. Ceramide inactivates cellular protein kinase Cα. J Biol Chem. 1996;271:13169–13174. doi: 10.1074/jbc.271.22.13169. [DOI] [PubMed] [Google Scholar]
- Lewy DS, Gauss CM, Soenen DR, Boger DL. Fostriecin: chemistry and biology. Curr Med Chem. 2000;9:2005–2032. doi: 10.2174/0929867023368809. [DOI] [PubMed] [Google Scholar]
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990;264:187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- MacKintosh C, Klumpp S. Tautomycin from the bacterium Streptomyces verticillatus. Another potent and specific inhibitor of protein phosphatases 1 and 2A. FEBS Lett. 1990;277:137–140. doi: 10.1016/0014-5793(90)80828-7. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mansuy IM, Shenolikar S. Protein serine/threonine phosphatases in neuronal plasticity and disorders of learning and memory. Trends Neurosci. 2006;29:679–686. doi: 10.1016/j.tins.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Morishita W, Connor JH, Xia H, Quinlan EM, Shenolikar S, Malenka RC. Regulation of synaptic strength by protein phosphatase 1. Neuron. 2001;32:1133–1148. doi: 10.1016/s0896-6273(01)00554-2. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Herron CE, Malenka RC. Essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Nicholls RE, Alarcon JM, Malleret G, Carroll RC, Grody M, Vronskaya S, Kandel ER. Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron. 2008;58:104–117. doi: 10.1016/j.neuron.2008.01.039. [DOI] [PubMed] [Google Scholar]
- Peters M, Bletsch M, Catapano R, Zhang X, Tully T, Bourtchouladze R. RNA interference in hippocampus demonstrates opposing roles for CREB and PP1alpha in contextual and temporal long-term memory. Genes Brain Behav. 2009;8:320–329. doi: 10.1111/j.1601-183X.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- Roberson ED, English JD, Sweat JD. A biochemist's view of long-term potentiation. Learn Memory. 1996;3:1–24. doi: 10.1101/lm.3.1.1. [DOI] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Sala C, Rudolph-Curreia S, Sheng M. Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons. J Neurosci. 2000;15:3529–3536. doi: 10.1523/JNEUROSCI.20-10-03529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenman KJ, Steinberg JP, Huganir R, Malinow R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci. 2003;23:9220–9228. doi: 10.1523/JNEUROSCI.23-27-09220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein AM, Barrow CA, Davis AJ, Mumby MC. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc Natl Acad Sci U S A. 2002;99:4221–4226. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States BA, Khatri L, Ziff EB. Stable synaptic retention of serine-880-phosphorylated GluR2 in hippocampal neurons. Mol Cell Neurosci. 2008;38:189–202. doi: 10.1016/j.mcn.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD, Atkins CM, Johnson J, English JD, Roberson ED, Chen S-J, Newton AC, Klann E. Protected-site phosphorylation of protein kinase C in hippocampal long-term potentiation. J Neurochem. 1998;71:1075–1085. doi: 10.1046/j.1471-4159.1998.71031075.x. [DOI] [PubMed] [Google Scholar]
- Thiels E, Barrionuevo G, Berger TW. Excitatory stimulation during postsynaptic inhibition induces long-term depression in hippocampus in vivo. J Neurophysiol. 1994;72:3009–3016. doi: 10.1152/jn.1994.72.6.3009. [DOI] [PubMed] [Google Scholar]
- Thiels E, Norman ED, Barrionuevo G, Klann E. Transient and persistent increases in protein phosphatase activity during long-term depression in the adult hippocampus in vivo. Neuroscience. 1998;86:1023–1029. doi: 10.1016/s0306-4522(98)00135-3. [DOI] [PubMed] [Google Scholar]
- Thiels E, Kanterewicz BI, Knapp LT, Barrionuevo G, Klann E. Protein phosphatase-mediated regulation of protein kinase C during long-term depression in the adult hippocampus in vivo. J Neurosci. 2000;20:7199–7207. doi: 10.1523/JNEUROSCI.20-19-07199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E, Kanterewicz BI, Norman ED, Trzaskos JM, Klann E. Long-term depression in the adult hippocampus in vivo involves activation of extracellular signal-regulated kinase and phosphorylation of Elk-1. J Neurosci. 2002;22:2054–2062. doi: 10.1523/JNEUROSCI.22-06-02054.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Wadzinski BE, Wheat WH, Jaspers S, Peruski LFJ, Lickteig RL, Johnson GL, Klemm DJ. Nuclear protein phosphatase 2A dephosphorylates protein kinase A-phosphorylated CREB and regulates CREB transcriptional stimulation. Molec Cell Biol. 1993;13:2822–2834. doi: 10.1128/mcb.13.5.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-Y, Orser BA, Brautigan DL, MacDonald JF. Regulation of NMDA receptors in cultured hippocampal neurons by protein phosphatases 1 and 2A. Nature. 1994;369:230–232. doi: 10.1038/369230a0. [DOI] [PubMed] [Google Scholar]
- Wheat WH, Roesler WJ, Klemm DJ. Simian virus 40 small tumor antigen inhibits dephosphorylation of protein kinase A-phosphorylated CREB and regulates CREB transcriptional stimulation. Mol Cell Biol. 1994;14:5881–5890. doi: 10.1128/mcb.14.9.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder DG, Sweatt JD. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat Rev. 2001;2:1–14. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA. 2001;98:2808–2813. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M-Y, Karpefors M, Gustafsson B, Wigström H. On the linkage between AMPA and NMDA receptor-mediated EPSPs in homosynaptic long-term depression in the hippocampal CA1 region of young rats. J Neurosci. 1995;15:4496–4506. doi: 10.1523/JNEUROSCI.15-06-04496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]