Abstract
OBJECTIVE
Although adipocyte-derived murine resistin links insulin resistance to obesity, the role of human resistin, predominantly expressed in mononuclear cells and induced by inflammatory signals, remains unclear. Given the mounting evidence that obesity and type 2 diabetes are inflammatory diseases, we sought to determine the relationship between inflammatory increases in human resistin and insulin resistance.
RESEARCH DESIGN AND METHODS
To investigate the role of human resistin on glucose homeostasis in inflammatory states, we generated mice lacking murine resistin but transgenic for a bacterial artificial chromosome containing human resistin (BAC-Retn), whose expression was similar to that in humans. The metabolic and molecular phenotypes of BAC-Retn mice were assessed after acute and chronic endotoxemia (i.e., exposure to inflammatory lipopolysaccharide).
RESULTS
We found that BAC-Retn mice have circulating resistin levels within the normal human range, and similar to humans, lipopolysaccharide markedly increased serum resistin levels. Acute endotoxemia caused hypoglycemia in mice lacking murine resistin, and this was attenuated in BAC-Retn mice. In addition, BAC-Retn mice developed severe hepatic insulin resistance under chronic endotoxemia, accompanied by increased inflammatory responses in liver and skeletal muscle.
CONCLUSIONS
These results strongly support the role of human resistin in the development of insulin resistance in inflammation. Thus, human resistin may link insulin resistance to inflammatory diseases such as obesity, type 2 diabetes, and atherosclerosis.
The prevalence of obesity has increased worldwide, and therefore, the burden of obesity on health is progressively growing (1). Obesity is associated with insulin resistance and an increased risk of type 2 diabetes. Recently, it has become clear that obesity promotes a state of chronic, low-grade inflammation in white adipose tissue (WAT), which leads to insulin resistance. Adipocytes and macrophages in WAT both are involved in the development of insulin resistance in obesity through the release of free fatty acids (FFAs), proinflammatory cytokines, and adipokines (2–4).
Resistin, predominantly expressed in adipocytes, mediates insulin resistance in rodents. Circulating levels of resistin are increased in obesity, and treatment with recombinant resistin or transgenic overexpression of resistin induces hepatic insulin resistance in mice (5–8). Furthermore, deletion of resistin enhances insulin sensitivity in livers of mice fed a high-fat diet, and in muscle and WAT in leptin-deficient ob/ob mice (9,10). In contrast, resistin is mainly expressed in mononuclear cells, including macrophages in humans (11,12). Human resistin is induced in response to various inflammatory stimuli such as lipopolysaccharide (LPS), tumor necrosis factor-α (TNF-α), or interleukin (IL)-6, and resistin itself induces proinflammatory cytokines, suggesting a role for resistin in inflammation in humans (13–16). Moreover, circulating resistin levels correlate with inflammatory markers in subjects with type 2 diabetes, coronary atherosclerosis, chronic kidney disease, rheumatoid arthritis, sepsis, and experimental endotoxemia (14,17–21).
Because it is widely accepted that obesity and type 2 diabetes are associated with chronic inflammation in WAT, and resistin is produced by macrophages in inflammation, hyperresistinemia might be a contributing factor to these pathophysiologic states in humans. However, studies relating resistin in humans to obesity, insulin resistance, or type 2 diabetes have shown conflicting results (22–28). Therefore, it remains uncertain whether the role of adipocyte-derived murine resistin in glucose homeostasis translates directly to the biology of macrophage-derived human resistin.
We have previously demonstrated that transgenic expression of human resistin in resistin-knockout (Rko) mice fed a high-fat diet results in insulin resistance in muscle and adipose tissue, indicating that macrophage-derived human resistin exacerbates insulin resistance (29). However, macrophage human resistin expression in that model was constitutive and not induced by inflammatory stimuli. Here we investigated the metabolic effects of human resistin induced by inflammation using a novel transgenic mouse that express human resistin from its human genomic elements (BAC-Retn mice). In contrast to the previous macrophage-specific humanized resistin mice, circulating resistin levels in this new BAC-Retn mouse are similar to those in healthy humans but increase in response to inflammatory signals such as LPS. Under experimental endotoxemia, BAC-Retn mice showed increased inflammation and hepatic insulin resistance. Thus, with a humanized expression pattern, human resistin contributes to the development of insulin resistance in inflammation.
RESEARCH DESIGN AND METHODS
Cell culture and transfections.
The BAC-Retn construct that included the human resistin gene plus 20 kb upstream and 4 kb downstream DNA regions was engineered and transfected into mouse macrophage RAW.264 cells by electroporation according to the manufacturer’s instructions (Lonza, Allendale, NJ). Primary mouse macrophages were obtained from male BAC-Retn mice by peritoneal lavage 3 days after an intraperitoneal injection with 1 mL of sterile 3% thioglycollate. RAW.264 cells or primary mouse macrophages were stimulated with LPS for 16 h. Cells or media not exposed to LPS served as the control. Human resistin gene expression was detected by quantitative PCR, and protein in the media was determined by ELISA (Millipore Corporation, Billerica, MA).
Derivation and treatment of animals.
For the derivation of mice that express the human resistin under the control of the human resistin regulatory DNA elements, we engineered a BAC as described previously (30). The engineered BAC contained the whole human resistin gene, including 21,300 bp upstream and 4,248 bp downstream of the human resistin start site. The fusion construct containing human resistin and the 25,548 bp surrounding it was then excised using NotI/BglII and injected into the pronucleus of fertilized C57BL/6 J mouse oocytes (Transgenic and Chimeric Mouse Facility, University of Pennsylvania). Transgenic founder mice were identified by PCR of tail DNA with the forward primer covering the 5′ end of the human resistin exon (5′-ACCAGTCTCTGGACATGAAG-3′) and the reverse primer covering the 3′-end of the human resistin exon (5′-TCGGTGGGCTCAGCTAACCA-3′). Positive mice were propagated by mating to Retn−/− mice (9). The progeny (Retn+/−BAChR) were then crossed back to the Retn−/− mice to produce the BAC-transgenic (BAC-Retn) mice that express human resistin without expressing murine resistin (Retn−/−BAChR). Retn−/− littermates were used as controls in this study.
Animals used in all experiments were age-matched (10- to 12-week-old) male mice housed four or five per cage under an ambient temperature of 22°C with 12 h light/dark cycle (lights on at 0700 h). Regular chow (LabDiet, Richmond, IN) and water were provided ad libitum. All protocols for animal use and euthanasia were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania School of Medicine and were in accordance with National Institutes of Health guidelines.
LPS administration.
Mice were weighed to determine the dose of LPS (Escherichia coli O 111:B4; Sigma-Aldrich, St. Louis, MO). For acute endotoxemia experiments, equivalent volumes of LPS (0.2 mg/kg body wt) or normal saline (0.9%) were intraperitoneally injected to both Rko and BAC-Retn mice. Blood was collected before and at 1, 3, and 6 h after injection. For chronic endotoxemia experiments, mice were implanted subcutaneously with an osmotic minipump (Alzet model 1002; Braintree Scientific Inc., Braintree, MA) as previously described (31). The pumps were filled with normal saline or LPS to infuse 2 mg/kg/day for 2 weeks.
Total RNA extraction and quantitative PCR.
Mice were killed and their tissues isolated and immediately placed in liquid nitrogen for later use. Total RNA from tissues was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. RNA integrity was determined with ultraviolet spectrophotometry. Reverse transcription was performed with Sprint RT complete-Oligo(dT)18 (Clontech Laboratories, Mountain View, CA) according to the manufacturer’s instructions. Expression of genes was analyzed using real-time (TaqMan) quantitative PCR (ABI Prism; Applied Biosystems, Foster City, CA) as previously described (29). The level of mRNA expression was normalized to 36B4.
Measurement of serum and tissue metabolites.
Blood glucose measurements were obtained from tail vein blood using the OneTouch Ultra Glucometer (Lifescan, Milpitas, CA). Serum was harvested by tail vein blood or cardiac puncture, spun at 4°C for 30 min, and assayed immediately or stored at −20°C. Serum alanine aminotransferase (ALT), TGs, cholesterol, β-hydroxybutyric acid, and FFAs levels were measured using colorimetric assays (Stanbio Laboratory, Boerne, TX; Wako Pure Chemical Industries, Osaka, Japan). Serum mouse insulin, resistin, leptin, high-molecular-weight adiponectin, and human resistin levels were measured by ELISA (Crystal Chem Inc., Downers Grove, IL; Millipore Corporation, Billerica, MA). Serum TNF-α and IL1β levels were measured with Luminex-100 multiplex immunoassay (Millipore). Lipids were extracted from liver for TGs and cholesterol measurements, as previously described (32). For diacylglycerol and ceramides levels, 100-mg samples of liver tissue were submitted to the Mouse Metabolic Phenotyping Center at Yale Medical School (New Haven, CT).
Glucose tolerance test.
On the 8th day after the minipump subcutaneous implantation, glucose tolerance testing was performed after an 8-h fast. Blood glucose concentrations were measured before and at 15, 30, 60, 90, and 120 min after an intraperitoneal injection of glucose (1 g/kg, 20% glucose solution). Tail blood samples collected before the glucose injection were used to measure basal levels of mouse insulin and human resistin.
Hyperinsulinemic-euglycemic clamp.
Hyperinsulinemic-euglycemic clamp analysis was performed as described previously (29). Clamp studies were performed at 6 h after LPS (0.2 mg/kg) or saline intraperitoneal injection, and also at 10 days after the minipump subcutaneous implantation. Mice were fasted for 4 h, placed in restrainers, and administered a bolus injection of 5 μCi of [3-3H]glucose, followed by continuous intravenous infusion at 0.05 μCi/min. Baseline glucose kinetics was measured for 60 min, followed by hyperinsulinemic clamp for 120 min. A priming dose of regular insulin (16 mU/kg, Humulin; Eli Lilly, Indianapolis, IN) was given intravenously, followed by continuous infusion at 2.5 mU/kg/min. Tail blood glucose was measured by glucometer at 10-min intervals, and blood glucose was maintained at 120–140 mg/dL by a variable infusion rate of 20% glucose. 2-Deoxy-D-[1-14C]glucose (10 μCi) was injected 45 min before the end of the clamp, and blood samples were collected to estimate glucose uptake. The mice were killed, and liver, perigonadal WAT, and gastrocnemius muscle were excised, frozen immediately in liquid nitrogen, and stored at −80°C for subsequent analysis of glucose uptake and other analysis.
Statistics.
Data are presented as means ± SEM. Changes of various parameters were analyzed with one-way ANOVA, followed by post hoc Tukey multiple comparison test or unpaired Student t test using Prism software (GraphPad Software, San Diego, CA). Values of P < 0.05 were considered as statistically significant.
RESULTS
Generation of mice that express human resistin with human-like inducible regulation.
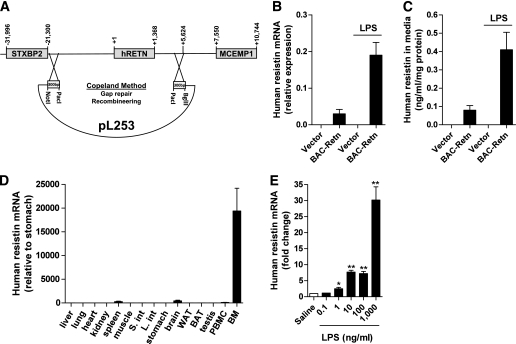
We generated a BAC that contains the human resistin gene plus all the DNA elements up to 21,300 bp upstream and 4,248 bp downstream relative to the human resistin start site (Fig. 1A). Human resistin was basally expressed and markedly induced by LPS treatment of RAW.264 murine macrophages transfected with this BAC fusion construct (Fig. 1B and C). To better understand the role of human resistin in the pathophysiology of inflammation and metabolism, we then derived transgenic mice on the C57BL/6 J Rko background using the BAC fusion construct (BAC-Retn mice). The expression pattern of human resistin in BAC-Retn mice was similar to that seen in humans, with the highest expression seen in bone marrow (Fig. 1D) (11). Moreover, human resistin expression was dramatically induced in peritoneal macrophages isolated from BAC-Retn mice (Fig. 1E).
FIG. 1.
Generation and characterization of BAC-transgenic (BAC-Retn) mice. A: A BAC that contains the human resistin gene plus all the DNA elements up to 21,300 bp upstream and 4,248 bp downstream relative to the human resistin start site is used to generate the mice. The fusion construct was excised using NotI/BglII and injected into the pronucleus of fertilized C57BL/6 J mouse oocytes. B: Induction of human resistin gene by LPS (100 ng/mL) in transfected RAW.264 macrophages. C: LPS (100 ng/mL) induced secretion of resistin in transfected RAW.264 macrophages. D: Expression profile of human resistin in different tissues in BAC-Retn mice. E: LPS induced human resistin in vitro in peritoneal macrophages from BAC-Retn mice (n = 5 per group). Data are presented with the SEM. BAT, brown adipose tissue; BM, bone marrow; L. int, large intestine; PBMC, peripheral blood mononuclear cells; S. int, small intestine. *P < 0.05, **P < 0.01 vs. saline.
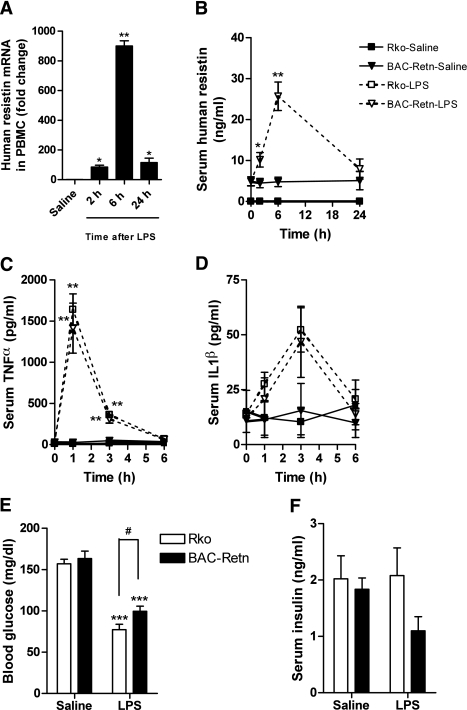
LPS-induced hypoglycemia is less severe in BAC-Retn mice.
In the mice fed normal chow, there were no appreciable differences between Rko and BAC-Retn mice in serum levels of TGs, FFAs, ketone, adiponectin, or leptin. LPS increased serum alanine aminotransferase (ALT), FFA, and leptin levels in both groups and decreased serum total and high-molecular-weight adiponectin levels (Table 1). Circulating levels of human resistin increased about fivefold from baseline within 6 h after LPS injection in BAC-Retn mice, which was consistent with the robust increase in human resistin expression and secretion observed in blood mononuclear cells (Fig. 2A and B). As has been previously described (33), LPS injection caused hypoglycemia acutely. Although LPS induced similar increases in serum TNF-α and IL1β levels in Rko and BAC-Retn mice (Fig. 2C and D), hypoglycemia was less severe in BAC-Retn compared with Rko mice (100 ± 6 vs. 77 ± 6 mg/dL, P < 0.05; Fig. 2E). Serum insulin levels were not different between groups (P = 0.32), suggesting human resistin attenuated hypoglycemia in acute endotoxemia via insulin resistance (Fig. 2F).
TABLE 1.
Serum metabolic profile 6 h after LPS administration
| Variable | Rko-Saline | BAC-Retn-Saline | Rko-LPS | BAC-Retn-LPS |
|---|---|---|---|---|
| Weight (g) | 29.4 ± 0.6 | 29.6 ± 0.8 | 29.5 ± 0.7 | 30.1 ± 0.5 |
| Triglyceride (mg/dL) | 40.3 ± 5.2 | 32.6 ± 1.8 | 38.0 ± 3.0 | 55.9 ± 6.7*† |
| Cholesterol (mg/dL) | 66.6 ± 5.3 | 67.5 ± 2.6 | 66.4 ± 2.5 | 72.1 ± 2.6 |
| FFAs (mEq/L) | 0.90 ± 0.05 | 0.85 ± 0.04 | 1.10 ± 0.05‡ | 1.22 ± 0.13* |
| Ketone (mg/dL) | 3.3 ± 0.5 | 5.5 ± 0.8 | 8.4 ± 1.0‡ | 7.2 ± 0.6 |
| Alanine aminotransferase (U/L) | 12 ± 1 | 14 ± 1 | 25 ± 2* | 44 ± 3*§ |
| Adiponectin (μg/mL) | 25.7 ± 0.9 | 26.3 ± 2.0 | 21.3 ± 1.3‡ | 20.2 ± 1.5‡ |
| HMW adiponectin (μg/mL) | 3.70 ± 0.13 | 3.45 ± 0.36 | 2.45 ± 0.28‡ | 2.25 ± 0.17‡ |
| Leptin (ng/mL) | 5.7 ± 1.0 | 4.8 ± 1.1 | 10.9 ± 1.7‡ | 10.6 ± 1.4‡ |
LPS (0.2 mg/kg) or normal saline (0.9%) was intraperitoneally injected in 12-week-old, normal-chow-fed Rko and BAC-Retn mice (n = 5–7 per group). Data are presented as means ± SEM.
BAC-Retn, BAC-transgenic; HMW, high-molecular-weight.
*P < 0.01 vs. saline;
†P < 0.05;
‡P < 0.05;
§P < 0.01 vs. Rko.
FIG. 2.
Changes in resistin, cytokines, and glucose in acute endotoxemia in BAC-Retn mice. Acute LPS (0.2 mg/kg) or normal saline (0.9%) intraperitoneal administration to Rko (□) and BAC-Retn (■) mice (n = 5–7 per group). A and B: Human resistin was induced in response to LPS in vivo in BAC-Retn mice. C and D: Serum TNF-α and IL1β levels increased similarly in Rko and BAC-Retn mice. E: LPS-induced hypoglycemia is less severe in BAC-Retn than in Rko mice at 6 h after LPS injection. F: Serum insulin levels in 6 h after LPS injection. PBMC, peripheral blood mononuclear cells. Data are presented with the SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. saline; #P < 0.05 vs. Rko.
Insulin resistance in BAC-Retn mice in acute endotoxemia.
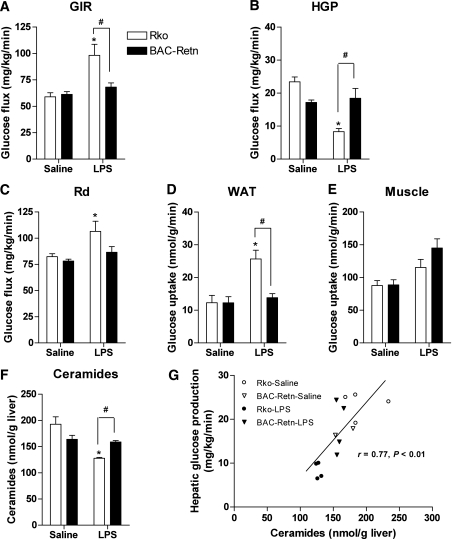
To examine the role of human resistin in glucose homeostasis in acute endotoxemia, we performed hyperinsulinemic-euglycemic clamp studies after LPS or saline injection. Compared with saline injection, LPS acutely suppressed the basal rate of hepatic glucose production in Rko mice by 61%, and in BAC-Retn mice by 40% (Supplementary Fig. 1A). The glucose infusion rate required to maintain euglycemia was 31% lower in BAC-Retn than in Rko mice, indicating that the BAC-Retn mice were more insulin resistant during acute endotoxemia (Fig. 3A). Hepatic insulin resistance in acute endotoxemia was manifested by a higher rate of glucose production (HGP) in BAC-Retn mice compared with Rko mice (Fig. 3B). In support of this result, both glucose-6-phosphatase (G6pase) and PEPCK expression levels were slightly higher in BAC-Retn mice than in Rko mice under clamp studies in acute endotoxemia, although the differences did not reach statistical significance (Supplementary Fig. 1B and C). Although the whole-body rate of glucose disposal (Rd) was not different between both groups, insulin-stimulated glucose uptake in WAT was 46% lower in BAC-Retn mice (Fig. 3C–E). Impaired glucose uptake in WAT in BAC-Retn mice was also consistent with significantly higher serum FFA levels compared with Rko mice, indicating less suppression of lipolysis by insulin during hyperinsulinemic clamp studies (0.13 ± 0.03 vs. 0.07 ± 0.03 mEq/L, P < 0.05).
FIG. 3.
Acute endotoxemia induces hepatic and adipose tissue insulin resistance in BAC-Retn mice. A–C: Hyperinsulinemic-euglycemic clamp analysis of BAC-Retn (■) vs. Rko (□) mice (n = 4 per group) in 6 h after LPS administration. D and E: Rate of insulin-stimulated glucose uptake in WAT (D) and skeletal muscle (E). F: Total ceramides content in liver. G: Correlation between hepatic ceramide content and hepatic glucose production (HGP) during the clamp study. Data are presented with the SEM. GIR, glucose infusion rate; Rd, whole-body rate of glucose disposal. *P < 0.05 vs. saline; #P < 0.05 vs. Rko.
To understand the possible mechanism of hepatic insulin resistance, we next measured lipids content in liver. Compared with Rko mice, BAC-Retn mice had a 24% higher total ceramides content in liver (Fig. 3F) during acute endotoxemia, with most ceramide species being elevated (Supplementary Fig. 1D). Moreover, there was a significant correlation between hepatic ceramide content and HGP during hyperinsulinemic clamp studies (r = 0.77, P < 0.01; Fig. 3G). However, there were no differences in hepatic diacylglycerol and TG levels between Rko and BAC-Retn mice (Supplementary Fig. 1E and F). Despite the changes in hepatic ceramide content, there were no differences in the expression of several genes involved in pathways of ceramide generation and salvage between Rko and BAC-Retn mice (Supplementary Fig. 1G) (34).
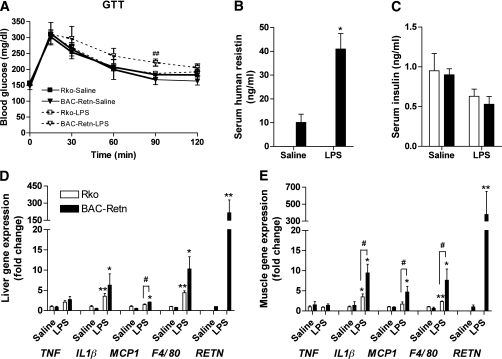
Impaired glucose tolerance in BAC-Retn mice in chronic endotoxemia.
BAC-Retn mice were studied under chronic experimental endotoxemia to mimic the chronic inflammation of obesity and type 2 diabetes. Body weight and blood glucose of chronic LPS-infused mice significantly decreased initially, similar to the effect of acute endotoxemia described above, but recovered later (Supplementary Fig. 2A and B). During chronic endotoxemia, glucose tolerance in BAC-Retn mice was significantly impaired relative to the Rko mice (Fig. 4A), while circulating resistin levels rose approximately fourfold in these mice (Fig. 4B). Serum insulin, TNF-α and IL1β levels were not different in both groups, suggesting that human resistin is responsible for glucose intolerance in chronic endotoxemia (Fig. 4C; Supplementary Fig. 2C and D).
FIG. 4.
Impaired glucose tolerance and exacerbated inflammation in chronically endotoxemic BAC-Retn mice. A: With chronic LPS (2 mg/kg/day) subcutaneous infusion, BAC-Retn mice show impaired glucose tolerance compared with Rko mice (n = 5–7 per group). B and C: Serum resistin (B) and insulin (C) levels in Rko (□) and BAC-Retn (■) mice. D and E: Gene-expression analysis in liver (D) and skeletal muscle (E). Data are presented with the SEM. GTT, glucose tolerance test; RETN, human resistin. *P < 0.05, **P < 0.01 vs. saline; #P < 0.05, ##P < 0.01 vs. Rko.
To explore the role of resistin on inflammation in chronic endotoxemia, we next performed gene-expression analysis. BAC-Retn mice had increased expression of proinflammatory cytokines as well as macrophage markers such as monocyte chemotactic protein-1 (MCP1) and F4/80 in liver and skeletal muscle (Fig. 4D and E), indicating resistin exacerbated inflammation and promoted tissue infiltration of macrophages in chronic endotoxemia but not in WAT (Supplementary Fig. 3). Although serum levels of TGs, FFA, and total and high-molecular-weight adiponectin decreased in chronic endotoxemia, there were no differences between the Rko and BAC-Retn mice (Table 2).
TABLE 2.
Serum metabolic profile in chronic endotoxemia
| Variable | Rko-Saline | BAC-Retn-Saline | Rko-LPS | BAC-Retn-LPS |
|---|---|---|---|---|
| Weight (g) | 28.0 ± 0.4 | 26.8 ± 0.8 | 26.2 ± 0.2 | 25.8 ± 0.3 |
| Triglycerides (mg/dL) | 85.7 ± 5.4 | 70.2 ± 1.5 | 64.0 ± 6.3* | 60.0 ± 2.8* |
| Cholesterol (mg/dL) | 80.8 ± 4.2 | 73.7 ± 1.2 | 72.3 ± 1.7 | 62.9 ± 5.2 |
| FFAs (mEq/L) | 0.77 ± 0.07 | 0.84 ± 0.08 | 0.41 ± 0.07† | 0.47 ± 0.04* |
| Ketone (mg/dL) | 2.6 ± 0.3 | 3.2 ± 0.8 | 2.0 ± 0.3 | 1.9 ± 0.1* |
| Alanine aminotransferase (U/L) | 7 ± 1 | 8 ± 1 | 6 ± 1 | 7 ± 2 |
| Adiponectin (μg/mL) | 25.2 ± 1.4 | 25.5 ± 1.2 | 15.9 ± 1.6† | 15.3 ± 0.7* |
| HMW adiponectin (μg/mL) | 3.60 ± 0.19 | 4.09 ± 0.99 | 1.57 ± 0.08† | 1.59 ± 0.11* |
LPS (2 mg/kg/day) or normal saline (0.9%) was subcutaneously infused with a minipump to 10-week-old, normal-chow-fed Rko and BAC-Retn mice for 10 days (n = 5–7 per group). Data are presented as means ± SEM.
BAC-Retn, BAC-transgenic; HMW, high-molecular-weight.
*P < 0.05;
†P < 0.01 vs. saline.
Hepatic insulin resistance in BAC-Retn mice in chronic endotoxemia.
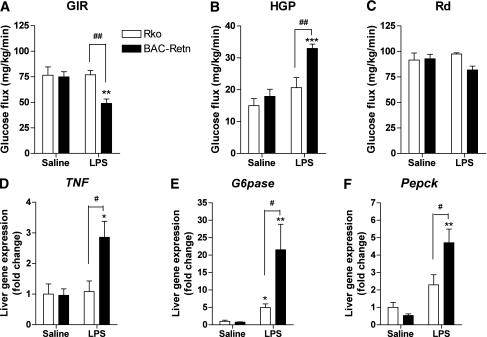
To determine the mechanism underlying the effect of humanized resistin induction on glucose homeostasis in chronic endotoxemia, we performed hyperinsulinemic-euglycemic clamp studies. The glucose infusion rate required to maintain euglycemia was significantly lower in BAC-Retn than in Rko mice (Fig. 5A). Although Rd was not different, HGP was 60% higher in BAC-Retn mice, indicating that human resistin induced severe hepatic insulin resistance in chronic endotoxemia (Fig. 5B and C). To understand the mechanism of hepatic insulin resistance, we examined gene-expression analysis in liver under clamp studies. TNF-α expression was approximately threefold higher in liver of BAC-Retn mice, suggesting resistin exacerbates inflammation in liver (Fig. 5D). Expression levels of G6pase and PEPCK were also significantly higher in BAC-Retn mice compared with Rko mice (fourfold and twofold, respectively; Fig. 5E and F), which is compatible with a higher rate of HGP in chronic endotoxemia. Measurements of lipids content in liver showed no differences in hepatic TG and total ceramides content between Rko and BAC-Retn mice (Supplementary Fig. 4A and B).
FIG. 5.
Hepatic insulin resistance in BAC-Retn mice in chronic endotoxemia. A–C: Hyperinsulinemic-euglycemic clamp analysis of BAC-Retn (■) mice vs. Rko (□) mice (n = 5–8 per group) in chronic endotoxemia. D–F: Gene-expression analysis in liver. Data are presented with the SEM. GIR, glucose infusion rate; HGP, hepatic glucose production; Rd, whole-body rate of glucose disposal. *P < 0.05, **P < 0.01, ***P < 0.001 vs. saline; #P < 0.05, ##P < 0.01 vs. Rko.
DISCUSSION
Murine resistin is secreted by adipocytes, whereas human resistin is secreted by mononuclear cells. Although genetic and pharmacologic studies strongly support links between resistin, obesity, and insulin resistance in mice, the role of human resistin as a modulator of metabolism in inflammatory states is not well understood. The present finding that induction of human resistin alters glucose metabolism in endotoxemia clearly links human resistin to inflammation-induced insulin resistance. Therefore, this study provides much needed insight into the pathophysiologic role of human resistin in various inflammatory states associated with insulin resistance, ranging from sepsis to type 2 diabetes and atherosclerosis. In this respect, it is noteworthy that several epidemiologic studies have recently showed close relationships between resistin and type 2 diabetes, cardiovascular disease, and inflammatory markers (17,18,21,35).
Early stages of sepsis and experimental endotoxemia are associated with hypoglycemia through increased glucose disposal by macrophage-rich tissues as well as decreased glucose production in humans as well as animals (33,36–38). In line with previous reports, LPS administration acutely caused hypoglycemia in Rko and BAC-Retn mice, with similar robust increases in serum proinflammatory cytokines, which are known to cause hypoglycemia (33,36). LPS increased serum leptin levels and decreased adiponectin, which can be explained by cytokine responses (39–42). During acute LPS-induced hypoglycemia, however, the induction of human resistin increased hepatic glucose production and decreased glucose uptake, thereby reducing the severity of hypoglycemia. Intriguingly, mice lacking resistin are more prone to fasting-induced hypoglycemia than wild-type mice (9), suggesting similar functions of resistin across species, albeit in different physiologic settings. Thus the human-specific expression pattern of resistin facilitates its function as a glucose counterregulatory hormone to mitigate hypoglycemia during acute endotoxemia. Of note, the metabolic effects of chronic endotoxemia in wild-type mice (31) are likely through a resistin-independent mechanism because murine resistin is not induced by LPS (Supplementary Fig. 5A–C) (43,44), although it was recently reported to be induced by homocysteine, which is proinflammatory among other pleiotropic effects (45).
Animal studies on the acute effect of LPS on insulin sensitivity have yielded conflicting results (46,47). In the setting of acute endotoxemia, we found that insulin sensitivity was enhanced in liver as well as in WAT of Rko mice and that human resistin abrogated this insulin-sensitizing effect of LPS, inducing insulin resistance. Our finding that hepatic ceramide content correlated with hepatic insulin resistance is consistent with a previous report (48). Ceramide has been shown to induce insulin resistance by inhibiting insulin signaling. Interestingly, TNF-α can acutely stimulate the accumulation of ceramide and various ceramide metabolites (49). Although there we did not detect differences in ceramide-related gene expression in liver of the BAC-Retn mice, the changes of hepatic ceramide content in acute endotoxemia could be due to changes in enzyme activity or to alterations in substrate flux.
During chronic endotoxemia, induction of human resistin promoted glucose intolerance and hepatic insulin resistance, suggesting its role as a link between innate immunity, inflammation, and insulin resistance. Epidemiologic studies provide strong evidence that chronic inflammation and obesity-induced insulin resistance are closely related (50–53). Experimental endotoxemia in humans, akin to states of infection and sepsis, also induces systemic insulin resistance as well as increased circulating resistin (16,37,54). Here we found that, in mice, chronic endotoxemia robustly induced inflammation in liver and skeletal muscle and was exacerbated by induction of human resistin. Our finding that human resistin promotes insulin resistance by inducing TNF-α and upregulating G6pase and PEPCK in liver are consistent with previous reports (15,55,56).
Although hepatic ceramide accumulation can at least partly explain hepatic insulin resistance in acute endotoxemia, tissue inflammation exacerbated by resistin, not hepatic ceramide, seems to be a plausible mechanism for insulin resistance in chronic endotoxemia. Recently, regulatory T-cells have been demonstrated to contribute to macrophage recruitment and adipose inflammation in obesity, and resistin has been shown to indirectly enhance regulatory T-cells (57,58). Thus, T-cells, in addition to macrophages, may possibly be involved in the enhanced inflammation of the BAC-Retn mice. It remains unanswered how resistin functions in various tissues or cells, because the receptor for resistin has not yet been uncovered.
In summary, we have demonstrated that human resistin modulates glucose homeostasis under inflammation using a humanized resistin mouse model. Human resistin is induced in response to inflammation. Resistin attenuates endotoxemia-induced hypoglycemia by inducing insulin resistance in liver and WAT and promotes hepatic insulin resistance by exacerbating inflammatory responses in chronic endotoxemia. Increased inflammation is accompanied by increased infiltration of macrophages, which can, in turn, augment resistin induction. Thus, induction of resistin is expected to be a component of the pathophysiology of inflammation-induced insulin resistance in humans. The mouse model of humanized resistin expression reported here can be used to provide a better understanding and potential amelioration of the metabolic pathology of inflammatory states in humans.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK P01-DK-49210 to M.A.L. and R.S.A.), and by the Cox Institute for Medical Research. M.Q. was supported by an ADA-Takeda Mentor-Based Research Fellowship, and H.-K.P. was supported by the Charles N. Cohen Fellowship.
M.Q. is currently employed by Merck & Co. M.A.L. is a board member of Eli Lilly and Co., Dr. Reddy's Pharmaceutical Company, and Novartis. No other potential conflicts of interest relevant to this article were reported.
H.-K.P. designed experiments, researched data, and wrote the manuscript. M.Q. designed experiments, researched data, and edited the manuscript. E.R.B. researched data and edited the manuscript. R.S.A. and M.A.L. designed experiments, wrote and edited the manuscript, and obtained funding supporting the research.
The authors thank Daniel Schwartz (University of Pennsylvania) for critically reading the manuscript, the Penn Diabetes and Endocrinology Research Center (NIH P30-DK-19525) for providing outstanding core services, and J. Richa of the Transgenic and Chimeric Mouse Core, H. Collins of the Radioimmunoassay/Biomarkers Core, R. Dhir of the Mouse Phenotyping, Physiology and Metabolism Core (also supported by NIDDK P01-DK-49210) for technical help, and the Yale Mouse Metabolic Phenotyping Center (U24-DK-59635) for diacylglycerol and ceramides assays.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1416/-/DC1.
M.Q. is currently affiliated with the Department of Metabolic Disorders, Merck Research Laboratories, Rahway, New Jersey.
REFERENCES
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782–787 10.1038/414782a [DOI] [PubMed] [Google Scholar]
- 2.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005;115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 10.1038/nature05482 [DOI] [PubMed] [Google Scholar]
- 5.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature 2001;409:307–312 10.1038/35053000 [DOI] [PubMed] [Google Scholar]
- 6.Rajala MW, Obici S, Scherer PE, Rossetti L. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J Clin Invest 2003;111:225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muse ED, Obici S, Bhanot S, et al. Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest 2004;114:232–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li FP, He J, Li ZZ, Luo ZF, Yan L, Li Y. Effects of resistin expression on glucose metabolism and hepatic insulin resistance. Endocrine 2009;35:243–251 10.1007/s12020-009-9148-4 [DOI] [PubMed] [Google Scholar]
- 9.Banerjee RR, Rangwala SM, Shapiro JS, et al. Regulation of fasted blood glucose by resistin. Science 2004;303:1195–1198 10.1126/science.1092341 [DOI] [PubMed] [Google Scholar]
- 10.Qi Y, Nie Z, Lee YS, et al. Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes 2006;55:3083–3090 10.2337/db05-0615 [DOI] [PubMed] [Google Scholar]
- 11.Patel L, Buckels AC, Kinghorn IJ, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun 2003;300:472–476 10.1016/S0006-291X(02)02841-3 [DOI] [PubMed] [Google Scholar]
- 12.Savage DB, Sewter CP, Klenk ES, et al. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes 2001;50:2199–2202 10.2337/diabetes.50.10.2199 [DOI] [PubMed] [Google Scholar]
- 13.Kaser S, Kaser A, Sandhofer A, Ebenbichler CF, Tilg H, Patsch JR. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun 2003;309:286–290 10.1016/j.bbrc.2003.07.003 [DOI] [PubMed] [Google Scholar]
- 14.Lehrke M, Reilly MP, Millington SC, Iqbal N, Rader DJ, Lazar MA. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med 2004;1:e45. 10.1371/journal.pmed.0010045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol 2005;174:5789–5795 [DOI] [PubMed] [Google Scholar]
- 16.Anderson PD, Mehta NN, Wolfe ML, et al. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab 2007;92:2272–2279 10.1210/jc.2006-2545 [DOI] [PubMed] [Google Scholar]
- 17.Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care 2004;27:2450–2457 10.2337/diacare.27.10.2450 [DOI] [PubMed] [Google Scholar]
- 18.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 2005;111:932–939 10.1161/01.CIR.0000155620.10387.43 [DOI] [PubMed] [Google Scholar]
- 19.Axelsson J, Bergsten A, Qureshi AR, et al. Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney Int 2006;69:596–604 10.1038/sj.ki.5000089 [DOI] [PubMed] [Google Scholar]
- 20.Senolt L, Housa D, Vernerová Z, et al. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann Rheum Dis 2007;66:458–463 10.1136/ard.2006.054734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundén-Cullberg J, Nyström T, Lee ML, et al. Pronounced elevation of resistin correlates with severity of disease in severe sepsis and septic shock. Crit Care Med 2007;35:1536–1542 10.1097/01.CCM.0000266536.14736.03 [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Chan JL, Yiannakouris N, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab 2003;88:4848–4856 10.1210/jc.2003-030519 [DOI] [PubMed] [Google Scholar]
- 23.Vozarova de Courten B, Degawa-Yamauchi M, Considine RV, Tataranni PA. High serum resistin is associated with an increase in adiposity but not a worsening of insulin resistance in Pima Indians. Diabetes 2004;53:1279–1284 10.2337/diabetes.53.5.1279 [DOI] [PubMed] [Google Scholar]
- 24.Youn BS, Yu KY, Park HJ, et al. Plasma resistin concentrations measured by enzyme-linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus. J Clin Endocrinol Metab 2004;89:150–156 10.1210/jc.2003-031121 [DOI] [PubMed] [Google Scholar]
- 25.Chen CC, Li TC, Li CI, Liu CS, Wang HJ, Lin CC. Serum resistin level among healthy subjects: relationship to anthropometric and metabolic parameters. Metabolism 2005;54:471–475 10.1016/j.metabol.2004.10.015 [DOI] [PubMed] [Google Scholar]
- 26.Gerber M, Boettner A, Seidel B, et al. Serum resistin levels of obese and lean children and adolescents: biochemical analysis and clinical relevance. J Clin Endocrinol Metab 2005;90:4503–4509 10.1210/jc.2005-0437 [DOI] [PubMed] [Google Scholar]
- 27.Hivert MF, Sullivan LM, Fox CS, et al. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocrinol Metab 2008;93:3165–3172 10.1210/jc.2008-0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazar MA. Resistin- and Obesity-associated metabolic diseases. Horm Metab Res 2007;39:710–716 10.1055/s-2007-985897 [DOI] [PubMed] [Google Scholar]
- 29.Qatanani M, Szwergold NR, Greaves DR, Ahima RS, Lazar MA. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest 2009;119:531–539 10.1172/JCI37273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomaru T, Steger DJ, Lefterova MI, Schupp M, Lazar MA. Adipocyte-specific expression of murine resistin is mediated by synergism between peroxisome proliferator-activated receptor gamma and CCAAT/enhancer-binding proteins. J Biol Chem 2009;284:6116–6125 10.1074/jbc.M808407200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–1772 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- 32.Imai Y, Varela GM, Jackson MB, Graham MJ, Crooke RM, Ahima RS. Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology 2007;132:1947–1954 10.1053/j.gastro.2007.02.046 [DOI] [PubMed] [Google Scholar]
- 33.Raetzsch CF, Brooks NL, Alderman JM, et al. Lipopolysaccharide inhibition of glucose production through the Toll-like receptor-4, myeloid differentiation factor 88, and nuclear factor kappa b pathway. Hepatology 2009;50:592–600 10.1002/hep.22999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowart LA. Sphingolipids: players in the pathology of metabolic disease. Trends Endocrinol Metab 2009;20:34–42 10.1016/j.tem.2008.09.004 [DOI] [PubMed] [Google Scholar]
- 35.Koch A, Gressner OA, Sanson E, Tacke F, Trautwein C. Serum resistin levels in critically ill patients are associated with inflammation, organ dysfunction and metabolism and may predict survival of non-septic patients. Crit Care 2009;13:R95. 10.1186/cc7925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oguri S, Motegi K, Iwakura Y, Endo Y. Primary role of interleukin-1 alpha and interleukin-1 beta in lipopolysaccharide-induced hypoglycemia in mice. Clin Diagn Lab Immunol 2002;9:1307–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agwunobi AO, Reid C, Maycock P, Little RA, Carlson GL. Insulin resistance and substrate utilization in human endotoxemia. J Clin Endocrinol Metab 2000;85:3770–3778 10.1210/jc.85.10.3770 [DOI] [PubMed] [Google Scholar]
- 38.van der Crabben SN, Blümer RM, Stegenga ME, et al. Early endotoxemia increases peripheral and hepatic insulin sensitivity in healthy humans. J Clin Endocrinol Metab 2009;94:463–468 10.1210/jc.2008-0761 [DOI] [PubMed] [Google Scholar]
- 39.Sarraf P, Frederich RC, Turner EM, et al. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med 1997;185:171–175 10.1084/jem.185.1.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1 beta mediates leptin induction during inflammation. Am J Physiol 1998;274:R204–R208 [DOI] [PubMed] [Google Scholar]
- 41.Fasshauer M, Kralisch S, Klier M, et al. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun 2003;301:1045–1050 10.1016/S0006-291X(03)00090-1 [DOI] [PubMed] [Google Scholar]
- 42.Bruun JM, Lihn AS, Verdich C, et al. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab 2003;285:E527–E533 [DOI] [PubMed] [Google Scholar]
- 43.Rajala MW, Lin Y, Ranalletta M, et al. Cell type-specific expression and coregulation of murine resistin and resistin-like molecule-alpha in adipose tissue. Mol Endocrinol 2002;16:1920–1930 10.1210/me.2002-0048 [DOI] [PubMed] [Google Scholar]
- 44.Brown R, Imran SA, Wilkinson M. Lipopolysaccharide (LPS) stimulates adipokine and socs3 gene expression in mouse brain and pituitary gland in vivo, and in N-1 hypothalamic neurons in vitro. J Neuroimmunol 2009;209:96–103 10.1016/j.jneuroim.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Jiang C, Xu G, et al. Homocysteine upregulates resistin production from adipocytes in vivo and in vitro. Diabetes 2008;57:817–827 10.2337/db07-0617 [DOI] [PubMed] [Google Scholar]
- 46.Lang CH, Spolarics Z, Ottlakan A, Spitzer JJ. Effect of high-dose endotoxin on glucose production and utilization. Metabolism 1993;42:1351–1358 10.1016/0026-0495(93)90137-D [DOI] [PubMed] [Google Scholar]
- 47.Virkamäki A, Yki-Järvinen H. Mechanisms of insulin resistance during acute endotoxemia. Endocrinology 1994;134:2072–2078 10.1210/en.134.5.2072 [DOI] [PubMed] [Google Scholar]
- 48.Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 2007;5:167–179 10.1016/j.cmet.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 49.Summers SA, Nelson DH. A role for sphingolipids in producing the common features of type 2 diabetes, metabolic syndrome X, and Cushing’s syndrome. Diabetes 2005;54:591–602 10.2337/diabetes.54.3.591 [DOI] [PubMed] [Google Scholar]
- 50.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 1999;19:972–978 [DOI] [PubMed] [Google Scholar]
- 51.Festa A, D’Agostino R, Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000;102:42–47 [DOI] [PubMed] [Google Scholar]
- 52.Pannacciulli N, Cantatore FP, Minenna A, Bellacicco M, Giorgino R, De Pergola G. C-reactive protein is independently associated with total body fat, central fat, and insulin resistance in adult women. Int J Obes Relat Metab Disord 2001;25:1416–1420 10.1038/sj.ijo.0801719 [DOI] [PubMed] [Google Scholar]
- 53.Hak AE, Pols HA, Stehouwer CD, et al. Markers of inflammation and cellular adhesion molecules in relation to insulin resistance in nondiabetic elderly: the Rotterdam study. J Clin Endocrinol Metab 2001;86:4398–4405 10.1210/jc.86.9.4398 [DOI] [PubMed] [Google Scholar]
- 54.Mehta NN, McGillicuddy FC, Anderson PD, et al. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 2010;59:172–181 10.2337/db09-0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun 2005;334:1092–1101 10.1016/j.bbrc.2005.06.202 [DOI] [PubMed] [Google Scholar]
- 56.Luo Z, Zhang Y, Li F, et al. Resistin induces insulin resistance by both AMPK-dependent and AMPK-independent mechanisms in HepG2 cells. Endocrine 2009;36:60–69 10.1007/s12020-009-9198-7 [DOI] [PubMed] [Google Scholar]
- 57.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009;15:930–939 10.1038/nm.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Son YM, Ahn SM, Kim GR, et al. Resistin enhances the expansion of regulatory T cells through modulation of dendritic cells. BMC Immunol 2010;11:33. 10.1186/1471-2172-11-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.