Abstract
Childhood obesity is a risk factor for the development of both type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD). One marker that can be used to predict T2DM is the metabolic syndrome (MetS). MetS, a cluster of cardiovascular factors associated with insulin resistance, is defined by central obesity, impaired fasting glucose, hypertension, elevated triglycerides (TG), and low levels of high-density lipoprotein cholesterol. Some have advocated using a diagnosis of MetS to trigger increased intervention in children. However, ethnic differences in MetS may hamper identification of at-risk children. For example, non-Hispanic blacks are diagnosed with MetS less frequently than non-Hispanic whites, despite having higher rates of T2DM and CVD. These differences in MetS are predominantly due to a low frequency of hypertriglyceridemia in non-Hispanic blacks. Compared with non-Hispanic whites and Mexican Americans, non-Hispanic blacks have lower TG levels at baseline but exhibit worsening insulin resistance with increasing TG. Therefore “normal” TG levels appear to be falsely reassuring among insulin-resistant non-Hispanic blacks. Ethnic-specific tools may be needed to more accurately predict risk for T2DM and CVD in minorities.
Keywords: Metabolic syndrome, Insulin resistance, Ethnic differences, Inflammation, Cardiovascular disease, Diabetes
Introduction
Pediatric obesity increases the risk of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) and threatens longevity in affected children [1]. Successful interventions such as weight control and exercise programs have been performed in large randomized trials [2, 3•] but are expensive and time-intensive, underscoring the need to choose targets judiciously for maximal effect. As not all obese children carry the same risk for developing future disease, there is value in identifying who would benefit most from intense lifestyle intervention. One marker that has been advocated for identifying children at increased risk for future T2DM and CVD is the metabolic syndrome (MetS) [4]. MetS represents a clustering of several cardiovascular factors associated with insulin resistance, including visceral adiposity (as assessed by waist circumference [WC]), glucose dysregulation (as assessed by fasting glucose), hypertension, elevated triglycerides (TG), and low levels of high-density lipoprotein–cholesterol (HDL–C) [5]. Children with MetS are likely to become adults with MetS and are 12 times as likely to develop T2DM over a 30-year period [6••], underscoring the potential benefit of targeting these children for weight loss and exercise efforts. Significant weight loss has been proven to result in a resolution of MetS [7].
Nevertheless, there are important considerations related to ethnicity in using MetS to identify children at increased risk for future disease. Although MetS performs well in identifying future risk among non-Hispanic whites, it does not identify risk well in non-Hispanic blacks [8]. Non-Hispanic blacks have higher rates of both T2DM and CVD despite having lower rates of MetS [9••]. In this review, we discuss the underlying causes and the individual components of MetS, including ethnic variation in these issues. We identify potential explanations for differences in the predictive ability of MetS among non-Hispanic blacks as well as potential solutions to better identify non-Hispanic black adolescents at increased risk.
MetS: Underlying Pathophysiology
MetS was first described as a cluster of variables that correlate with insulin resistance more often than would be expected by chance [4]. Intense research since this initial description has revealed many of the underlying cellular and metabolic processes that result in MetS. In particular, visceral adipose tissue appears to have a central role in the process. In a large proportion of individuals, increases in visceral obesity result in dysfunction of hypertrophied adipocytes [10••], causing multiple downstream effects that influence findings related to MetS.
A key factor in the pathophysiology of visceral obesity is a decrease in the production of adiponectin, an adipokine associated with insulin sensitivity. In animal models, administration of adiponectin results in an increase in insulin sensitivity [11, 12] and suppression of inflammatory cytokine expression [13] and action [14]. In the setting of visceral obesity, however, adiponectin production is decreased, contributing to insulin resistance and other features of MetS [15–18].
As central adiposity develops, visceral adipocytes release more proinflammatory molecules such as tumor necrosis factor-α (TNF-α), promoting phosphorylation of the insulin receptor and worsening insulin resistance [19]. Visceral adipocytes also release chemoattractants such as monocyte chemoattractant protein-1 (MCP-1), which result in the recruitment of macrophages to adipose tissue, further worsening the inflammatory state [20, 21•, 22]. The inflammatory state associated with visceral obesity leads to increased hepatic production of C-reactive protein (CRP), which is also an independent risk factor for the development of T2DM and CVD [10••, 23, 24].
A multitude of other processes also contribute to insulin resistance and continue to be described, including dysfunction of the endoplasmic reticulum and mitochondria [10••, 25–27], production of reactive oxygen species [28, 29], and nitric oxide-related modification of enzymes [30–32]. The combined result of all of these processes is worsening resistance to insulin, related to alterations in function of the insulin receptor and its downstream signaling [33••], as well as increased hepatic glucose production [33••].
Components of MetS
In addition to producing insulin resistance, the underlying processes described here result in clinical signs such as elevated TG, low HDL–C, hypertension, and elevated fasting glucose, making these laboratory findings a convenient means to identify at risk individuals. TG levels are elevated in the setting of insulin resistance because of increased production and decreased removal of TG from the circulation. Production is increased by impairment of insulin-mediated suppression of lipolysis in visceral adipocytes, causing an increase in the release of free fatty acids (FFA) that are converted to TG in the liver [34, 35]. Moreover, insulin resistance is associated with decreased function of lipoprotein lipase, impairing removal of TG from the circulation [36]. HDL levels are low due to increased activity of hepatic lipase in the setting of insulin resistance and low levels of adiponectin [37]. Hypertension in MetS, particularly represented by increases in systolic blood pressure [38], is caused at least in part by hypertrophied visceral adipocytes producing higher levels of angiotensinogen (leading to increased activation of the rennin-angiotensin system [39]) and 11-β hydroxysteroid type 1 (leading to increased conversion of inactive cortisone to active cortisol) [40, 41•]. Fasting glucose levels are elevated due to the cumulative effects of elevated FFA and/or glucose on the β cell [42] and the liver [43, 44]. Insufficient release of insulin and excess hepatic glucose production ultimately lead to elevated fasting glucose.
The frequency of these clinical findings among individuals with insulin resistance and the association of these findings with future T2DM and CVD make the concept of MetS an intriguing tool for patient care. Therefore, several different sets of criteria to diagnose MetS in children and adolescents have been proposed, using cut-off values for each of the above components (along with WC as a surrogate for visceral obesity), an example of which is shown in Table 1 [45]. These sets of criteria essentially use elevations in the components of MetS to identify individuals likely to be experiencing the biological processes underlying insulin resistance.
Table 1.
One proposed set of criteria for the diagnosis of metabolic syndrome in children
| Components needed for MetS diagnosis | Obesity | Hypertension | Low HDL | Elevated TG | Elevated fasting glucose | |
|---|---|---|---|---|---|---|
| Ford et al. [45] | ≥3 | WC ≥90% for age, sex [85] | SBP ≥90% for age, sex, height | HDL <40 mg/dL | TG ≥110 mg/dL | ≥100 mg/dL (fasting) |
HDL high-density lipoprotein; MetS metabolic syndrome; SBP systolic blood pressure; TG triglyceride; WC waist circumference
Further implied by a diagnosis of MetS is that individuals exhibiting elevations in MetS components will have an increase in risk for CVD and T2DM. Altogether, a diagnosis of MetS in adults predicts future disease, with an odds ratio (OR) of 4 for developing T2DM over a 5-year to 10-year period [46, 47] and an OR of 1.6 for predicting CVD events [47] among MetS-positive adults compared with MetS-negative adults. Among children, prospective studies have confirmed that MetS-positive children are likely to become MetS-positive adults, with an OR of 9.4 compared with MetS-negative children [6••]. Even more importantly, prospective studies have shown that MetS- positive children have an OR of 12.1 for developing T2DM over a 24-year to 31-year period [6••]. Nevertheless, it is uncertain which is most responsible for the long-term risks associated with MetS: the underlying processes, the insulin resistance that they confer, or the combination of abnormalities in MetS components.
Ethnic Differences in Insulin Resistance, Adiponectin, and Inflammation
MetS is diagnosed among non-Hispanic blacks at rates that are 43–55% lower among men compared with Mexican Americans and non-Hispanic whites, and 9–28% lower among non-Hispanic black women compared with Mexican Americans and non-Hispanic whites [6••, 48–50•]. This is paradoxical, since it has been long noted that non-Hispanic black adolescents [51–53] and adults [54–56•] have a degree of insulin resistance that is increased by 29–42% at any given level of adiposity or pubertal state [53]. Consistent with the increase in insulin resistance, non-Hispanic blacks have lower levels of adiponectin despite having less visceral adipose tissue [57, 58]. Ethnic differences in insulin resistance disappear after adjusting for levels of adiponectin, suggesting an etiologic role [58]. Compared with non-Hispanic whites, non-Hispanic blacks also have equal [59, 60] or even higher [61, 62] concentrations of CRP. Thus some findings related to the underlying processes in MetS are present and in some cases more common in non-Hispanic blacks (Fig. 1). More importantly, there is a twofold higher prevalence of T2DM among non-Hispanic black adolescents [63] and adults [64] compared with non-Hispanic whites. Non-Hispanic blacks are also 24% more likely to die from CVD than non-Hispanic whites [65]. Altogether, these data suggest that among non-Hispanic blacks, the presence of a more severe insulin resistance, lower levels of adiponectin, and increased inflammation (all processes underlying MetS) have clinical significance.
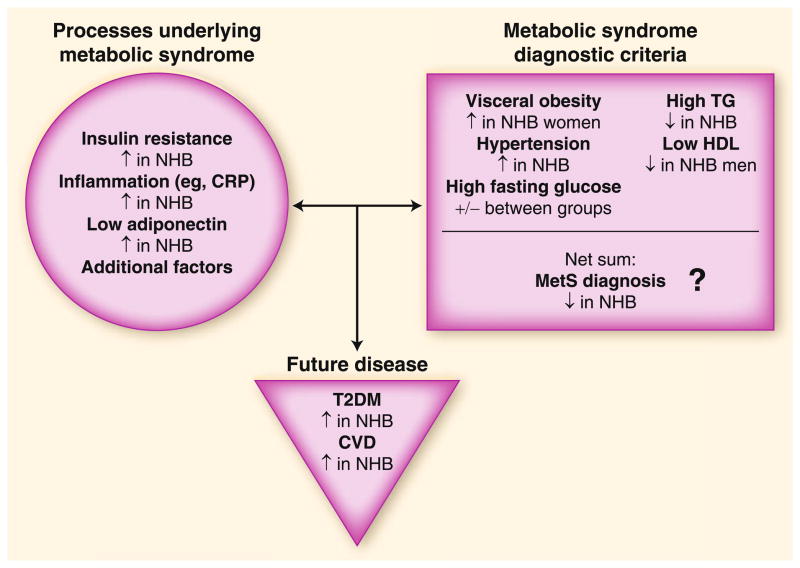
Fig. 1.
Ethnic differences in metabolic syndrome (MetS). Non-Hispanic black (NHB) individuals are more likely to exhibit many of the processes associated with MetS and are more likely to develop adverse health outcomes but are less likely to be diagnosed with MetS. The difference in MetS diagnosis largely rests on a lower prevalence of lipid abnormalities, particularly with respect to triglyceride (TG) levels, in NHB individuals. CRP—C-reactive protein; CVD—cardiovascular disease; HDL—high-density lipoprotein; T2DM—type 2 diabetes mellitus
Yet, it remains unclear whether non-Hispanic blacks are truly less likely to have MetS (i.e., the biological processes linking visceral adiposity, inflammation, insulin resistance) or are simply less likely to be diagnosed with MetS using current criteria. To examine these issues, we consider similarities and differences in individual components of MetS between ethnic groups.
Mets Components: Similarities and Differences Between Ethnic Groups
As mentioned previously, MetS is diagnosed based on the presence of the specific components shown in Table 1. Non-Hispanic black children and adults have several similarities, and a few important differences, in these components compared with non-Hispanic whites.
Obesity
Obesity (body mass index [BMI] >95th percentile for age) is more common among non-Hispanic black girls and adolescents than non-Hispanic white girls starting at a young age [66, 67]. In longitudinal studies, the difference in BMI widens over time [68, 69]; furthermore, non-Hispanic black women are nearly 50% more likely to be obese compared with non-Hispanic white women [70, 71]. Non-Hispanic black adolescent boys are more likely than non-Hispanic white adolescent boys to have a BMI greater than the 97th percentile, with an overall trend toward increased obesity in both children and adults [66, 70, 71].
Elevated Waist Circumference
Elevated WC is the most commonly used measure of central obesity. Non-Hispanic black women are more likely to have elevated WC [72], whereas non-Hispanic black adolescent girls, boys, and men are either equally or less likely to have high WC than non-Hispanic whites [50•, 73].
Hypertension
Hypertension is more common in non-Hispanic black women than non-Hispanic white women across the age spectrum in adolescence and adults [8]. Although similar trends toward higher rates of hypertension exist among non-Hispanic black men, these differences only reach statistical significance at higher age ranges [8, 74].
Fasting Glucose
Fasting glucose measurements are similar between non-Hispanic blacks and non-Hispanic whites, with a trend toward being less common among non-Hispanic black adolescents and men without diabetes [50•]. This occurs despite at the higher prevalence of T2DM among non-Hispanic blacks than non-Hispanic whites.
Low HDL–C
Low HDL–C levels are less common among non-Hispanic black adolescent boys and men [8]. This lower prevalence is less consistent among non-Hispanic black women [8].
Elevated TG
Elevated TG levels are a key feature of MetS that separates ethnic groups. The prevalence of elevated TG levels in non-Hispanic black adolescents is one third that observed in non-Hispanic whites [50•], and although this gap declines with age, non-Hispanic black adults continue to have a 30–45% lower prevalence of hypertriglyceridemia than non-Hispanic whites.
Other Similarities and Differences
In contrast, Mexican American adolescents are diagnosed with MetS at a rate similar to non-Hispanic whites and higher than non-Hispanic blacks [50•]. Mexican Americans have a tendency for an increased WC compared with non-Hispanic whites and blacks, and Mexican American adolescents are less likely to exhibit hypertension [50•]. Although Hispanics have a higher lifetime prevalence of diabetes than seen in non-Hispanic whites, there is still a higher prevalence of diabetes among non-Hispanic blacks [75].
In summary, even though non-Hispanic black adolescents have higher rates of obesity and hypertension, MetS is diagnosed at strikingly lower rates than Mexican Americans and non-Hispanic whites. Presumably, this is because non-Hispanics have lower rates of dyslipidemia.
The Relationship of Triglycerides to Insulin Resistance
The sharp differences in TG levels between non-Hispanic black individuals and non-Hispanic white individuals raise important issues regarding the accuracy of the current sets of MetS criteria for identifying non-Hispanic blacks with MetS. Specifically, it is unclear whether lower TG levels may be falsely reassuring among non-Hispanic black individuals who 1) exhibit underlying processes causative of MetS and/or 2) are at higher risk for T2DM and CVD. Unfortunately, prospective studies addressing this issue are lacking. However, regarding whether underlying processes may exist among non-Hispanic blacks with normal TG concentrations, studies suggest this is a common finding. Non-Hispanic blacks with TG in the normal range are nearly twice as likely as non-Hispanic whites to exhibit insulin resistance (Fig. 2) [56•]. In addition, increasing TG levels in non-Hispanic blacks, including levels well in the range of normal, are associated with worse insulin resistance (Fig. 3) [56•]. This suggests that the baseline TG levels in non-Hispanic blacks (i.e., the level seen in individuals without insulin resistance) is intrinsically lower than in non-Hispanic whites, but that elevations above this baseline, even if still within normal ranges for the entire population, may reflect more severe insulin resistance.
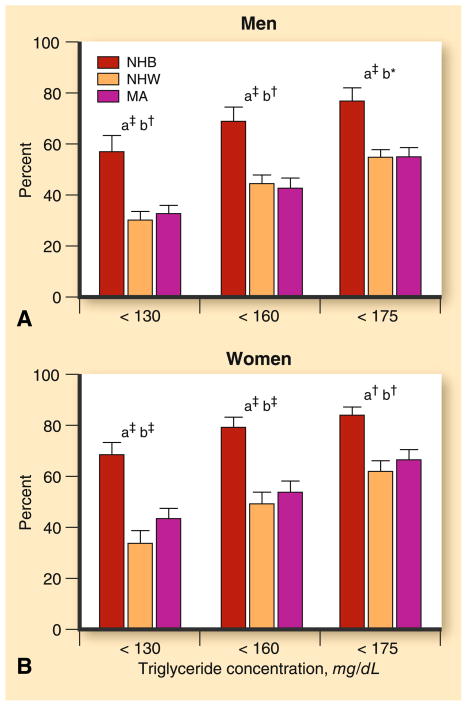
Fig. 2.
Percent of insulin-resistant individuals with triglyceride levels below threshold values by sex and ethnicity. Subjects were 2,804 participants of the National Health and Nutrition Examination Survey (NHANES) 1999–2000, including 569 non-Hispanic blacks (NHB), 1,485 non-Hispanic whites (NHW), and 750 Mexican Americans (MA). Insulin resistance was estimated as having a homeostasis model assessment (HOMA) in the upper tertile for the cohort. The letter “a” indicates a significant difference between NHB versus NHW; “b” indicates a significant difference between NHB versus MA; and “c” indicates a significant difference between NHW versus MA. Asterisk indicates a level of significance of <0.05; dagger indicates a level of significance of <0.01; and double dagger indicates a level of significance of <0.001. (From Sumner and Cowie [56•]; with permission)
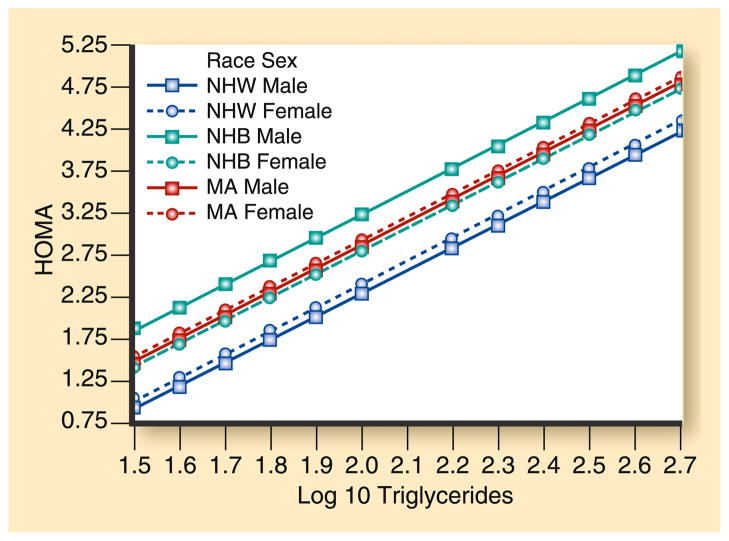
Fig. 3.
Regression of insulin resistance on fasting triglyceride (TG) levels. Participants are from the National Health and Nutrition Examination Survey 1999–2000. A multiple linear regression model was formed, predicting insulin resistance (as measured by homeostasis model assessment [HOMA]) versus log TG. R2 for the final regression is 0.17. The parallel regression lines demonstrate that insulin resistance rose at a similar rate with increasing TG levels in all sex and ethnic groups. However, the y-intercepts were different, being highest for non-Hispanic black (NHB) women, followed by similar y-intercepts for Mexican American (MA) women, NHB men, and MA men. Non-Hispanic white (NHW) men and women had the lowest y-intercepts. (From Sumner and Cowie [56•]; with permission)
Current sets of MetS criteria that use population-based cutoffs to define elevated TG levels do not take these ethnic differences into account and may give false reassurances regarding the presence of high TG levels in the population. Future investigations will be needed to identify TG thresholds in non-Hispanic blacks that are associated with the processes underlying MetS and/or with future risk. A similar logic may hold true regarding low HDL–C levels among non-Hispanic black adolescents, although these inter-ethnicity differences narrow in adulthood [8, 76], suggesting either a time-based effect or that the difference in baseline (low risk) level of HDL–C is not as striking between non-Hispanic blacks and whites as is seen with TG levels.
Approaches to Detect Increased Risk
The goal of accurate identification of future risk is twofold: 1) to use this information to convince families of their need to change their lifestyle to avoid medical complications in the future and 2) to convince the health insurance industry of the need for investment. With respect to motivating families, a demonstration of predicting future disease may increase a family’s compliance with the treatment regimen [77, 78]. Goetz [79] has written that “a diagnosis of the metabolic syndrome can get [people] off the couch in a way that the doctor tut-tutting about their pounds cannot.” Similarly, with regard to convincing medical payers of the importance of funding obesity interventions, the most logical way to do this would be in the demonstration of a decrease in risk status, which is most likely to be done among subgroups of highest risk, including adolescents with MetS.
If MetS is to be used as a trigger for intervention, as has been proposed [80], then many at-risk non-Hispanic black adolescents will be missed with the current criteria. Alternative means of better detecting risk in non-Hispanic blacks remain to be determined. As mentioned earlier, one means of achieving improved accuracy of MetS would be determining ethnic-specific cut-off points for TG and HDL to better identify non-Hispanic black adolescents with early evidence of MetS. These studies would optimally be performed using cohort data looking at levels TG associated with increased risk of future disease in non-Hispanic blacks. Such long-term data with adequate subject numbers are usually lacking given the amount of time necessary to follow individuals from adolescence until they develop adult disease. However, new ethnic-specific cut-offs could also be determined using cross-sectional data looking at TG and HDL thresholds associated with increased insulin resistance in non-Hispanic black adolescents.
An alternate approach to improving the predictive ability of MetS through all ethnicities would be to construct a linear MetS scale [81–83]. Instead of requiring that individuals have elevations in a specified number of components, a set of continuous MetS criteria would require the sum of elevated components reach a certain threshold. If, for example, fasting insulin level represented one of these components, then non-Hispanic black adolescents might be expected to reach the threshold for MetS diagnosis at a lower level of TG than seen among non-Hispanic white adolescents, given the higher rates of elevated insulin among non-Hispanic blacks.
Finally, biomarkers associated with the processes underlying MetS may ultimately prove to be better markers of disease risk than a diagnosis of MetS itself. The role of CRP has been examined among adults in this regard [23, 24]. Future studies would be needed to tightly link CRP to future risk among adolescents.
A potentially better marker of risk may be levels of adiponectin in children [84]. As discussed earlier, decreasing levels of adiponectin are associated with insulin resistance in adolescents [15–18]. Given the strength of these of these associations, adiponectin has been suggested as a screening test to identify children at risk for long-term sequelae of MetS [15–18]. Because low adiponectin levels correlate well with insulin resistance in non-Hispanic blacks [58], screening for adiponectin may prove a superior estimate of long-term risk than MetS itself.
Future studies are necessary to determine whether early identification of risk among adolescents using an ethnic-specific set of MetS criteria would more completely predict risk than current sets of criteria. Initiating intervention among individuals at increased risk (beginning with lifestyle changes to promote better food choices and more physical activity) are likely to be beneficial for all individuals but are also likely to be costly if done at the level of intensity needed to be effective [2, 3•]. Nevertheless, a diagnosis of MetS itself may prove motivating to some individuals and their families to make appropriate lifestyle adjustments. If future research links ethnic-specific criteria to increased risk, this may justify additional resources being placed at intervention. This is particularly true given the intensity of the effort necessary to reverse MetS [7].
Conclusions
In summary, although non-Hispanic blacks have lower rates of MetS diagnosis, there is no evidence that they exhibit a decrease in the underlying processes behind MetS or the long-term consequences. The major finding separating non-Hispanic blacks from non-Hispanic whites with insulin resistance is that TG levels among non-Hispanic blacks are unlikely to exceed the cut-off values used in most MetS criteria. Therefore, additional diagnostic criteria are likely to be necessary to identify increased risk among non-Hispanic blacks. In addition to ethnic-specific MetS criteria, use of a biomarker such as adiponectin level may be of value as a screening test to identify individuals at high risk for the future development of CVD and T2DM. The more detailed information we have on the predictive ability of such tests, the better we will be able to provide appropriate intervention to individuals most likely to benefit.
Acknowledgments
The author has received funding from the National Institutes of Health (grant HD060739-01)
Footnotes
Disclosure No potential conflict of interest relevant to this article was reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Narayan KM, Boyle JP, Thompson TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 2.Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007;297:2697–2704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- 3•.Wilfley DE, Stein RI, Saelens BE, et al. Efficacy of maintenance treatment approaches for childhood overweight: a randomized controlled trial. JAMA. 2007;298:1661–1673. doi: 10.1001/jama.298.14.1661. This randomized trial demonstrated that by using time-intensive efforts, children and their families were able to lose weight and maintain weight loss (change in body mass index z-score of 0.24) over a 30-month period. Such programs are likely to be more expensive that clinical programs that are currently available. [DOI] [PubMed] [Google Scholar]
- 4.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 5.DeBoer MD, Gurka MJ. Ability among adolescents for the metabolic syndrome to predict elevations in factors associated with type 2 diabetes and cardiovascular disease: Data from the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Metab Syndrome Related Disord. 2010 doi: 10.1089/met.2010.0008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6••.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25–30 years later. J Pediatr. 2008;152:201–206. doi: 10.1016/j.jpeds.2007.09.010. This is a prospective study that demonstrated that MetS in childhood predicts future T2DM. Children had metabolic measurements at 6–18 years of age and were evaluated 25–30 years later for the development of diabetes. Children with MetS were 12 times as likely to develop T2DM. [DOI] [PubMed] [Google Scholar]
- 7.Coppen AM, Risser JA, Vash PD. Metabolic syndrome resolution in children and adolescents after 10 weeks of weight loss. J Cardiometab Syndr. 2008;3:205–210. doi: 10.1111/j.1559-4572.2008.00016.x. [DOI] [PubMed] [Google Scholar]
- 8.Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr. 2009;155:S7. e7–S7.e11. doi: 10.1016/j.jpeds.2009.04.049. This review details ethnic differences in individual components of MetS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. This review describes molecular features of processes underlying insulin resistance in MetS. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 12.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 13.Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 14.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 15.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 16.Stefan N, Bunt JC, Salbe AD, et al. Plasma adiponectin concentrations in children: relationships with obesity and insulinemia. J Clin Endocrinol Metab. 2002;87:4652–4656. doi: 10.1210/jc.2002-020694. [DOI] [PubMed] [Google Scholar]
- 17.Huang KC, Lue BH, Yen RF, et al. Plasma adiponectin levels and metabolic factors in nondiabetic adolescents. Obes Res. 2004;12:119–124. doi: 10.1038/oby.2004.16. [DOI] [PubMed] [Google Scholar]
- 18.Martin LJ, Woo JG, Daniels SR, et al. The relationships of adiponectin with insulin and lipids are strengthened with increasing adiposity. J Clin Endocrinol Metab. 2005;90:4255–4259. doi: 10.1210/jc.2005-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguirre V, Uchida T, Yenush L, et al. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 20.Harman-Boehm I, Blüher M, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92:2240–2247. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- 21•.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–231. doi: 10.2119/2007-00119.Tilg. This review highlights molecular processes linking inflammation, adipokines, and reduced insulin signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 24.Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 25.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregor MF, Hotamisligil GS. Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res. 2007;48:1905–1914. doi: 10.1194/jlr.R700007-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 28.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21:1443–1455. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 30.Carvalho-Filho MA, Ueno M, Hirabara SM, et al. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes. 2005;54:959–967. doi: 10.2337/diabetes.54.4.959. [DOI] [PubMed] [Google Scholar]
- 31.Kaneki M, Shimizu N, Yamada D, Chang K. Nitrosative stress and pathogenesis of insulin resistance. Antioxid Redox Signal. 2007;9:319–329. doi: 10.1089/ars.2006.1464. [DOI] [PubMed] [Google Scholar]
- 32.Pauli JR, Ropelle ER, Cintra DE, et al. Acute physical exercise reverses S-nitrosation of the insulin receptor, insulin receptor substrate 1 and protein kinase B/Akt in diet-induced obese Wistar rats. J Physiol. 2008;586:659–671. doi: 10.1113/jphysiol.2007.142414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. This fantastic treatise extensively details processes related to insulin resistance and β-cell failure leading to T2DM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 35.Mittelman SD, Van Citters GW, Kirkman EL, Bergman RN. Extreme insulin resistance of the central adipose depot in vivo. Diabetes. 2002;51:755–761. doi: 10.2337/diabetes.51.3.755. [DOI] [PubMed] [Google Scholar]
- 36.Maheux P, Azhar S, Kern PA, et al. Relationship between insulin-mediated glucose disposal and regulation of plasma and adipose tissue lipoprotein lipase. Diabetologia. 1997;40:850–858. doi: 10.1007/s001250050759. [DOI] [PubMed] [Google Scholar]
- 37.Schneider JG, von Eynatten M, Schiekofer S, et al. Low plasma adiponectin levels are associated with increased hepatic lipase activity in vivo. Diabetes Care. 2005;28:2181–2186. doi: 10.2337/diacare.28.9.2181. [DOI] [PubMed] [Google Scholar]
- 38.Li C, Ford ES. Is there a single underlying factor for the metabolic syndrome in adolescents? A confirmatory factor analysis. Diabetes Care. 2007;30:1556–1561. doi: 10.2337/dc06-2481. [DOI] [PubMed] [Google Scholar]
- 39.Pausova Z. From big fat cells to high blood pressure: a pathway to obesity-associated hypertension. Curr Opin Nephrol Hypertens. 2006;15:173–178. doi: 10.1097/01.mnh.0000214775.42103.a5. [DOI] [PubMed] [Google Scholar]
- 40.Paulmyer-Lacroix O, Boullu S, Oliver C, et al. Expression of the mRNA coding for 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue from obese patients: an in situ hybridization study. J Clin Endocrinol Metab. 2002;87:2701–2705. doi: 10.1210/jcem.87.6.8614. [DOI] [PubMed] [Google Scholar]
- 41•.Mathieu P, Poirier P, Pibarot P, et al. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53:577–584. doi: 10.1161/HYPERTENSIONAHA.108.110320. This review demonstrates many adverse roles that visceral adipocytes play in worsening MetS. [DOI] [PubMed] [Google Scholar]
- 42.Paolisso G, Tagliamonte MR, Rizzo MR, et al. Lowering fatty acids potentiates acute insulin response in first degree relatives of people with type II diabetes. Diabetologia. 1998;41:1127–1132. doi: 10.1007/s001250051041. [DOI] [PubMed] [Google Scholar]
- 43.Clore JN, Stillman J, Sugerman H. Glucose-6-phosphatase flux in vitro is increased in type 2 diabetes. Diabetes. 2000;49:969–974. doi: 10.2337/diabetes.49.6.969. [DOI] [PubMed] [Google Scholar]
- 44.Gastaldelli A, Baldi S, Pettiti M, et al. Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans: a quantitative study. Diabetes. 2000;49:1367–1373. doi: 10.2337/diabetes.49.8.1367. [DOI] [PubMed] [Google Scholar]
- 45.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 46.Hanley AJ, Williams K, Gonzalez C, et al. San Antonio Heart Study; Mexico City Diabetes Study; Insulin Resistance Atherosclerosis Study: Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. Diabetes. 2003;52:463–469. doi: 10.2337/diabetes.52.2.463. (Published erratum appears in Diabetes 2003; 52:1306.) [DOI] [PubMed] [Google Scholar]
- 47.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 48.Cook S, Weitzman M, Auinger P, et al. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 49.Ford ES, Li C, Zhao G, et al. Prevalence of the metabolic syndrome among U.S. adolescents using the definition from the International Diabetes Federation. Diabetes Care. 2008;31:587–589. doi: 10.2337/dc07-1030. [DOI] [PubMed] [Google Scholar]
- 50•.Johnson WD, Kroon JJ, Greenway FL, et al. Prevalence of risk factors for metabolic syndrome in adolescents: National Health and Nutrition Examination Survey (NHANES), 2001–2006. Arch Pediatr Adolesc Med. 2009;163:371–377. doi: 10.1001/archpediatrics.2009.3. This report from NHANES provides the most current evaluation of individual components of MetS among adolescents. [DOI] [PubMed] [Google Scholar]
- 51.Arslanian S, Suprasongsin C, Janosky JE. Insulin secretion and sensitivity in black versus white prepubertal healthy children. J Clin Endocrinol Metab. 1997;82:1923–1927. doi: 10.1210/jcem.82.6.4002. [DOI] [PubMed] [Google Scholar]
- 52.Gower BA, Granger WM, Franklin F, et al. Contribution of insulin secretion and clearance to glucose-induced insulin concentration in African–American and Caucasian children. J Clin Endocrinol Metab. 2002;87:2218–2224. doi: 10.1210/jcem.87.5.8498. [DOI] [PubMed] [Google Scholar]
- 53.Klein DJ, Aronson Friedman L, Harlan WR, et al. Obesity and the development of insulin resistance and impaired fasting glucose in black and white adolescent girls: a longitudinal study. Diabetes Care. 2004;27:378–383. doi: 10.2337/diacare.27.2.378. [DOI] [PubMed] [Google Scholar]
- 54.Haffner SM, D’Agostino R, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African–Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 55.Osei K, Rhinesmith S, Gaillard T, Schuster D. Impaired insulin sensitivity, insulin secretion, and glucose effectiveness predict future development of impaired glucose tolerance and type 2 diabetes in pre-diabetic African Americans: implications for primary diabetes prevention. Diabetes Care. 2004;27:1439–1446. doi: 10.2337/diacare.27.6.1439. [DOI] [PubMed] [Google Scholar]
- 56•.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196:696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. This evaluation of NHANES data among adults demonstrates that increasing TG levels are associated with increased insulin resistance among non-Hispanic blacks, although non-Hispanic blacks are likely to have TG levels in the “normal”range, even with significant insulin resistance. [DOI] [PubMed] [Google Scholar]
- 57.Degawa-Yamauchi M, Dilts JR, Bovenkerk JE, et al. Lower serum adiponectin levels in African–American boys. Obes Res. 2003;11:1384–1390. doi: 10.1038/oby.2003.187. [DOI] [PubMed] [Google Scholar]
- 58.Lee S, Bacha F, Gungor N, Arslanian SA. Racial differences in adiponectin in youth: relationship to visceral fat and insulin sensitivity. Diabetes Care. 2006;29:51–56. doi: 10.2337/diacare.29.1.51. [DOI] [PubMed] [Google Scholar]
- 59.Ford ES, Giles WH, Mokdad AH, Myers GL. Distribution and correlates of C-reactive protein concentrations among adult US women. Clin Chem. 2004;50:574–581. doi: 10.1373/clinchem.2003.027359. [DOI] [PubMed] [Google Scholar]
- 60.Ford ES, Giles WH, Myers GL, et al. C-reactive protein concentration distribution among US children and young adults: findings from the National Health and Nutrition Examination Survey, 1999–2000. Clin Chem. 2003;49:1353–1357. doi: 10.1373/49.8.1353. [DOI] [PubMed] [Google Scholar]
- 61.Albert MA, Glynn RJ, Buring J, Ridker PM. C-reactive protein levels among women of various ethnic groups living in the United States (from the Women’s Health Study) Am J Cardiol. 2004;93:1238–1242. doi: 10.1016/j.amjcard.2004.01.067. [DOI] [PubMed] [Google Scholar]
- 62.Khera A, McGuire DK, Murphy SA, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 63.SEARCH for Diabetes in Youth Study Group. Liese AD, D’Agostino RB, Jr, Hamman RF, et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 64.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 65.Yancy CW, Benjamin EJ, Fabunmi RP, Bonow RO. Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: executive summary. Circulation. 2005;111:1339–1349. doi: 10.1161/01.CIR.0000157740.93598.51. [DOI] [PubMed] [Google Scholar]
- 66.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 67.Lieb DC, Snow RE, DeBoer MD. Socioeconomic factors in the development of childhood obesity and diabetes. Clin Sports Med. 2009;28:349–378. doi: 10.1016/j.csm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kimm SY, Barton BA, Obarzanek E, et al. Racial divergence in adiposity during adolescence: The NHLBI Growth and Health Study. Pediatrics. 2001;107:E34. doi: 10.1542/peds.107.3.e34. [DOI] [PubMed] [Google Scholar]
- 69.Thompson DR, Obarzanek E, Franko DL, et al. Childhood overweight and cardiovascular disease risk factors: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2007;150:18–25. doi: 10.1016/j.jpeds.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 71.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 72.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 73.Katzmarzyk PT, Srinivasan SR, Chen W, et al. Body mass index, waist circumference, and clustering of cardiovascular disease risk factors in a biracial sample of children and adolescents. Pediatrics. 2004;114:e198–e205. doi: 10.1542/peds.114.2.e198. [DOI] [PubMed] [Google Scholar]
- 74.Winkleby MA, Robinson TN, Sundquist J, Kraemer HC. Ethnic variation in cardiovascular disease risk factors among children and young adults: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. JAMA. 1999;281:1006–1013. doi: 10.1001/jama.281.11.1006. [DOI] [PubMed] [Google Scholar]
- 75.CDC. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 76.Sumner AE. “Half the dsylipidemia of insulin resistance” is the dsylipidemia of insulin-resistant Blacks. Ethn Dis. 2009;19:462–465. [PMC free article] [PubMed] [Google Scholar]
- 77.Butterworth SW. Influencing patient adherence to treatment guidelines. J Manag Care Pharm. 2008;14(6 Suppl B):21–24. doi: 10.18553/jmcp.2008.14.S6-B.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suarez M, Mullins S. Motivational interviewing and pediatric health behavior interventions. J Dev Behav Pediatr. 2008;29:417–428. doi: 10.1097/DBP.0b013e31818888b4. [DOI] [PubMed] [Google Scholar]
- 79.Goetz T. 75 million Americans may have something called metabolic syndrome. How Big Pharma turned obesity into a disease—then invented the drugs to cure it. Wired. 2006;10:152–157. [Google Scholar]
- 80.Zimmet P, Alberti G, Kaufman F, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–2061. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 81.Andersen LB, Harro M, Sardinha LB, et al. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study) Lancet. 2006;368:299–304. doi: 10.1016/S0140-6736(06)69075-2. [DOI] [PubMed] [Google Scholar]
- 82.Bao W, Srinivasan SR, Wattigney WA, Berenson GS. Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood. The Bogalusa Heart Study. Arch Intern Med. 1994;154:1842–1847. [PubMed] [Google Scholar]
- 83.Eisenmann JC. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc Diabetol. 2008;7:17. doi: 10.1186/1475-2840-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee KK, Fortmann SP, Fair JM, et al. Insulin resistance independently predicts the progression of coronary artery calcification. Am Heart J. 2009;157:939–945. doi: 10.1016/j.ahj.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 85.Li C, Ford ES, Mokdad AH, Cook S. Recent trends in waist circumference and waist-height ratio among US children and adolescents. Pediatrics. 2006;118:e1390–e1398. doi: 10.1542/peds.2006-1062. [DOI] [PubMed] [Google Scholar]