Abstract
OBJECTIVE
The reversible attachment of small ubiquitin-like modifier (SUMO) proteins controls target localization and function. We examined an acute role for the SUMOylation pathway in downstream events mediating insulin secretion.
RESEARCH DESIGN AND METHODS
We studied islets and β-cells from mice and human donors, as well as INS-1 832/13 cells. Insulin secretion, intracellular Ca2+, and β-cell exocytosis were monitored after manipulation of the SUMOylation machinery. Granule localization was imaged by total internal reflection fluorescence and electron microscopy; immunoprecipitation and Western blotting were used to examine the soluble NSF attachment receptor (SNARE) complex formation and SUMO1 interaction with synaptotagmin VII.
RESULTS
SUMO1 impairs glucose-stimulated insulin secretion by blunting the β-cell exocytotic response to Ca2+. The effect of SUMO1 to impair insulin secretion and β-cell exocytosis is rapid and does not require altered gene expression or insulin content, is downstream of granule docking at the plasma membrane, and is dependent on SUMO-conjugation because the deSUMOylating enzyme, sentrin/SUMO-specific protease (SENP)-1, rescues exocytosis. SUMO1 coimmunoprecipitates with the Ca2+ sensor synaptotagmin VII, and this is transiently lost upon glucose stimulation. SENP1 overexpression also disrupts the association of SUMO1 with synaptotagmin VII and mimics the effect of glucose to enhance exocytosis. Conversely, SENP1 knockdown impairs exocytosis at stimulatory glucose levels and blunts glucose-dependent insulin secretion from mouse and human islets.
CONCLUSIONS
SUMOylation acutely regulates insulin secretion by the direct and reversible inhibition of β-cell exocytosis in response to intracellular Ca2+ elevation. The SUMO protease, SENP1, is required for glucose-dependent insulin secretion.
Small ubiquitin-like modifier (SUMO) proteins belong to the family of ubiquitin-like peptides (1,2). The attachment of SUMO, called SUMOylation, modifies target localization or function and is implicated in mitosis, DNA repair, nuclear import, and the control of transcription factors, including pancreatic and duodenal homeobox-1 and MafA, in pancreatic β-cells (3–8). Recent evidence also suggests a role for SUMOylation in signaling from the insulin granule back to the nucleus (6,9). There are three known functional SUMO isoforms (SUMO1, -2, and -3), which are conjugated to target proteins through a well-established series of reactions (10). The final step in this series involves the transfer of SUMO from the ubiquitin-conjugating enzyme E2I (UBE2I, also called Ubc9) to a target lysine. The action of sentrin/SUMO-specific proteases (SENPs) reverses this process.
SUMOylation also regulates membrane proteins, including K+ channels (11–14), nonselective cation channels (15), and kainate receptors (16), and is suggested to control Ca2+ influx at nerve terminals (17). Membrane proteins are critical to insulin secretion from β-cells. After translocation from the cell interior, secretory granules are docked to the plasma membrane by formation of a soluble NSF attachment receptor (SNARE) protein complex in conjunction with Munc18a (18–20). This complex interacts closely with the voltage-dependent Ca2+ channels (21,22). Ca2+ sensing at the site of exocytosis is mediated, at least in part, by synaptotagmin VII (23,24). An interaction between the SUMOylation and exocytotic pathways, and the impact of this interaction on glucose-dependent insulin secretion, has not been explored.
In the present work, we find that SUMO1 impairs glucose-stimulated insulin secretion but not insulin content or Ca2+ responses. Rather, SUMOylation directly inhibits the β-cell exocytotic response to membrane depolarization or infusion of Ca2+, an effect that is acute, not requiring altered gene expression, and reversible by the SUMO protease SENP1. This inhibitory effect is downstream of granule docking at the plasma membrane. A role for SUMO1 in downstream Ca2+-dependent exocytosis is further suggested by its interaction with synaptotagmin VII. This association is transiently lost upon glucose-stimulation but returns within 30–60 min, correlating with glucose-dependent exocytosis. Overexpression of SENP1 prevents interaction of SUMO1 with synaptotagmin VII and augments exocytosis at low glucose and after prolonged glucose stimulation. Finally, knockdown of the deSUMOylating enzyme inhibits β-cell exocytosis and glucose-dependent insulin secretion from mouse and human islets. Thus, SUMO1 impairs insulin exocytosis, possibly through a direct interaction with the exocytotic machinery, and the deSUMOylating enzyme SENP1 is required for glucose-dependent insulin secretion.
RESEARCH DESIGN AND METHODS
Cells and cell culture.
Human embryonic kidney (HEK)-293 cells were cultured in DMEM with 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin at 37°C and 5% CO2. INS-1 832/13 cells (a gift from Dr. Christopher Newgard, Duke University) were cultured in RPMI-1640 containing 11.1 mmol/L glucose and supplemented with 10% FBS, 10 mmol/L HEPES, 2 mmol/L l-glutamine, 1 mmol/L sodium pyruvate, 50 μmol/L β-mercaptoethanol, and 100 units/mL penicillin/streptomycin at 37°C and 5% CO2. Islets from male C57/BL6 mice were isolated by collagenase digestion. Human islets from 32 healthy donors (mean age 48.4 ± 2.3 years) were provided by the clinical islet laboratory at the University of Alberta. Islets were dispersed to single cells by shaking in Ca2+-free buffer and plated in 35-mm culture dishes. Mouse islets and cells were cultured in RPMI with l-glutamine, 10% FBS, and 100 units/mL penicillin/streptomycin. Human islets and cells were cultured in low-glucose (1 g/L) DMEM with l-glutamine, 110 mg/L sodium pyruvate, 10% FBS, and 100 units/mL penicillin/streptomycin. All studies were approved by the animal care and use committee and the human research ethics board at the University of Alberta.
Constructs, adenoviruses, and recombinant peptides.
The human SUMO1-YFP (25) in the pEYFP-C1 vector (Clontech, Palo Alto, CA) was a gift from Dr. Heidi McBride (University of Ottawa). The human SUMO1, Ubc9, and SENP1 constructs were in the pCMV6-XL4 vector and were from Origene Technologies (Rockville, MD). The pIRES-EGFP vector (Clontech) or pcDNA3.1 were used as controls. Recombinant human SUMO1 protein was from GeneTex (San Antonio, TX); SENP1 and Ubc9 enzymes were from Enzo Life Sciences (Plymouth Meeting, PA). Glutathione S-transferase (GST) was from Sigma-Aldrich Canada (Oakville, Canada). Recombinant adenoviruses expressing green fluorescent protein (GFP) (Ad-GFP), SUMO1 (Ad-SUMO1), or SENP1 (Ad-SENP1) were created using pAdtrackCMV and the AdEasy system (www.coloncancer.org). Three separate SENP1-targeted small-hairpin RNA (shRNA) constructs and a scrambled control were designed against identical human and mouse sequences using Genscript siRNA target-finder software (Genscript, Piscataway, NJ). These were synthesized as hairpin oligos with BamHI and HindIII restriction sites on the 5′ and 3′ ends and ligated into the pRNATH1.1/shuttle vector. Knockdown was tested by quantitative real-time PCR and Western blot, followed by the production of the recombinant adenovirus (Ad-shSENP1 and Ad-shScramble) in HEK-293 cells using the Adeno-X Expression System 1 (Clontech).
Insulin secretion measurements.
Insulin secretion measurements were performed at 37°C in Krebs-Ringer buffer (KRB) (in mmol/L: 115 NaCl, 5 KCl, 24 NaHCO3, 2.5 CaCl2, 1 MgCl2, 10 HEPES; and 0.1% BSA, pH 7.4). Twenty-five islets per group were preincubated for 2 h in 1 mmol/L glucose KRB then for 1 h in KRB at 1 mmol/L glucose followed by 1 h with 16.7 mmol/L glucose. INS-1 832/13 cells were cultured overnight in RPMI with 5 mmol/L glucose then preincubated in KRB for 30 min followed by a 1-h incubation in KRB with 2.5 or 15 mmol/L glucose and acid/ethanol extraction for insulin content. Islet perifusion was performed at 37°C using a Brandel SF-06 system (Gaithersburg, MD) after a 2-h preincubation in KRB with 1 mmol/L glucose. Twenty-five islets per lane were perifused (0.25 mL/min) with KRB. Glucose was increased as indicated. Samples were stored at −20°C and assayed for insulin via enzyme-linked immunosorbent assay (Alpco, Salem, NH).
Immunoprecipitation and immunoblotting.
INS-1 832/13 cells or human islets, after treatment or transfection as indicated, were lysed in either SNARE immunoprecipitation buffer (in mmol/L: 100 KCl, 1 EDTA, and 20 HEPES, pH 7.4, with 1.5% Triton-X100 and protease inhibitor cocktail) (Fig. 2C), or SUMO lysis buffer (in mmol/L: 100 NaCl, 40 KCl, 1 EDTA, and 20 HEPES, pH 7.4, with 10% glycerol, 1% Triton X-100, and 25 mmol/L N-ethylmaleimide and protease inhibitor cocktail) (Fig. 5). For immunoprecipitation, 500 μg total cell lysates were precleared with 20 μL of protein G-Sepharose or 30 μL protein A agarose. Lysates were incubated with 5 μL rabbit antisynaptotagmin VII (Synaptic Systems, Goettingen, Germany) and 100 μL protein G slurry (4°C overnight), 5 μg immobilized anti–SUMO1-agarose (4°C overnight; Santa Cruz Biotechnology, Santa Cruz, CA), or 4 μg protein A agarose cross-linked anti–syntaxin-1A (40°C for 40 min; Synaptic Systems). Immunoprecipitates were washed four times with PBS with 0.5% NP-40. These, or whole cell lysates, were separated using SDS-PAGE, transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA), probed with primary antibodies (Living Colors AV JL-8 antibody, Clontech; anti-SUMO1, anti-Ubc9, anti–syntaxin-1A, and anti–β-actin, Santa Cruz Biotechnology; antisyaptotagmin VII, Synaptic Systems; anti-Munc18a, Transduction Laboratory; anti–synaptosomal-associated protein 25 (SNAP-25), Sternberger; anti-SENP1, AbCam; and anti–vesicle-associated membrane protein 2 (VAMP2), a gift from Dr. Anson Lowe, Stanford University), and detected with peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology).
Total internal reflection fluorescence, electron microscopy, and Ca2+ imaging.
Total internal reflection fluorescence (TIRF) imaging was performed as described (26) on INS-1 832/13 cells transfected with islet amyloid polypeptide–mCherry that localizes to insulin granules together with a control plasmid (pcDNA3.1) or SUMO1. Electron microscopy was performed (26) on INS-1 832/13 cells infected with either Ad-GFP or Ad-SUMO1 and cultured for 48 h. For Ca2+ imaging, islets infected with either Ad-GFP or Ad-SUMO1 and cultured for 48 h were incubated for 45 min with 3 μmol/L Fura-2-AM (Invitrogen, Carlsbad, CA) and 0.06% pluronic acid (Invitrogen) in an extracellular calcium-imaging solution containing (in mmol/L) 130 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 NaHCO3, and 10 HEPES (pH 7.4 with NaOH). Islets were then imaged in fresh imaging solution with 0.5 mmol/L glucose and without Fura-2-AM or pluronic acid at 37°C with constant bath perfusion. Glucose and KCl were increased as indicated. Fluorescence recordings were obtained every 5 s. Images were analyzed with Image Pro Plus version 6.2 (Media Cybernetics) or Ratio Cam software (Metamorph).
Electrophysiology.
We used the standard whole-cell technique with the sine plus DC lockin function of an EPC10 amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany). Experiments were performed at 32–35°C. Bath solution for depolarization trains contained (in mmol/L) 118 NaCl, 20 TEA, 5.6 KCl, 1.2 MgCl2 ⋅ 6H2O, 2.6 CaCl2, 5 glucose, and 5 HEPES (pH 7.4 with NaOH). The pipette solution for depolarization trains contained (in mmol/L) 125 Cs-glutamate, 10 CsCl, 10 NaCl, 1 MgCl2 ⋅ 6H2O, 0.05 EGTA, 5 HEPES, 0.1 cAMP, and 3 MgATP (pH 7.15 with CsOH). When exocytosis was initiated by dialysis of a Ca2+/EGTA buffer (200 nmol/L free Ca2+) the intracellular solution contained (in mmol/L) 125 K-glutamate, 10 NaCl, 10 KCl, 1 MgCl2 ⋅ 6H2O, 5 CaCl2, 10 EGTA, 5 HEPES, and 3 MgATP (pH 7.1 with KOH). The extracellular solution contained (in mmol/L) 138 NaCl, 5.6 KCl, 1.2 MgCl2 ⋅ 6H2O, 2.6 CaCl2, 5 glucose, and 5 HEPES (pH 7.4 with NaOH). Patch pipettes, pulled from borosilicate glass and coated with Sylgard, had resistances of 3–4 MΩ when filled with pipette solution. Whole-cell capacitance responses were normalized to initial cell size and expressed as femtofarad per picofarad (fF/pF). Mouse β-cells were identified by size and the presence of a voltage-gated Na+ current that inactivates at approximately –90 mV (27), whereas human β-cells were positively identified after the experiment by insulin immunostaining.
Data analysis.
Data were analyzed using FitMaster version 2.32 (HEKA Electronik) and SigmaPlot 10 (Systat Software, Point Richmond, CA) and compared by multiple ANOVA and Student t test. Data are expressed as means ± SE, and P < 0.05 is considered significant.
RESULTS
SUMO1 inhibits downstream events in glucose-stimulated insulin secretion.
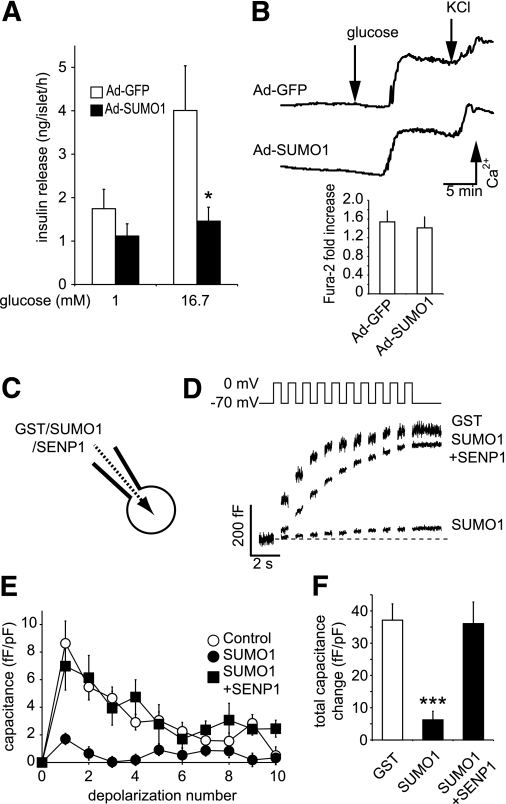
Overexpression of SUMO1 inhibits voltage-dependent K+ (Kv) currents and increases action potential duration in rodent and human β-cells (13). However, unlike the insulinotropic effect of Kv current blockade (28,29), we find that overexpression of SUMO1 inhibited glucose-stimulated insulin secretion from mouse islets (n = 3, P < 0.05) (Fig. 1A). This was not attributable to reduced islet insulin content (data not shown) or impaired intracellular Ca2+ responses to glucose (n = 6) (Fig. 1B), suggesting that the inhibitory effect lies downstream of Ca2+ entry.
FIG. 1.
SUMOylation impairs glucose-stimulated insulin secretion by acute inhibition of β-cell exocytosis. A: Insulin secretion from isolated mouse islets expressing either GFP alone (Ad-GFP, □) or SUMO1 (Ad-SUMO1, ■). B: Intracellular Ca2+ responses to glucose and KCl in these islets by ratiometric imaging of Fura-2-AM. The glucose-stimulated increase in the Fura-2 ratio is shown at the bottom. C: Single-cell experiments were performed on β-cells by whole-cell patch clamp, allowing the direct and acute intracellular dialysis of a control peptide (GST), SUMO1, or SENP1. D: Mouse β-cell exocytosis, measured as an increase in capacitance (cell size) during a train of ten 500-ms depolarizations (top), after ~4 min of dialysis with GST, SUMO1, or SUMO1 + SENP1. E: Average capacitance response to each step-wise depolarization. F: The total capacitance response over the train of depolarizations. *P < 0.05; ***P < 0.001 vs. controls.
To examine whether acute SUMOylation affects the downstream mechanism of insulin secretion, recombinant proteins were dialyzed directly into mouse β-cells for ~4 min via a patch-clamp pipette (Fig. 1C). Exocytosis was then measured as the whole-cell capacitance response to a train of membrane depolarizations (Fig. 1D–F). β-Cells dialyzed with a control GST peptide (2 μg/mL) displayed robust exocytosis (n = 41), whereas recombinant SUMO1 (2 μg/mL) blunted the response by 85% (P < 0.001, n = 19). This required SUMO conjugation because it could be readily reversed by codialysis of the SUMO protease SENP1 (6 μg/mL; n = 19).
SUMOylation increases granule docking and SNARE complex formation.
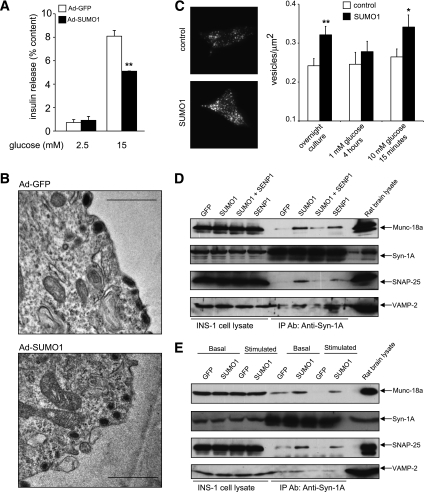
A lack of effect of SUMO1 on the intracellular Ca2+ response, together with the acute inhibition of exocytosis by SUMO1, suggests a role in regulating downstream events in insulin secretion. Similar to mouse islets, glucose-stimulated insulin secretion also was impaired in INS-1 832/13 cells overexpressing SUMO1 (Fig. 2A). Dense-core granules were clearly seen to be morphologically docked at the plasma membrane by electron microscopy of cells overexpressing either GFP or SUMO1 (Fig. 2B; representative of 33 and 31 images, respectively). Indeed, membrane-associated secretory granule density, monitored by TIRF microscopy, was increased by expression of SUMO1 (n = 79) compared with control (n = 50, P < 0.01) (Fig. 2C). This was normalized by a 4-h culture at 1 mmol/L glucose and increased again in SUMO1-expressing (n = 67) versus control (n = 57, P < 0.05) cells following a 15-min glucose stimulation (Fig. 2C).
FIG. 2.
SUMOylation does not impair secretory granule docking. A: Insulin secretion from INS-1 832/13 cells expressing either GFP alone (Ad-GFP, □) or SUMO1 (Ad-SUMO1, ■). B: Representative electron micrographs of INS-1 832/13 cells expressing either GFP alone (Ad-GFP) or SUMO1 (Ad-SUMO1). Scale bar represents 1 μm. C: Membrane-localized secretory granules (labeled with IAPP-mCherry) imaged by TIRF microscopy of INS-1 832/13 cells expressing SUMO1 or control vector. Representative images obtained under standard culture conditions (left). Average secretory granule density at the plasma membrane (right) following standard overnight culture at 11.1 mmol/L glucose or 4-h culture at 1 mmol/L glucose followed by acute (15 min) stimulation at 10 mmol/L glucose. D: Munc-18a/SNARE complex formation in INS-1 832/13 cells expressing GFP, SUMO1, and/or SENP1, assessed by immunoprecipitation (IP) of syntaxin 1A (Syn-1A) and blotting for Munc-18a, Syn-1A, SNAP-25, and VAMP2 under standard culture conditions. E: Same as D but after a 2-h incubation at 1 mmol/L glucose and following 15 min at 15 mmol/L glucose (basal and stimulated). *P < 0.05; **P < 0.01.
Consistent with increased granule docking, Munc18a/SNARE complex formation was increased in INS-1 832/13 cells transfected with SUMO1 (Fig. 2D). This could be partially reversed by coexpression of SENP1, whereas total SNARE protein levels were unchanged. Cells expressing GFP alone showed the glucose-stimulated disassembly of the complex consistent with the release of docked granules, but this was blunted after overexpression of SUMO1 (Fig. 2E). Some Munc18a may be pulled down with syntaxin-1A independent of the SNARE complex. However, changes in Munc18a occurred in parallel with SNAP-25 and VAMP2, consistent with Munc18a participation in a “SNAREpin” structure (30). Therefore, SUMO1 does not impair insulin secretion by reducing insulin granule recruitment and docking at the plasma membrane. Inhibition of the secretory process by SUMO1 occurs downstream of the docking event.
SUMOylation inhibits Ca2+-dependent exocytosis.
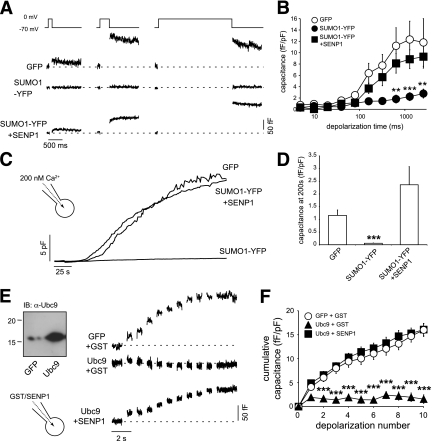
We examined whether Ca2+-dependent exocytosis, per se, is impaired by SUMO1. We thus examined voltage-dependent calcium channel (VDCC) function and capacitance responses in INS-1 832/13 cells overexpressing a conjugation-competent SUMO1-YFP (25) alone or together with SENP1. Upon depolarization of cells to 0 mV, the peak VDCC current in control cells was −20.9 ± 2.9 pA/pF (n = 30) and after SUMO1-YFP expression was −31.3 ± 4.6 pA/pF (n = 16). This approached statistical significance (P = 0.054), although it should be noted that an increase in Ca2+ current cannot account for the inhibitory effect of SUMO1-YFP on the exocytotic response.
Insulin granules are physically coupled to VDCCs (31), and loss of this interaction can reduce insulin secretion and β-cell exocytosis (32). To examine VDCC-granule coupling, cells were subjected to depolarizations of increasing duration (Fig. 3A and B). Short pulses allow local Ca2+ signals, whereas longer pulses mediate increases in bulk cytosolic Ca2+ (21,33). Compared with GFP alone (n = 14), expression of SUMO1-YFP impaired the exocytotic response to even the longest (~2.5 s) depolarization (inhibited by 76%; n = 16, P < 0.01). This was rescued by coexpression of SENP1 (n = 14). This suggests that decoupling of secretory granules from VDCCs does not account for the impaired exocytotic response. Indeed, exocytosis could not be rescued by the direct infusion of 200 nmol/L free Ca2+ and remained impaired in cells expressing SUMO1-YFP (by 95%, n = 15, P < 0.001) (Fig. 3C and D). Again, this was rescued by coexpression of SENP1 (n = 15). The exocytotic response of INS-1 832/13 cells also was impaired by overexpression of the SUMOylating enzyme Ubc9 (by 90%, n = 7, P < 0.001) rather than SUMO1 itself (Fig. 3E and F). Importantly, this was rapidly rescued by intracellular dialysis of SENP1 (6 μg/mL, n = 10). Taken together, these results demonstrate that SUMOylation inhibits Ca2+-dependent exocytosis distal to granule docking and Ca2+ entry.
FIG. 3.
SUMOylation impairs Ca2+-dependent exocytosis. A: Exocytotic capacitance responses to depolarizations of increasing duration (10, 320, and 2560 ms shown) of INS-1 832/13 cells expressing GFP, SUMO1-YFP, or SUMO1-YFP + SENP1. B: Average capacitance responses from A plotted vs. depolarization duration. C: The whole-cell capacitance response of INS-1 832/13 cells measured during direct application of 200 nmol/L free Ca2+ via the patch pipette. D: The average capacitance response of cells expressing GFP, SUMO1-YFP, or SUMO1-YFP + SENP1 at 200 s following initiation of Ca2+ infusion and normalized to initial cell size. E: Endogenous and overexpressed Ubc9 in INS-1 832/13 cells (left). Exocytotic capacitance responses (right) in INS-1 832/13 cells expressing GFP or Ubc9 and dialyzed with either GST or SENP1. F: Average cumulative capacitance responses over the series of 10 depolarizations. **P < 0.01; ***P < 0.001 vs. GFP.
SUMOylation inhibits exocytosis in human β-cells.
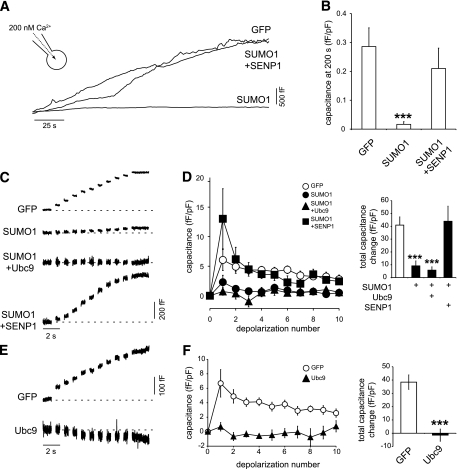
We next examined the effect of upregulating SUMOylation in human β-cells positively identified by insulin immunostaining. Similar to the effect observed in the INS-1 832/13 cells, overexpression of SUMO1-YFP inhibited the exocytotic response of human β-cells elicited both by infusion of 200 nmol/L free Ca2+ (by 94%, n = 13 and 5 from two donors, P < 0.05) (Fig. 4A and B) and by trains of membrane depolarization (by 71%, n = 18 and 10 from three donors, P < 0.001) (Fig. 4C and D). This could be rescued by coexpression of SENP1 (n = 13 and 19, respectively) (Fig. 4C and D). Overexpression of SUMO1-YFP had a maximal inhibitory effect on human β-cell exocytosis that was not further enhanced by coexpression of the SUMO-conjugating enzyme Ubc9 (77% inhibition, n = 17). However, Ubc9 alone was sufficient to inhibit exocytosis in human β-cells (n = 25 and 11, P < 0.001) (Fig. 4E and F).
FIG. 4.
SUMOylation inhibits exocytosis in human β-cells. A: The exocytotic response of single human β-cells to direct intracellular dialysis of 200 nmol/L free Ca2+ measured as increased cell capacitance. B: The average capacitance response, at 200 s following Ca2+ infusion and normalized to initial cell size, in human β-cells expressing GFP, SUMO1, or SUMO1 + SENP1. C: Exocytosis elicited by a series of ten 500-ms depolarizations in human β-cells expressing GFP, SUMO1, and SUMO1 + Ubc or SUMO1 + SENP1. D: Average capacitance responses to each depolarization (left) and the total capacitance response over the train (right). E: Exocytotic responses of human β-cells expressing GFP or the SUMO-conjugating enzyme Ubc9 alone. F: Average capacitance responses to each depolarization (left) and the total capacitance response over the train (right). ***P < 0.001 vs. GFP alone.
SUMO1 interacts dynamically with synaptotagmin VII.
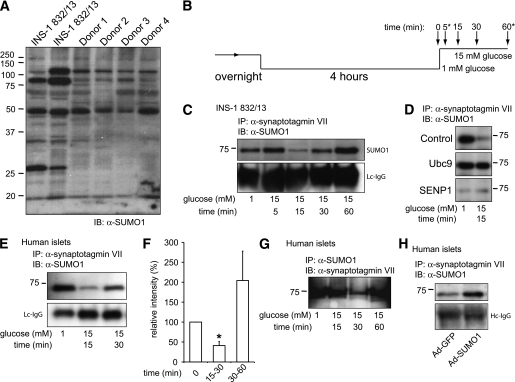
Both INS-1 832/13 cells and human islets possess numerous SUMOylated proteins (Fig. 5A). Because SUMOylation prevents Ca2+-dependent exocytosis in β-cells at a point distal to Munc18a/SNARE complex assembly, we postulated that SUMO1 might interact with synaptotagmin VII, a likely mediator of exocytotic Ca2+-sensing in β-cells (23,24) that possesses a potential type II nonconsensus SUMOylation site (34). We find that SUMO1 coimmunoprecipitates with synaptotagmin VII, and vice versa, from INS-1 832/13 and human islets (Fig. 5C–H). SUMO1 was not pulled down with synaptotagmin IX (data not shown), which also is reported to contribute to the control of Ca2+-dependent exocytosis in β-cells (35,36). The association with synaptotagmin VII was SUMOylation dependent because it could be enhanced by overexpression of Ubc9 or SUMO1 (Fig. 5D and H) and could be prevented by SENP1 (Fig. 5D). Furthermore, the endogenous SUMO1/synaptotagmin VII interaction is transiently disrupted after glucose stimulation (Fig. 5B–G) in both INS-1 832/13 cells (n = 3) and human islets (n = 5 donors). This generally occurred at 15 min poststimulation (although in human islets this was occasionally observed at 30 min as shown in Fig. 5G). Additionally, in one of five human donors the glucose-dependent loss of this interaction was not observed; islets from this donor displayed neither exocytosis nor glucose-stimulated insulin secretion (data not shown).
FIG. 5.
SUMO1 associates with the exocytotic Ca2+ sensor synaptotagmin VII in a glucose-dependent manner. A: SUMOylation profile of whole-cell lysates from INS-1 832/13 cells and four human donors showing numerous SUMOylated proteins. B: INS-1 832/13 cells and human islets were cultured overnight, preincubated at 1 mmol/L glucose for 4 h, and stimulated with 15 mmol/L glucose for varying times, as indicated. At some time points (*) lysates were not always collected. C: Immunoprecipitation of synaptotagmin VII from INS-1 832/13 cells collected at time points following glucose-stimulation indicated in B, followed by blotting for SUMO1. Light-chain (Lc) or heavy chain (Hc) IgG is shown as a loading control. D: Same as in C, collected at time = 0 and 15 min following glucose stimulation. The interaction between SUMO1 and synaptotagmin VII in INS-1 832/13 cells is increased by the SUMO-conjugating enzyme Ubc9 and lost upon expression of the SUMO protease SENP1, in comparison with cells transfected with control vector. Ubc9 prevents, whereas SENP1 mimics, the glucose-dependent disruption of the interaction. E: Same as in C but with human islets. F: Densitometry demonstrates a >50% reduction in SUMO1 coimmunoprecipitation at 15 min following glucose stimulation of human islets. G: As in D but with SUMO1 immunoprecipitation followed by blotting for synaptotagmin VII. H: As in D, after infection with Ad-GFP or Ad-SUMO1, demonstrating that increasing SUMO1 expression enhances the interaction with synaptotagmin VII. *P <0.05 vs. time = 0. (A high-quality digital representation of this figure is available in the online issue.)
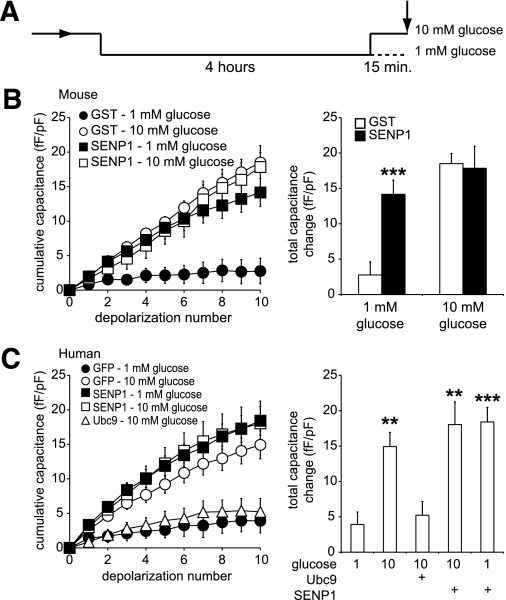
(De)SUMOylation and glucose-dependent β-cell exocytosis.
Because glucose stimulation transiently disrupts the SUMO1/synaptotagmin VII interaction, we considered whether the SUMOylation pathway is an important determinant of the glucose dependence of β-cell exocytosis. We examined whether SUMOylation acts to limit Ca2+-dependent exocytosis at low glucose and after prolonged glucose stimulation, conditions where we observed a strong SUMOylation-dependent interaction between SUMO1 and synaptotagmin VII. Preincubation of cells at 1 mmol/L glucose for 4 h was followed by acute (15 min) exposure to either 1 or 10 mmol/L glucose (Fig. 6A). The exocytotic response of mouse β-cells at 1 mmol/L glucose was low (Fig. 6B and C, ●) and was significantly enhanced in response to 10 mmol/L glucose (Fig. 6B and C, ○). At 1 mmol/L glucose, the direct intracellular dialysis (6 μg/mL; mouse, n = 40, P < 0.001) or overexpression (human, n = 38 from six donors, P < 0.01) of the deSUMOylating enzyme SENP1 was sufficient to enhance exocytosis but had no further effect at 10 mmol/L glucose (n = 9 and 25) (Fig. 6B and C). In addition, overexpression of the SUMO-conjugating enzyme Ubc9 prevents the glucose-stimulated increase in the exocytotic response of human β-cells (n = 21) (Fig. 6C).
FIG. 6.
SUMOylation and deSUMOylation enzymes modulate glucose enhancement of exocytosis in mouse and human β-cells. A: Cells were preincubated at 1 mmol/L glucose for 4 h prior to a 15-min exposure to either 1 or 10 mmol/L glucose, after which exocytosis was measured as the whole-cell capacitance response to a series of membrane depolarizations (arrow). B: Exocytosis in mouse β-cells was blunted at 1 mmol/L (●), and enhanced at 10 mmol/L (○) glucose in cells dialyzed with GST. Dialysis of SENP1 peptide at 1 mmol/L glucose recapitulated the effect of glucose stimulation (■), but had no further effect at 10 mmol/L glucose (□). The total capacitance response under these conditions (right). C: Same as in B but human β-cells by overexpression rather than acute dialysis. Also, expression of the SUMO ligase, Ubc9, prevented glucose enhancement of exocytosis in these cells (△). **P < 0.01; ***P < 0.001 vs. 1 mmol/L glucose control.
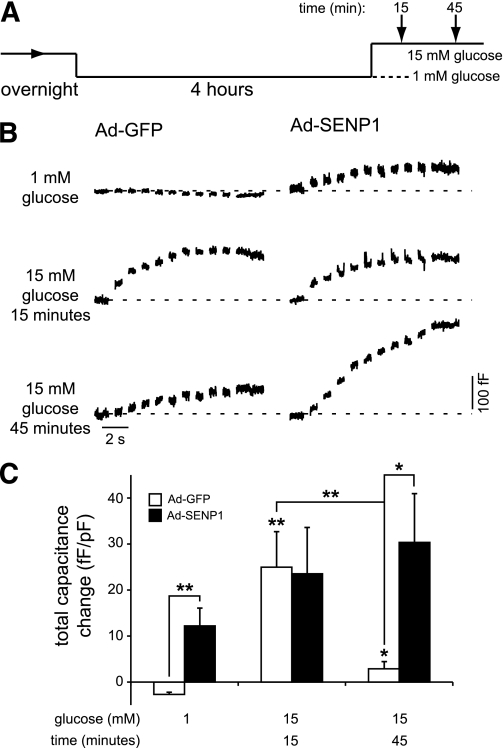
In a separate set of experiments in human β-cells expressing GFP or SENP1 by recombinant adenovirus (Ad-GFP and Ad-SENP1), we examined exocytotic responses at 1 mmol/L glucose and after stimulation with 15 mmol/L glucose for 15 or 45 min (Fig. 7A). As above, little or no exocytosis was seen GFP-expressing cells at 1 mmol/L glucose (n = 7 from two donors) (Fig. 7B and C), and the response was enhanced either by 15 min of glucose stimulation (n = 7, P < 0.01) or expression of SENP1 (n = 7, P < 0.01). SENP1 overexpression at 15 min of glucose stimulation did not further increase the exocytotic response (n = 8) (Fig. 7B and C). Interestingly, the exocytotic response following a 45-min glucose stimulation, which correlates with a return of the SUMO1/synaptotagmin VII interaction (Fig. 5), was reduced compared with acute stimulation (n = 13, P < 0.01). Under this condition, SENP1 was again able to enhance the exocytotic response (n = 13, P < 0.05) (Fig. 7B and C).
FIG. 7.
SUMOylation limits exocytosis in human β-cells following prolonged glucose stimulation. A: Cells were preincubated at 1 mmol/L glucose for 4 h prior to a 15-min exposure to either 1 or 15 mmol/L glucose or a 45-min exposure to 15 mmol/L glucose, after which exocytosis was measured (arrows). B: Exocytotic capacitance responses of human β-cells expressing GFP (Ad-GFP) or SENP1 (Ad-SENP1) under the indicated glucose conditions. C: The total capacitance response under these conditions. *P < 0.05; **P < 0.01 vs. 1 mmol/L glucose control or as indicated.
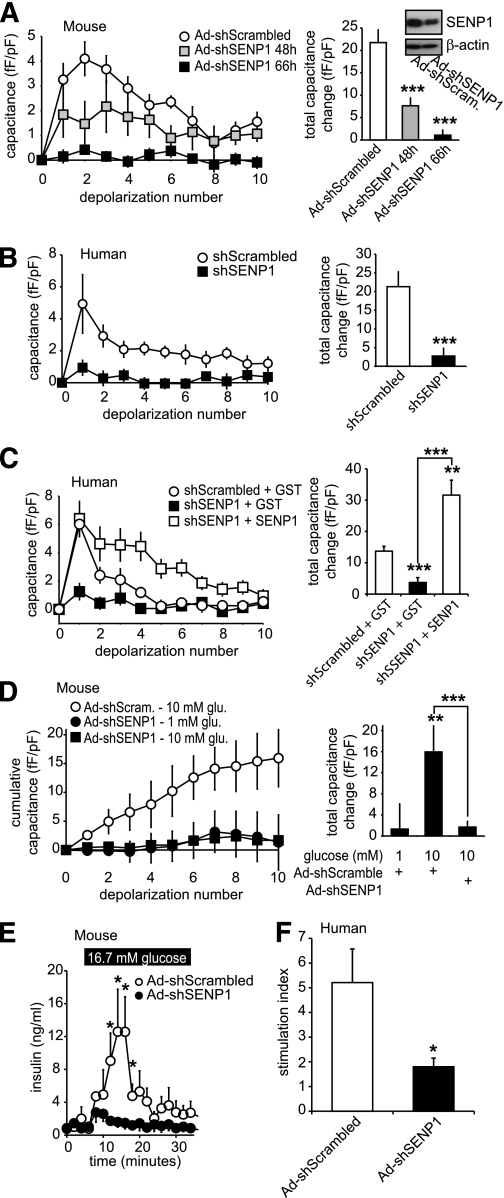
SENP1 is required for glucose-stimulated exocytosis and insulin secretion.
To investigate whether SENP1 is required in glucose- and depolarization-stimulated exocytosis and insulin secretion, we used an shRNA strategy to knock down this deSUMOylating enzyme. Our recombinant adenovirus expressing SENP1 shRNA (Ad-shSENP1) knocked down the endogenous enzyme (58.3 ± 17.2%, n = 3) measured by Western blot (Fig. 8A). The exocytotic response of mouse β-cells was impaired by 65 and 95%, at 48 and 66 h, respectively, after infection with Ad-shSENP1 (n = 14–18, P < 0.001) (Fig. 8A). Similar results were observed in human β-cells, where knockdown of SENP1 impaired exocytosis by 87% at 66 h (n = 17–26 from three donors, P < 0.001) (Fig. 8B). The inhibitory effect of SENP1 knockdown could be rapidly rescued by intracellular dialysis with recombinant SENP1 (6 μg/mL) in both human (n = 12) (Fig. 8C) and mouse (n = 8) (data not shown) β-cells. Furthermore, SENP1 knockdown completely prevented the ability of glucose to enhance mouse β-cell exocytosis (n = 16, P < 0.001) (Fig. 8D) and blunted glucose-stimulated insulin release from both mouse (n = 3) (Fig. 8E) and human (n = 3 donors, P < 0.05) (Fig. 8F) islets.
FIG. 8.
SENP1 is required for glucose-stimulated β-cell exocytosis and insulin secretion from mouse and human islets. A: The capacitance response of mouse β-cells, infected with recombinant adenovirus expressing either a scrambled shRNA (Ad-shScrambled) or an shRNA targeting SENP1 (Ad-shSENP1) to a series of membrane depolarizations. The total capacitance response (right) and SENP1 knockdown by Western blot (inset) are shown. B: Same as in A but following lipid transfection of shScrambled or shSENP1 into human β-cells. C: Same as in B but upon intracellular dialysis of recombinant GST or SENP1 peptides. D: The exocytotic response of mouse β-cells, infected as in A, to a series of membrane depolarizations following preincubated at 1 mmol/L glucose and subsequent exposure to 1 or 10 mmol/L glucose for 15 min (as in Fig. 6A). The total capacitance response is shown (right). E: Insulin secretion measured by perifusion of mouse islets infected with Ad-shScrambled or Ad-shSENP1. F: Glucose-stimulated insulin responses, shown as the stimulation index (fold increase from 1 to 16.7 mmol/L glucose) of human islets infected with Ad-shScrambled or Ad-shSENP1. *P < 0.05; **P < 0.01; ***P < 0.001 vs. control or as indicated.
DISCUSSION
SUMOylation regulates transcription factor targeting and function, including that of MafA and pancreatic and duodenal homeobox-1 in pancreatic β-cells (7,8). More recently, extranuclear roles for SUMO have emerged, including the regulation of mitochondrial and ion channel function (11–15,25,37), which implicate SUMOylation in the acute control of cellular function without requiring altered gene transcription. Here, we demonstrate that SUMOylation acutely and reversibly controls glucose-dependent insulin secretion in rodents and humans through the regulation of Ca2+-dependent exocytosis downstream of secretory granule docking at the plasma membrane. Furthermore, the deSUMOylating enzyme SENP1 augments β-cell exocytosis and is required for glucose-dependent insulin secretion.
Posttranslational SUMOylation is suggested to regulate key plasma membrane proteins, including ion channels (11–15) and receptors (16), although in one case this has been questioned (38). We showed previously that overexpression of SUMO1 in INS-1 and human β-cells inhibits voltage-dependent K+ (Kv) currents and modulates excitability (13). However, in contrast with the expected insulinotropic effect associated with Kv inhibition (28,39), SUMO1 overexpression impaired glucose-stimulated insulin secretion. It is possible that mitochondrial function is impacted by SUMO1 (25,40). However, that SUMO1 primarily inhibits insulin secretion through a downstream mechanism is suggested by 1) the lack of effect on intracellular Ca2+ responses, 2) the inability of direct Ca2+ infusion to stimulate exocytosis after SUMO1 overexpression, and 3) the rapidity at which direct intracellular SUMO1 dialysis blocks exocytosis. Furthermore, although SUMOylation modulates transcription factor function in many cell types, including β-cells (7,8), altered gene expression or reduced insulin content is not required for the inhibitory effect of SUMO1 on β-cell exocytosis. This is evidenced again by the ability of acute SUMO1 infusion to inhibit exocytosis, as well as the lack of effect of SUMO1 on SNARE protein expression and preserved insulin content.
SUMO1 exerts the majority of its effects via its covalent and reversible conjugation to target proteins (41). SUMO conjugation is indeed required for SUMO1 to inhibit β-cell exocytosis because the SUMO protease, SENP1, rescues the exocytotic response. A role for endogenous SUMOylation in insulin exocytosis is demonstrated by manipulation of the SUMO conjugation and protease machinery. Upregulation of the SUMO ligase, Ubc9, or knockdown of the SUMO protease, SENP1, inhibits β-cell exocytosis, both of which are rescued by dialysis with SENP1. Thus, SUMOylation acutely and reversibly inhibits exocytosis in human and rodent β-cells.
The point at which SUMOylation inhibits β-cell exocytosis lies downstream of insulin granule trafficking to the plasma membrane and physical docking, as indicated by our electron microscopy and TIRF studies and the abundant Munc18a/SNARE complex assembly following SUMO1 overexpression. The ubiquitin/proteosome pathway is implicated in the regulation of β-cell voltage-dependent Ca2+ channels (42), and SUMOylation is reported to modulate Ca2+ influx at presynaptic nerve terminals (17). However, SUMOylation impairs β-cell exocytosis at the point of Ca2+ triggering of membrane fusion rather than Ca2+ entry or Ca2+ channel/granule coupling, which is most clearly shown by an inability of direct Ca2+ application to elicit exocytosis after SUMO1 upregulation. Consistent with an effect on exocytotic Ca2+ sensing, we find that endogenous SUMO1 interacts with synaptotagmin VII.
Synaptotagmin VII represents a major exocytotic Ca2+ sensor in β-cells (23,24). The SUMO1-positive band at 75 kDa pulled down with synaptotagmin VII is consistent with synaptotagmin VII splice variants (SYTVIIα or -β) expressed in β-cells (43) and corresponds to the synaptotagmin VII–positive band pulled down with a SUMO1 antibody. Additional work is required to determine whether synaptotagmin VII is SUMOylated directly, perhaps at its predicted nonconsensus SUMOylation site, or whether the coimmunoprecipitated protein is a SUMOylated binding partner. Certainly, the SUMO1/synaptotagmin VII interaction, whether direct or indirect, is SUMOylation dependent because it is upregulated by Ubc9 and lost upon SENP1 overexpression.
The SUMO1/synaptotagmin VII interaction is transiently lost upon glucose stimulation and returns within 30–60 min. Similarly, SUMOylation of MafA in β-cells increases at low glucose (7). Although the mechanism(s) underlying these effects remains to be elucidated, the present data suggest a key role for (de)SUMOylation in glucose-dependent insulin secretion. This is supported by our findings that SENP1 disrupts the SUMO1/synaptotagmin VII interaction and mimics the ability of glucose to enhance exocytosis. Furthermore, SENP1 knockdown demonstrates that this deSUMOylating enzyme is required for β-cell exocytosis and glucose-stimulated insulin secretion, and upregulation of Ubc9, which prevents dissociation of SUMO1 from synaptotagmin VII, prevents glucose-dependent exocytosis. Finally, we find that restoration of the SUMO1/synaptotagmin VII interaction correlates with the suppression of exocytosis during longer glucose stimulations, and robust exocytosis is restored by SENP1, demonstrating that SUMOylation is a limiting factor under this condition. Although it is tempting to speculate that the time course of SUMOylation effects on exocytosis relates to secretory capacity during first- and/or second-phase insulin secretion, it is clear that additional work is required to delineate the exact temporal relationship between SUMOylation and insulin secretion.
We conclude that the SUMOylation pathway plays an important and acute role in glucose-dependent insulin secretion in rodents and humans via the acute and dynamic regulation of Ca2+-dependent exocytosis. At low glucose, and following prolonged glucose stimulation, SUMOylation acts as a “brake” to prevent the Ca2+-induced exocytotic release of insulin. Conversely, the deSUMOylating enzyme, SENP1, augments Ca2+-dependent exocytosis and is required for robust glucose-stimulated insulin secretion.
ACKNOWLEDGMENTS
Funding for this research was provided by operating grants from the Canadian Institutes of Health Research to P.E.M. (MOP-97845) and H.Y.G. (MOP-86544). X.-Q.D. is supported by a fellowship from the Alberta Heritage Foundation for Medical Research (AHFMR). C.H. is supported by the Gladys Wirtanen nee Woodrow Studentship from the Alberta Diabetes Foundation. P.E.M. holds scholarships from the Canadian Diabetes Association and AHFMR and is the Canada Research Chair in Islet Biology. No potential conflicts of interest relevant to this article were reported.
X.-Q.D. and G.P. researched data and contributed to discussion. M.C., Y.K., and C.H. researched data. H.Y.G. contributed to discussion and reviewed and edited the manuscript. J.E.M.F. researched data and contributed to discussion. P.E.M. contributed to discussion and wrote the manuscript.
The authors are grateful to Nancy Smith (University of Alberta) for technical assistance, to Dr. Chris Newgard (Duke University) for providing INS-1 832/13 cells, and to Dr. James Shapiro and the Clinical Islet Laboratory at the University of Alberta for human donor islets.
REFERENCES
- 1.Hilgarth RS, Murphy LA, Skaggs HS, Wilkerson DC, Xing H, Sarge KD. Regulation and function of SUMO modification. J Biol Chem 2004;279:53899–53902 [DOI] [PubMed] [Google Scholar]
- 2.Hay RT. SUMO: a history of modification. Mol Cell 2005;18:1–12 [DOI] [PubMed] [Google Scholar]
- 3.Müller S, Ledl A, Schmidt D. SUMO: a regulator of gene expression and genome integrity. Oncogene 2004;23:1998–2008 [DOI] [PubMed] [Google Scholar]
- 4.Verger A, Perdomo J, Crossley M. Modification with SUMO: a role in transcriptional regulation. EMBO Rep 2003;4:137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seeler JS, Dejean A. Nuclear and unclear functions of SUMO. Nat Rev Mol Cell Biol 2003;4:690–699 [DOI] [PubMed] [Google Scholar]
- 6.Ehninger A, Mziaut H, Solimena M. Emerging role of SUMO in pancreatic beta-cells. Horm Metab Res 2007;39:658–664 [DOI] [PubMed] [Google Scholar]
- 7.Shao C, Cobb MH. Sumoylation regulates the transcriptional activity of MafA in pancreatic beta cells. J Biol Chem 2009;284:3117–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishi A, Nakamura T, Nishio Y, Maegawa H, Kashiwagi A. Sumoylation of Pdx1 is associated with its nuclear localization and insulin gene activation. Am J Physiol Endocrinol Metab 2003;284:E830–E840 [DOI] [PubMed] [Google Scholar]
- 9.Trajkovski M, Mziaut H, Altkrüger A, et al. Nuclear translocation of an ICA512 cytosolic fragment couples granule exocytosis and insulin expression in β-cells. J Cell Biol 2004;167:1063–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson ES. Protein modification by SUMO. Annu Rev Biochem 2004;73:355–382 [DOI] [PubMed] [Google Scholar]
- 11.Rajan S, Plant LD, Rabin ML, Butler MH, Goldstein SA. Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell 2005;121:37–47 [DOI] [PubMed] [Google Scholar]
- 12.Benson MD, Li QJ, Kieckhafer K, et al. SUMO modification regulates inactivation of the voltage-gated potassium channel Kv1.5. Proc Natl Acad Sci USA 2007;104:1805–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai XQ, Kolic J, Marchi P, Sipione S, Macdonald PE. SUMOylation regulates Kv2.1 and modulates pancreatic β-cell excitability. J Cell Sci 2009;122:775–779 [DOI] [PubMed] [Google Scholar]
- 14.Plant LD, Dementieva IS, Kollewe A, Olikara S, Marks JD, Goldstein SA. One SUMO is sufficient to silence the dimeric potassium channel K2P1. Proc Natl Acad Sci USA 2010;107:10743–10748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruse M, Schulze-Bahr E, Corfield V, et al. Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest 2009;119:2737–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin S, Nishimune A, Mellor JR, Henley JM. SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature 2007;447:321–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feligioni M, Nishimune A, Henley JM. Protein SUMOylation modulates calcium influx and glutamate release from presynaptic terminals. Eur J Neurosci 2009;29:1348–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jewell JL, Oh E, Thurmond DC. Exocytosis mechanisms underlying insulin release and glucose uptake: conserved roles for Munc18c and syntaxin 4. Am J Physiol Regul Integr Comp Physiol 2010;298:R517–R531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rorsman P, Renström E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003;46:1029–1045 [DOI] [PubMed] [Google Scholar]
- 20.Rutter GA, Hill EV. Insulin vesicle release: walk, kiss, pause...then run. Physiology (Bethesda) 2006;21:189–196 [DOI] [PubMed] [Google Scholar]
- 21.Barg S, Ma X, Eliasson L, et al. Fast exocytosis with few Ca(2+) channels in insulin-secreting mouse pancreatic B cells. Biophys J 2001;81:3308–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiser O, Trus M, Hernández A, et al. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc Natl Acad Sci USA 1999;96:248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustavsson N, Lao Y, Maximov A, et al. Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci USA 2008;105:3992–3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauthier BR, Wollheim CB. Synaptotagmins bind calcium to release insulin. Am J Physiol Endocrinol Metab 2008;295:E1279–E1286 [DOI] [PubMed] [Google Scholar]
- 25.Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol 2004;14:340–345 [DOI] [PubMed] [Google Scholar]
- 26.Pigeau GM, Kolic J, Ball BJ, et al. Insulin granule recruitment and exocytosis is dependent on p110γ in insulinoma and human β-cells. Diabetes 2009;58:2084–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Göpel SO, Kanno T, Barg S, Weng XG, Gromada J, Rorsman P. Regulation of glucagon release in mouse cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J Physiol 2000;528:509–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDonald PE, Ha XF, Wang J, et al. Members of the Kv1 and Kv2 voltage-dependent K(+) channel families regulate insulin secretion. Mol Endocrinol 2001;15:1423–1435 [DOI] [PubMed] [Google Scholar]
- 29.Roe MW, Worley JF, 3rd, Mittal AA, et al. Expression and function of pancreatic beta-cell delayed rectifier K+ channels. Role in stimulus-secretion coupling. J Biol Chem 1996;271:32241–32246 [DOI] [PubMed] [Google Scholar]
- 30.Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science 2009;323:474–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atlas D. Functional and physical coupling of voltage-sensitive calcium channels with exocytotic proteins: ramifications for the secretion mechanism. J Neurochem 2001;77:972–985 [DOI] [PubMed] [Google Scholar]
- 32.Hoppa MB, Collins S, Ramracheya R, et al. Chronic palmitate exposure inhibits insulin secretion by dissociation of Ca(2+) channels from secretory granules. Cell Metab 2009;10:455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanno T, Ma X, Barg S, et al. Large dense-core vesicle exocytosis in pancreatic beta-cells monitored by capacitance measurements. Methods 2004;33:302–311 [DOI] [PubMed] [Google Scholar]
- 34.Ren J, Gao X, Jin C, et al. Systematic study of protein sumoylation: development of a site-specific predictor of SUMOsp 2.0. Proteomics 2009;9:3409–3412 [DOI] [PubMed] [Google Scholar]
- 35.Iezzi M, Kouri G, Fukuda M, Wollheim CB. Synaptotagmin V and IX isoforms control Ca2+-dependent insulin exocytosis. J Cell Sci 2004;117:3119–3127 [DOI] [PubMed] [Google Scholar]
- 36.Iezzi M, Eliasson L, Fukuda M, Wollheim CB. Adenovirus-mediated silencing of synaptotagmin 9 inhibits Ca2+-dependent insulin secretion in islets. FEBS Lett 2005;579:5241–5246 [DOI] [PubMed] [Google Scholar]
- 37.Braschi E, Zunino R, McBride HM. MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep 2009;10:748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feliciangeli S, Bendahhou S, Sandoz G, et al. Does sumoylation control K2P1/TWIK1 background K+ channels? Cell 2007;130:563–569 [DOI] [PubMed] [Google Scholar]
- 39.Jacobson DA, Kuznetsov A, Lopez JP, Kash S, Ammälä CE, Philipson LH. Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metab 2007;6:229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, McBride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci 2007;120:1178–1188 [DOI] [PubMed] [Google Scholar]
- 41.Yeh ET. SUMOylation and De-SUMOylation: wrestling with life’s processes. J Biol Chem 2009;284:8223–8227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawaguchi M, Minami K, Nagashima K, Seino S. Essential role of ubiquitin-proteasome system in normal regulation of insulin secretion. J Biol Chem 2006;281:13015–13020 [DOI] [PubMed] [Google Scholar]
- 43.Gauthier BR, Duhamel DL, Iezzi M, et al. Synaptotagmin VII splice variants alpha, beta, and delta are expressed in pancreatic beta-cells and regulate insulin exocytosis. FASEB J 2008;22:194–206 [DOI] [PubMed] [Google Scholar]