Abstract
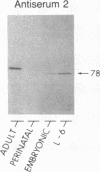
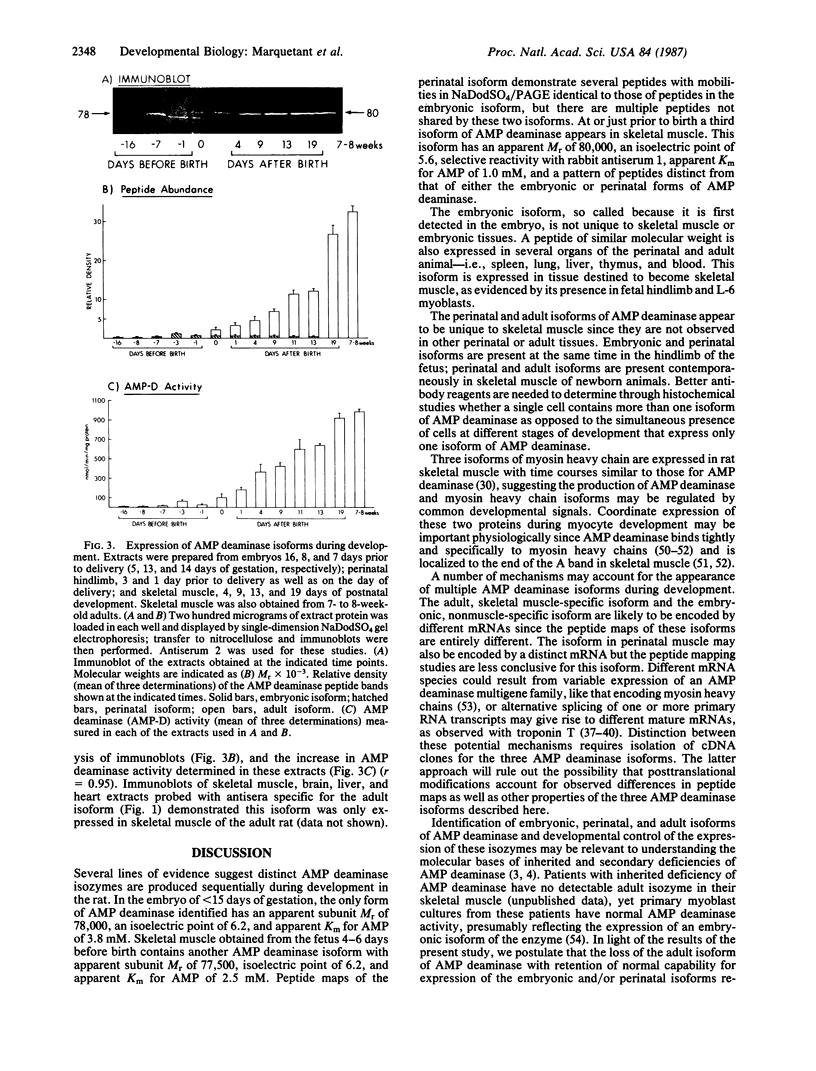
AMP deaminase (myoadenylate deaminase; EC 3.5.4.6) is an integral part of the myofibril in skeletal muscle, and this enzyme plays an important role in energy metabolism in this tissue. We report here the identification of three AMP deaminase isoforms during skeletal muscle development in the rat. An embryonic isoform is expressed in the developing hindlimb of the rat between 7 and 14 days of gestation. This isoform is not unique to skeletal muscle or the embryo as it is also expressed in many nonmuscle tissues of the perinatal and adult rat. A perinatal isoform of AMP deaminase that is restricted to skeletal muscle is produced 4-6 days before birth and persists for 2-3 weeks of postnatal life. An adult, skeletal muscle-specific isoform of AMP deaminase appears at birth and reaches maximal levels after 3 weeks of postnatal development. We conclude from these studies there is a developmentally controlled program that leads to the sequential expression of AMP deaminase isoforms during the transition from embryonic to adult skeletal muscle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby B., Frieden C. Interaction of AMP-aminohydrolase with myosin and its subfragments. J Biol Chem. 1977 Mar 25;252(6):1869–1872. [PubMed] [Google Scholar]
- Ashby B., Holmsen H. Platelet AMP deaminase. Purification and kinetic studies. J Biol Chem. 1981 Oct 25;256(20):10519–10523. [PubMed] [Google Scholar]
- Basi G. S., Boardman M., Storti R. V. Alternative splicing of a Drosophila tropomyosin gene generates muscle tropomyosin isoforms with different carboxy-terminal ends. Mol Cell Biol. 1984 Dec;4(12):2828–2836. doi: 10.1128/mcb.4.12.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C., Crettol-Järvinen A. Peptide mapping of heterogeneous protein samples. J Biol Chem. 1979 Apr 25;254(8):2565–2567. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Nguyen H. T., Medford R. M., Destree A. T., Mahdavi V., Nadal-Ginard B. Intricate combinatorial patterns of exon splicing generate multiple regulated troponin T isoforms from a single gene. Cell. 1985 May;41(1):67–82. doi: 10.1016/0092-8674(85)90062-5. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Fiszman M. Y., Eppenberger H. M. Molecular and cell isoforms during development. Science. 1983 Sep 2;221(4614):921–927. doi: 10.1126/science.6348946. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Coffee C. J., Kofke W. A. Rat muscle 5'-adenylic acid aminohydrolase. I. Purification and subunit structure. J Biol Chem. 1975 Sep 10;250(17):6653–6658. [PubMed] [Google Scholar]
- Cooper J., Trinick J. Binding and location of AMP deaminase in rabbit psoas muscle myofibrils. J Mol Biol. 1984 Jul 25;177(1):137–152. doi: 10.1016/0022-2836(84)90061-5. [DOI] [PubMed] [Google Scholar]
- Cooper T. A., Ordahl C. P. A single cardiac troponin T gene generates embryonic and adult isoforms via developmentally regulated alternate splicing. J Biol Chem. 1985 Sep 15;260(20):11140–11148. [PubMed] [Google Scholar]
- Cooper T. A., Ordahl C. P. A single troponin T gene regulated by different programs in cardiac and skeletal muscle development. Science. 1984 Nov 23;226(4677):979–982. doi: 10.1126/science.6095446. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Miranda A. F., Hays A. P., Franck W. A., Hoffman G. S., Schoenfeldt R. S., Singh N. Myoadenylate deaminase deficiency--muscle biopsy and muscle culture in a patient with gout. J Neurol Sci. 1980 Aug;47(2):191–202. doi: 10.1016/0022-510x(80)90003-9. [DOI] [PubMed] [Google Scholar]
- Firtel R. A. Multigene families encoding actin and tubulin. Cell. 1981 Apr;24(1):6–7. doi: 10.1016/0092-8674(81)90494-3. [DOI] [PubMed] [Google Scholar]
- Fishbein W. N. Myoadenylate deaminase deficiency: inherited and acquired forms. Biochem Med. 1985 Apr;33(2):158–169. doi: 10.1016/0006-2944(85)90024-9. [DOI] [PubMed] [Google Scholar]
- Kaletha K. Hen heart AMP-deaminase--the combined effect of ATP, ADP and orthophosphate on the enzyme activity. Int J Biochem. 1984;16(1):83–85. doi: 10.1016/0020-711x(84)90054-5. [DOI] [PubMed] [Google Scholar]
- Kaletha K. Regulatory properties of 14-day embryo and adult hen skeletal muscle AMP-deaminase. The influence of pH on the enzyme activity. Biochim Biophys Acta. 1984 Jan 18;784(1):90–92. doi: 10.1016/0167-4838(84)90177-8. [DOI] [PubMed] [Google Scholar]
- Kaletha K., Skladanowski A. Regulatory properties of 14 day embryo and adult hen heart AMP-deaminase. Int J Biochem. 1984;16(1):75–81. doi: 10.1016/0020-711x(84)90053-3. [DOI] [PubMed] [Google Scholar]
- Kaletha K., Skladanowski A., Zydowo M. Temperature- and pH-induced changes of the enzyme-substrate affinity and the reaction velocity catalysed by rabbit skeletal muscle AMP-Deaminase. Int J Biochem. 1978;9(2):97–101. doi: 10.1016/0020-711x(78)90018-6. [DOI] [PubMed] [Google Scholar]
- Kaletha K., Składanowski A., Bogdanowicz S., Zydowo M. Purification and some regulatory properties of human heart adenylate deaminase. Int J Biochem. 1979;10(11):925–929. doi: 10.1016/0020-711x(79)90125-3. [DOI] [PubMed] [Google Scholar]
- Kaletha K., Składanowski A. Regulatory properties of rat heart AMP deaminase. Biochim Biophys Acta. 1979 May 10;568(1):80–90. doi: 10.1016/0005-2744(79)90275-4. [DOI] [PubMed] [Google Scholar]
- Karlik C. C., Mahaffey J. W., Coutu M. D., Fyrberg E. A. Organization of contractile protein genes within the 88F subdivision of the D. melanogaster third chromosome. Cell. 1984 Jun;37(2):469–481. doi: 10.1016/0092-8674(84)90377-5. [DOI] [PubMed] [Google Scholar]
- Koretz J. F., Frieden C. Adenylate deaminase binding to synthetic thick filaments of myosin. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7186–7188. doi: 10.1073/pnas.77.12.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE Y. P. 5'-Adenylic acid deaminase. II. Homogeneity and physicochemical properties. J Biol Chem. 1957 Aug;227(2):993–998. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leinwand L. A., Fournier R. E., Nadal-Ginard B., Shows T. B. Multigene family for sarcomeric myosin heavy chain in mouse and human DNA: localization on a single chromosome. Science. 1983 Aug 19;221(4612):766–769. doi: 10.1126/science.6879174. [DOI] [PubMed] [Google Scholar]
- Makarewicz W., Stankiewicz A. AMP-aminohydrolase of human skeletal muscle: partial purification and properties. Biochem Med. 1974 Jun;10(2):180–197. doi: 10.1016/0006-2944(74)90021-0. [DOI] [PubMed] [Google Scholar]
- Medford R. M., Nguyen H. T., Destree A. T., Summers E., Nadal-Ginard B. A novel mechanism of alternative RNA splicing for the developmentally regulated generation of troponin T isoforms from a single gene. Cell. 1984 Sep;38(2):409–421. doi: 10.1016/0092-8674(84)90496-3. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ogasawara N., Goto H., Watanabe T. Isozymes of rat AMP deaminase. Biochim Biophys Acta. 1975 Oct 22;403(2):530–537. doi: 10.1016/0005-2744(75)90081-9. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Goto H., Watanabe T., Kawamura Y., Yoshino M. AMP deaminase from rat brain: purification and characterization of multiple forms. Biochim Biophys Acta. 1974 Oct 17;364(2):353–364. doi: 10.1016/0005-2744(74)90020-5. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Goto H., Watanabe T., Kawamura Y., Yoshino M. Multiple forms of AMP deaminase in various rat tissues. FEBS Lett. 1974 Aug 15;44(1):63–66. doi: 10.1016/0014-5793(74)80306-6. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Goto H., Yamada Y. AMP deaminase isozymes in rabbit red and white muscles and heart. Comp Biochem Physiol B. 1983;76(3):471–473. doi: 10.1016/0305-0491(83)90277-8. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Goto H., Yamada Y., Watanabe T., Asano T. AMP deaminase isozymes in human tissues. Biochim Biophys Acta. 1982 Feb 2;714(2):298–306. doi: 10.1016/0304-4165(82)90337-3. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Goto H., Yamada Y., Watanabe T. Distribution of AMP deaminase isozymes in various human blood cells. Int J Biochem. 1984;16(3):269–273. doi: 10.1016/0020-711x(84)90099-5. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Goto H., Yamada Y., Watanabe T. Distribution of AMP-deaminase isozymes in rat tissues. Eur J Biochem. 1978 Jun 15;87(2):297–304. doi: 10.1111/j.1432-1033.1978.tb12378.x. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Yoshino M., Kawamura Y. Multiple forms of AMP deaminase in rat brain. Biochim Biophys Acta. 1972 Feb 28;258(2):680–684. doi: 10.1016/0005-2744(72)90261-6. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Eldridge J. D. alpha-Cardiac actin is the major sarcomeric isoform expressed in embryonic avian skeletal muscle. Science. 1984 Jun 29;224(4656):1436–1438. doi: 10.1126/science.6729461. [DOI] [PubMed] [Google Scholar]
- Periasamy M., Strehler E. E., Garfinkel L. I., Gubits R. M., Ruiz-Opazo N., Nadal-Ginard B. Fast skeletal muscle myosin light chains 1 and 3 are produced from a single gene by a combined process of differential RNA transcription and splicing. J Biol Chem. 1984 Nov 10;259(21):13595–13604. [PubMed] [Google Scholar]
- Raggi A., Bergamini C., Ronca G. Isozymes of AMP deaminase in red and white skeletal muscles. FEBS Lett. 1975 Oct 15;58(1):19–23. doi: 10.1016/0014-5793(75)80216-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg U. B., Kunz G., Frischauf A., Lehrach H., Mähr R., Eppenberger H. M., Perriard J. C. Molecular cloning and expression during myogenesis of sequences coding for M-creatine kinase. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6589–6592. doi: 10.1073/pnas.79.21.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Opazo N., Weinberger J., Nadal-Ginard B. Comparison of alpha-tropomyosin sequences from smooth and striated muscle. Nature. 1985 May 2;315(6014):67–70. doi: 10.1038/315067a0. [DOI] [PubMed] [Google Scholar]
- Sabina R. L., Swain J. L., Olanow C. W., Bradley W. G., Fishbein W. N., DiMauro S., Holmes E. W. Myoadenylate deaminase deficiency. Functional and metabolic abnormalities associated with disruption of the purine nucleotide cycle. J Clin Invest. 1984 Mar;73(3):720–730. doi: 10.1172/JCI111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammons D. W., Chilson O. P. AMP deaminase: stage-specific isozymes in differentiating chick muscle. Arch Biochem Biophys. 1978 Dec;191(2):561–570. doi: 10.1016/0003-9861(78)90394-6. [DOI] [PubMed] [Google Scholar]
- Smiley K. L., Jr, Berry A. J., Suelter C. H. An improved purification, crystallization, and some properties of rabbit muscle 5'-adenylic acid deaminase. J Biol Chem. 1967 May 25;242(10):2502–2506. [PubMed] [Google Scholar]
- Spychała J., Kaletha K., Makarewicz W. Developmental changes of chicken liver AMP deaminase. Biochem J. 1985 Oct 15;231(2):329–333. doi: 10.1042/bj2310329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M., Butler-Browne G. S., Schwartz K., Bouveret P., Pinset-Härstöm I. Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nature. 1981 Aug 27;292(5826):805–809. doi: 10.1038/292805a0. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S., Suelter C. H. Human erythrocyte 5'-AMP aminohydrolase. Purification and characterization. J Biol Chem. 1978 Jan 25;253(2):404–408. [PubMed] [Google Scholar]