Abstract
Michael-type conjugate additions of γ-chiral aldehyde-derived acyclic nitrosoalkenes have been explored using a series of carbon and hetero nucleophiles. In all cases examined, these reactions are stereoselective, leading exclusively to the anti products.
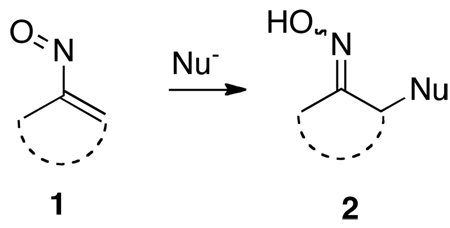
Vinylnitroso compounds 1 are highly reactive, short-lived species which have found only sporadic use in organic synthesis.1 To date, very few of these intermediates have actually been isolated and characterized, and they are usually generated and trapped in situ. One potentially valuable reaction of nitrosoalkenes involves intermolecular conjugate additions of hetero and carbon nucleophiles in a Michael-type of transformation to produce adducts such as 2 (eq 1). By this process nitrosoalkenes 1 can act as enolonium ion equivalents, thereby allowing a simple method for the umpolung of the usual enolate reactivity.2 However, these vinylnitroso species have been the object of surprisingly little systematic study and have not seen application to the synthesis of complex molecules. We have recently been exploring the scope of both inter-3 and intramolecular4 conjugate additions of these reactive intermediates with a variety of nucleophiles. Recently, we have also begun to investigate various stereochemical issues relevant to these reactions which have never been addressed, and some of this work is the subject of this communication.
 |
(1) |
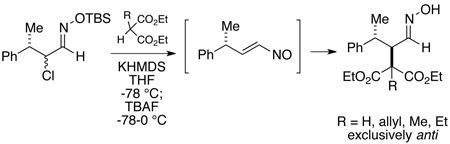
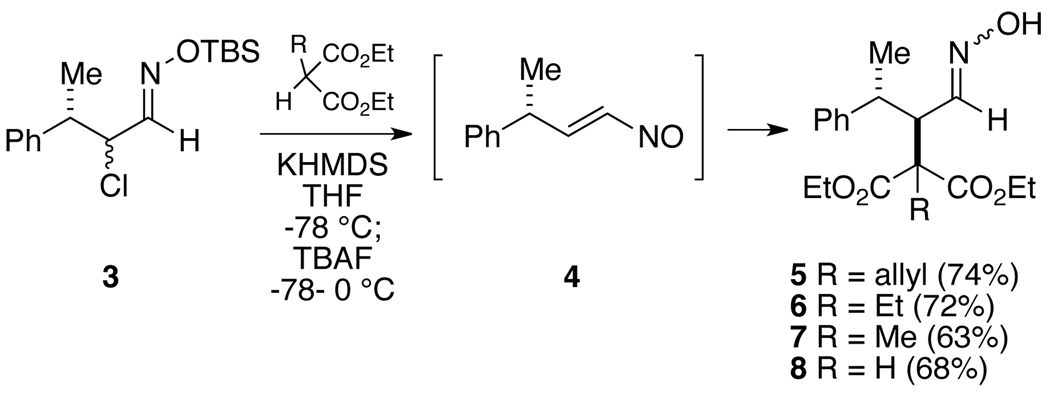
Our initial studies were designed to probe the stereoselectivity of conjugate additions of acyclic aldehyde-derived nitrosoalkenes bearing a γ-stereogenic center (Cf 4, Scheme 1). It should also be noted that relatively few examples exist in the literature of reactions of nitrosoalkenes generated from aldehydes.3,5 For this work, we have opted to generate the requisite nitrosoalkene by the Denmark method, which involves exposure of an O-TBS-α-chlorooxime to a fluoride source.6 Therefore, known α-chloro-γ-methyldihydrocinnamaldehyde7 (racemic, mixture of diastereomers) was converted to O-TBS-oxime 3 with commercially available O-TBS-hydroxylamine (see Supporting Information). Conjugate additions to the vinylnitroso compound derived from 3 were then effected with a series of four malonate anions as the nucleophiles (Scheme 1). Thus, the malonate (2 equiv) was first deprotonated with potassium hexamethyldisilazide in THF at low temperature followed by addition of O-silyloxime 3 (1 equiv). Tetrabutylammonium fluoride (2 equiv) in THF was then added at −78 °C and the mixture was warmed to 0 °C to generate the nitrosoalkene 4. We were pleased to find that in each case the malonate enolate addition was completely stereoselective, producing only the anti stereoisomeric adducts 5–8. Oximes 7 and 8 appear to be single geometric isomers assumed to be (E), whereas 5 and 6 are ~9–10:1 (E/Z) mixtures.8,9
Scheme 1.
Conjugate Additions to a γ-Chiral Aldehyde-Derived Nitrosoalkene
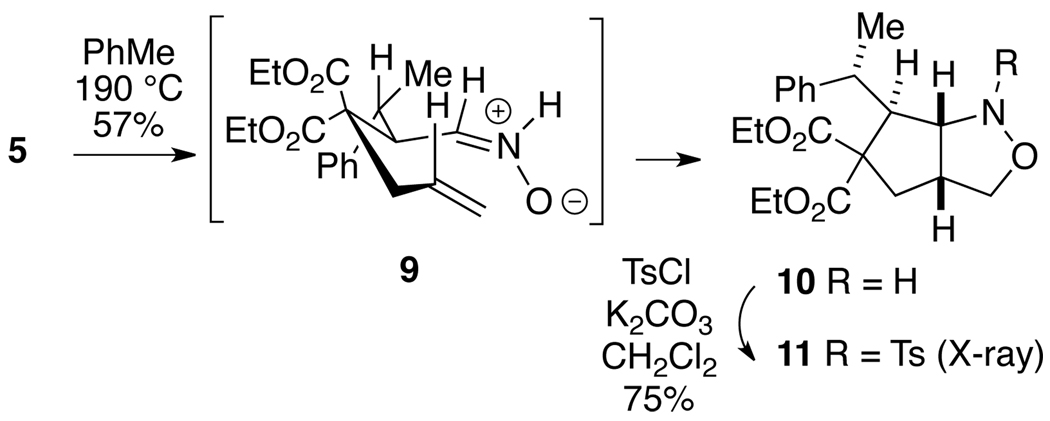
In order to establish the configuration of these adducts, α-allylmalonate oxime 5 was heated in toluene at 190 °C (sealed tube) to generate nitrone 9, which then cyclizes via the conformation shown to afford cis-fused bicyclic isoxazolidine 10 (Scheme 2).5c,e,g Treatment of 10 with tosyl chloride gave sulfonamide 11, whose structure was determined by X-ray crystallography, thereby proving the anti configuration of 5. It seems quite reasonable to assume that the other adducts 6–8 also have this same anti arrangement.
Scheme 2.
Intramolecular Nitrone/Olefin Cycloaddition of Allyl Oxime 5 to Isoxazolidine 10
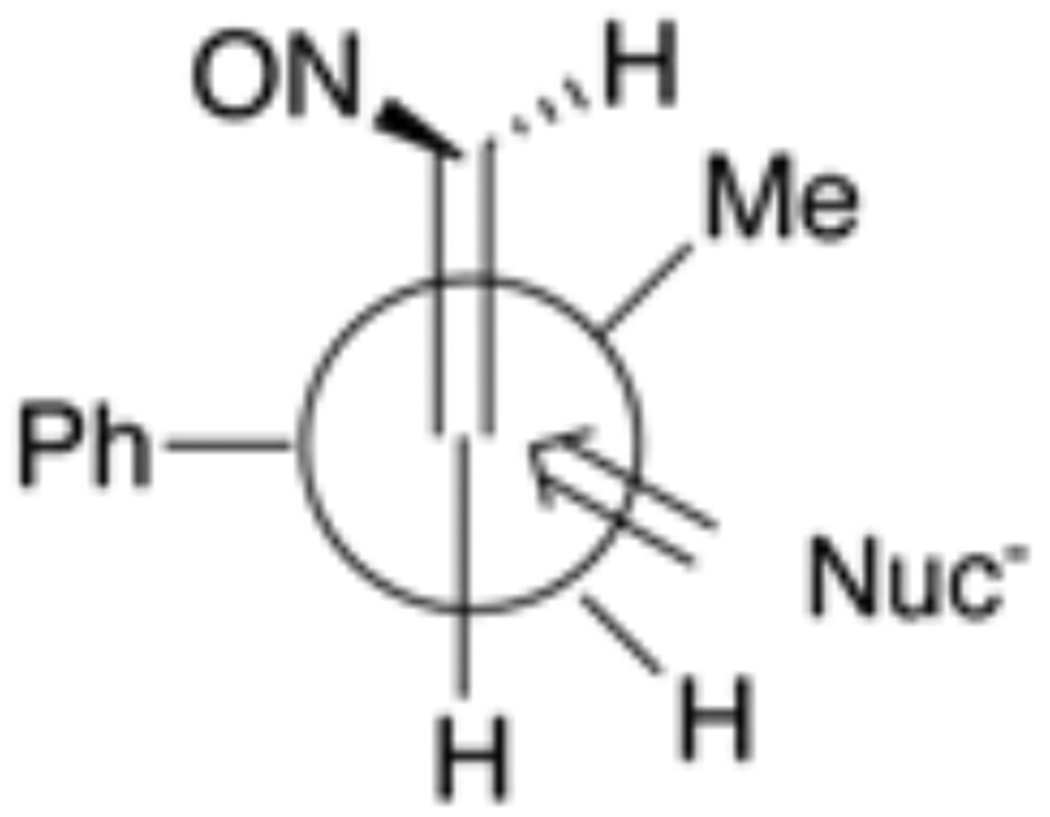
Since simple nitrosoalkenes such as 4 have never been isolated, the double bond configuration of these species has not been established, but we speculate that these reactions might well occur via the (E)-geometric isomer. If so, the outcome of these nitrosoalkene additions can be nicely rationalized based upon a Felkin-Ahn-type transition state (Figure 1). Thus, one would anticipate that the γ-phenyl group would be perpendicular to the olefinic double bond of the nitrosoalkene and the methyl substitutent would be “inside.” Burgi-Dunitz attack on this conformation as shown in the figure leads to the observed anti products 5–8.
Figure 1.
Felkin-Ahn-type Attack of Nucleophiles on Nitrosoalkene 4
A good analogy for this process would be the conjugate additions of organometallics and other nucleophiles to acyclic γ-chiral α,β-unsaturated esters, which have been extensively studied both experimentally10 and theoretically.11 For example, with (E)-α,β-unsaturated esters having γ-substituents as in 4, the reaction usually proceeds via a transition state similar to that in Figure 1 and therefore the major addition product is anti (with the corresponding (Z)-isomer, syn is usually preferred). However, in additions to related γ-alkoxy systems, both syn and anti products have been observed, depending upon the nucleophile and substrate. The formation of the syn products has been rationalized using a modified Felkin-Ahn model with the alkoxy group perpendicular to the π-bond, or one involving metal chelation to the oxygen. We therefore decided to explore a conjugate addition of a vinylnitroso compound related to 4 bearing a γ-alkoxy group in place of methyl to see if this substitution leads to formation of any of the syn product.
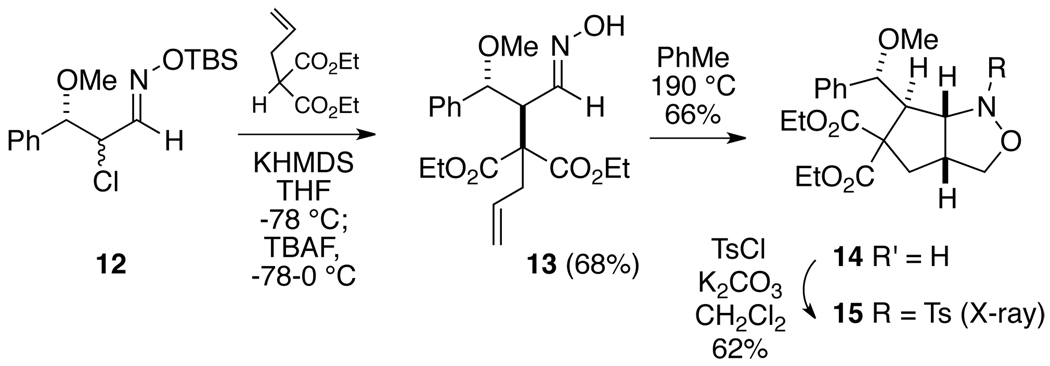
For this work, substrate 12 was prepared,12 and again using the Denmark protocol the potassium enolate of diethyl α-allylmalonate was added to the derived nitrosoalkene to give only the anti product 13 in 68% isolated yield (Scheme 3). The structure of this adduct was established as before by thermolysis to the isoxazolidine 14, which was converted to the sulfonamide 15. X-ray analysis of this compound indicated its composition to be as shown, thus confirming the anti configuration of 13. In addition, to see if there are any chelation effects in the conjugate addition, the corresponding lithium salt of the allylmalonate was used, but the anti product 13 was again produced exclusively, albeit in somewhat lower yield (52%) than with the potassium salt.
Scheme 3.
Conjugate Additions to a γ-Methoxy Nitrosoalkene
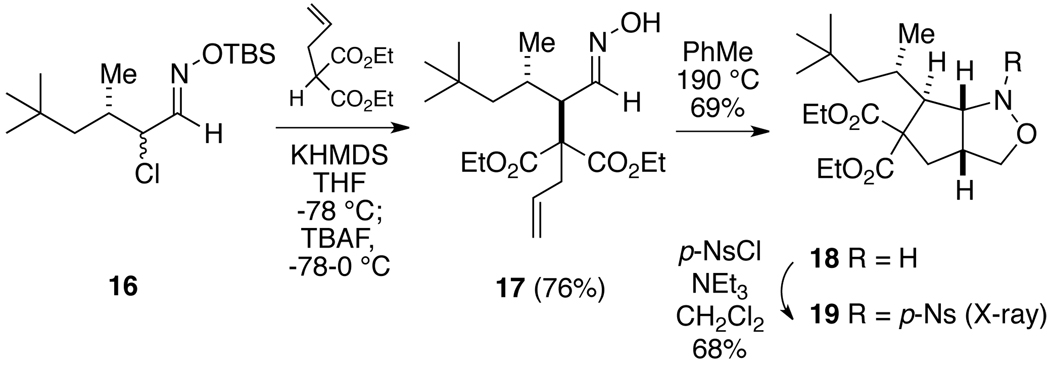
In order to probe whether replacing the γ-phenyl substituent in nitrosoalkene 4 with a bulky alkyl group has any affect on the stereochemistry of the addition, the neopentyl system 16 was prepared.13 Using the standard procedure, addition of potassium diethyl α-allylmalonate to the corresponding nitrosoalkene was totally stereoselective, giving the anti compound 17 as the only product (Scheme 4). Once again, the stereochemistry was established by thermal conversion to the isoxazolidine 18, followed by X-ray analysis of the derived p-nosyl compound 19.
Scheme 4.
Conjugate Additions to a γ-Neopentyl Nitrosoalkene
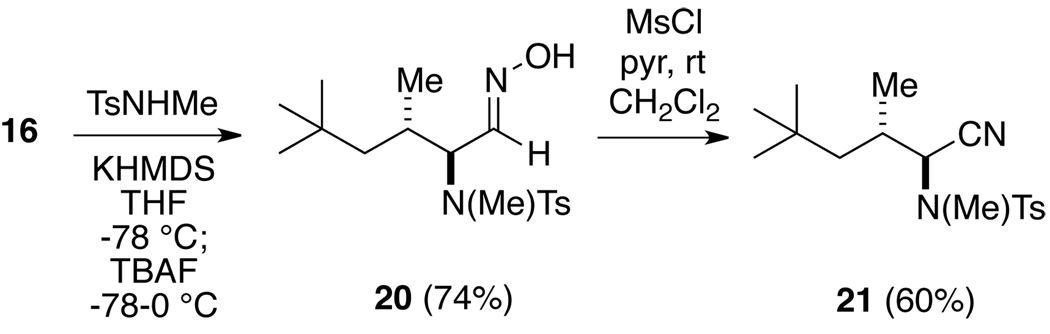
Finally, we have examined the stereochemistry of a heteronucleophile addition to one of these nitrosoalkenes. Therefore, the potassium salt of N-methyl-p-toluenesulfonamide was first combined with α-chloro-O-silyoxime 16, followed by addition of TBAF in THF, to produce a single stereoisomeric adduct 20 (Scheme 5). The oxime 20 was dehydrated with methanesulfonyl chloride/pyridine in methylene chloride to afford the corresponding nitrile 21. Based upon NMR proton coupling data for 21 in comparison with some closely related compounds,14,15 we have assigned the anti stereochemistry to the sulfonamide adduct as shown.
Scheme 5.
Conjugate Addition of a Sulfonamide Nucleophile to a Nitrosoalkene
In conclusion, the stereochemical outcome of conjugate additions to a series of in situ-generated γ-chiral aldehyde-derived nitrosoalkenes has been examined using a number of malonate enolates as nucleophiles. These reactions are totally stereoselective in all of the examples tested, leading exclusively to the anti products. Moreover, a similar reaction using a sulfonamide anion as a heteronucleophile also cleanly led to the anti adduct. We are currently exploring extensions of this methodology as well as applications to synthesis of complex molecules.
Supplementary Material
Acknowledgment
We are grateful to the National Institutes of Health (GM-087733) and the National Science Foundation (CHE-0806807) for financial support of this research. We also thank Dr. H. Yennawar (Penn State Small Molecule X-ray Cystallographic Facility) for the X-ray crystal structure determinations.
Footnotes
Supporting Information Available Experimental procedures for preparation of new compounds including spectral data. X-ray data for compounds 11, 15 and 19 are also provided. This material is available free of charge on the Internet at http://pubs.acs.org.
References
- 1.For reviews of vinylnitroso compounds and lead references see: Gilchrist TL. Chem. Soc. Rev. 1983;11:53. Lyapkalo IM, Ioffe SL. Russ. Chem. Rev. 1998;67:467. For related chemistry of vinyldiazo compounds see: Attanasi OA, De Crescentini L, Favi G, Filippone P, Matellini F, Perrulli FR, Santeusanio S. Eur. J. Org. Chem. 2009:3109.
- 2.For representative examples of enolonium ion equivalents see: Sacks CE, Fuchs PL. J. Am. Chem. Soc. 1975;97:7372. Fuchs PL. J. Org. Chem. 1976;41:2935. Stork G, Ponaras AA. J. Org. Chem. 1976;41:2937. Wender PA, Erhardt JM, Letendre LJ. J. Am. Chem. Soc. 1981;103:2114. Hatcher JM, Coltart DM. J. Am. Chem. Soc. 2010;132:4546. doi: 10.1021/ja100932q. Miyoshi T, Miyakawa T, Ueda M, Miyata O. Angew. Chem. Int. Ed. 2011;50:928. doi: 10.1002/anie.201004374.
- 3.Li P, Majireck MM, Witek JA, Weinreb SM. Tetrahedron Lett. 2010;51:2032. doi: 10.1016/j.tetlet.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Korboukh I, Kumar P, Weinreb SM. J. Am. Chem. Soc. 2007;129:10342. doi: 10.1021/ja074108r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kumar P, Li P, Korboukh I, Wang TL, Yennawar H, Weinreb SM. J. Org. Chem. doi: 10.1021/jo1024392. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.See inter alia: Hassner A, Murthy KSK. Tetrahedron Lett. 1987;28:683. Padwa A, Chiacchio U, Dean DC, Schoffsatll AM, Hassner A, Murthy KSK. Tetrahedron Lett. 1988;29:4169. Hassner A, Maurya R, Mesko E. Tetrahedron Lett. 1988;29:5313. Hassner A, Murthy KSK, Padwa A, Bullock WH, Stull PD. J. Org. Chem. 1988;53:5063. Hassner A, Maurya R. Tetrahedron Lett. 1989;30:5803. Hassner A, Murthy KSK, Padwa A, Chiacchio U, Dean DC, Schoffstall AM. J. Org. Chem. 1989;54:5277. Hassner A, Maurya R, Friedman O, Gottlieb HE, Padwa A, Austin D. J. Org. Chem. 1993;58:4539. Artman GD, III, Waldman JH, Weinreb SM. Synthesis. 2002:2057.
- 6.(a) Denmark SE, Dappen MS. J. Org. Chem. 1984;49:798. [Google Scholar]; (b) Denmark SE, Dappen MS, Sternberg JA. J. Org. Chem. 1984;49:4741. [Google Scholar]; (c) Denmark SE, Dappen MS, Sear NL, NJacobs RT. J. Am. Chem. Soc. 1990;112:3466. [Google Scholar]
- 7.Brochu MP, Brown SP, MacMillan DWC. J. Am. Chem. Soc. 2004;126:4108. doi: 10.1021/ja049562z. [DOI] [PubMed] [Google Scholar]
- 8.Dehydration of the mixture of oxime geometric isomers of 6 to the corresponding nitrile with MsCl/pyr/CH2Cl2 led to a single anti diastereomer in 73% yield.
- 9.In a control experiment, α-chloro-O-silyloxime 3 was found to be unreactive towards potassium diethyl malonate at room temperature in the absence of TBAF.
- 10.See for example: Yamamoto Y, Chounan Y, Nishii S, Ibuka T, Kitahara H. J. Am. Chem. Soc. 1992;114:7652. Costa JS, Dias AG, Anholeto AL, Monteiro MD, Patrocinio VL, Casta PRR. J. Org. Chem. 1997;62:4002. Raczko J. Tetrahedron: Asymmetry. 1997;8:3821. Yamamoto K, Ogura H, Jukuta J, Inoue H, Hamada K, Sugiyama Y, Yamada S. J. Org. Chem. 1998;63:4449. Chounan Y, Ono Y, Nishii S, Kitahara H, Ito S, Yamamoto Y. Tetrahedron. 2000;56:2821.
- 11.(a) Dorigo A, Morokuma K. J. Am. Chem. Soc. 1989;111:6524. [Google Scholar]; (b) Bernardi A, Capelli AM, Gennari C, Scolastico C. Tetrahedron: Asymmetry. 1990;10:21. [Google Scholar]
- 12.Synthesized from the corresponding known α-chloroaldehyde: Emling BL, Vogt RR, Hennion GF. J. Am. Chem. Soc. 1941;63:1624.
- 13.Prepared from the corresponding commercially available aldehyde (see Supporting Information).
- 14.Belsito EL, De Marco R, Di Gioia ML, Liguori A, Perri F, Viscomi MC. Eur. J. Org. Chem. 2010:4245. doi: 10.1021/jo901643f. [DOI] [PubMed] [Google Scholar]
- 15.For synthesis of other compounds closely related to nitrile 21 see: Tka N, Kraiem J, Kacem Y, Hajri A, Hassine BB. C. R. Chim. 2009;12:1066. Harms K, Marsch M, Oberthur M, Schuler P. Acta Cryst. 2009;E65:o2742. doi: 10.1107/S1600536809041245. Portonovo P, Liang B, Joullie MM. Tetrahedron: Asymmetry. 1999;10:1451.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.