Abstract
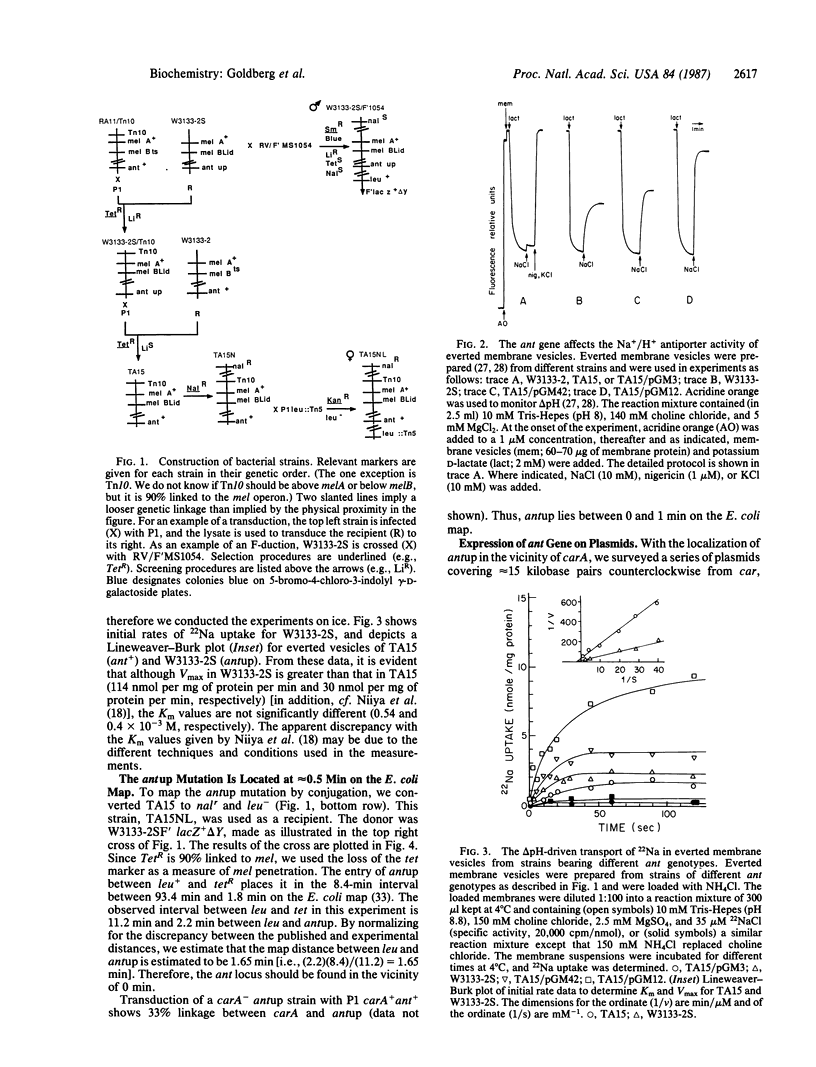
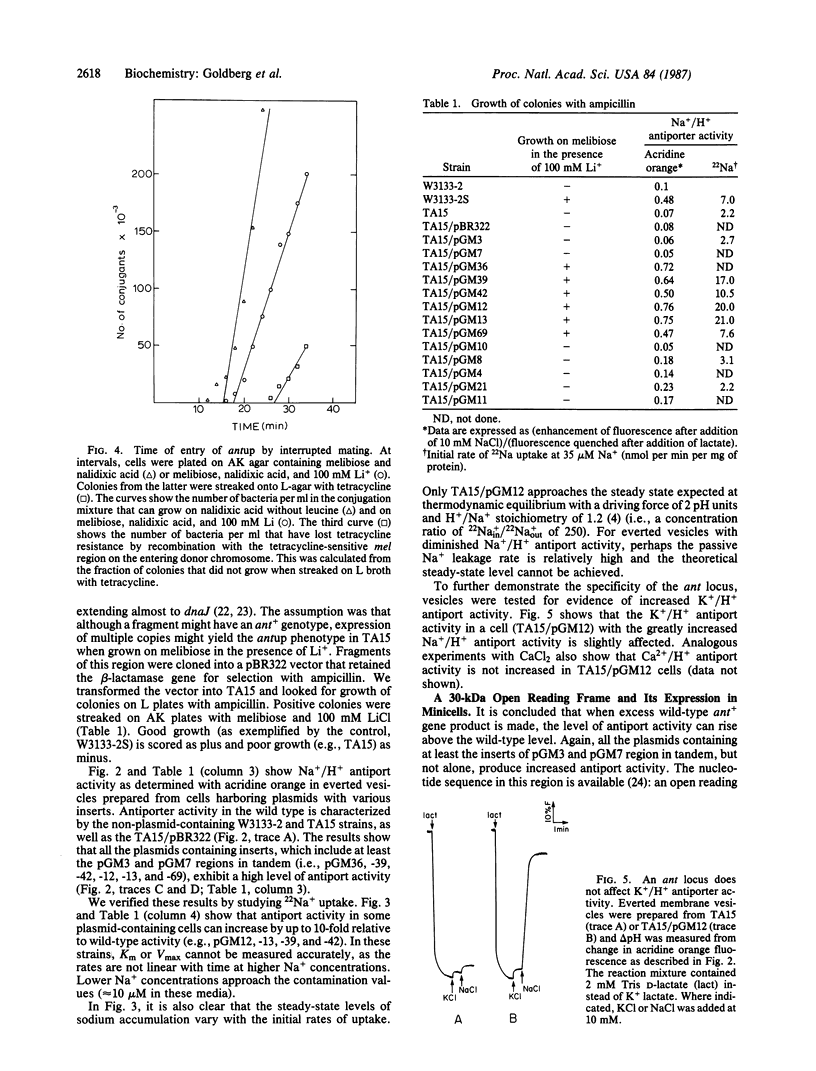
A mutant of Escherichia coli with increased Na+/H+ antiport activity was isolated and found by other workers to harbor two mutations [Niiya, S., Yamasaki, K., Wilson, T.H. & Tsuchiya, T. (1982) J. Biol. Chem. 257, 8902-8906]. The mutation that leads to increased Na+/H+ antiport (antup) has now been separated and mapped. antup maps in the vicinity of 0.5 min on the E. coli map, and the presence of this mutation alone in an isogenic pair raises antiport activity (Vmax) by approximately 4-fold. We also characterized cells hearing plasmids containing fragments of a 15-kilobase-pair segment of DNA between carA and dnaJ. A wild-type gene located within 2 kilobase pairs counterclockwise of rpsT increases the Na+/H+ antiport activity when present in multiple copies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambudkar S. V., Zlotnick G. W., Rosen B. P. Calcium efflux from Escherichia coli. Evidence for two systems. J Biol Chem. 1984 May 25;259(10):6142–6146. [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassilana M., Damiano E., Leblanc G. Relationships between the Na+-H+ antiport activity and the components of the electrochemical proton gradient in Escherichia coli membrane vesicles. Biochemistry. 1984 Feb 28;23(5):1015–1022. doi: 10.1021/bi00300a033. [DOI] [PubMed] [Google Scholar]
- Beck J. C., Rosen B. P. Cation/proton antiport systems in escherichia coli: properties of the sodium/proton antiporter. Arch Biochem Biophys. 1979 Apr 15;194(1):208–214. doi: 10.1016/0003-9861(79)90611-8. [DOI] [PubMed] [Google Scholar]
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown I. I., Galperin MYu, Glagolev A. N., Skulachev V. P. Utilization of energy stored in the form of Na+ and K+ ion gradients by bacterial cells. Eur J Biochem. 1983 Aug 1;134(2):345–349. doi: 10.1111/j.1432-1033.1983.tb07573.x. [DOI] [PubMed] [Google Scholar]
- Castle A. M., Macnab R. M., Shulman R. G. Measurement of intracellular sodium concentration and sodium transport in Escherichia coli by 23Na nuclear magnetic resonance. J Biol Chem. 1986 Mar 5;261(7):3288–3294. [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffard P. M., Rowland G. C., Kroll R. G., Stewart L. M., Bakker E. P., Booth I. R. Phenotypic properties of a unique rpoA mutation (phs) of Escherichia coli. J Bacteriol. 1985 Nov;164(2):904–910. doi: 10.1128/jb.164.2.904-910.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. II. Proton and sodium extrusion. J Membr Biol. 1972;8(1):45–62. doi: 10.1007/BF01868094. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A. Bioenergetics of alkalophilic bacteria. J Membr Biol. 1986;89(2):113–125. doi: 10.1007/BF01869707. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Guffanti A. A., Fong M. Y., Falk L., Hicks D. B. Alkalophilic Bacillus firmus RAB generates variants which can grow at lower Na+ concentrations than the parental strain. J Bacteriol. 1986 Mar;165(3):884–889. doi: 10.1128/jb.165.3.884-889.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopilato J., Tsuchiya T., Wilson T. H. Role of Na+ and Li+ in thiomethylgalactoside transport by the melibiose transport system of Escherichia coli. J Bacteriol. 1978 Apr;134(1):147–156. doi: 10.1128/jb.134.1.147-156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi J. K., Greene R. V. Sodium-stimulated glutamate uptake in membrane vesicles of Escherichia coli: the role of ion gradients. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3167–3170. doi: 10.1073/pnas.74.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie G. A. Cloning of fragments of lambda dapB2 DNA and identification of the dapB gene product. J Biol Chem. 1980 Sep 25;255(18):8928–8935. [PubMed] [Google Scholar]
- Mackie G. A., Parsons G. D. Identification of gene products from cloned fragments of the left arm of lambda dapB2. Can J Biochem. 1982 Mar;60(3):338–346. doi: 10.1139/o82-040. [DOI] [PubMed] [Google Scholar]
- Mackie G. A. Structure of the DNA distal to the gene for ribosomal protein S20 in Escherichia coli K12: presence of a strong terminator and an IS1 element. Nucleic Acids Res. 1986 Sep 11;14(17):6965–6981. doi: 10.1093/nar/14.17.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy M. H. Frameshift mutations in the lactose operon of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:189–201. doi: 10.1101/sqb.1966.031.01.027. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Hsu C., Rosen B. P. Cation/proton antiport systems in Escherichia coli. Solubilization and reconstitution of delta pH-driven sodium/proton and calcium/proton antiporters. J Biol Chem. 1986 Jan 15;261(2):678–683. [PubMed] [Google Scholar]
- Niiya S., Yamasaki K., Wilson T. H., Tsuchiya T. Altered cation coupling to melibiose transport in mutants of Escherichia coli. J Biol Chem. 1982 Aug 10;257(15):8902–8906. [PubMed] [Google Scholar]
- Padan E., Arbel T., Rimon A., Shira A. B., Cohen A. Biosynthesis of the lactose permease in Escherichia coli minicells and effect of carrier amplification on cell physiology. J Biol Chem. 1983 May 10;258(9):5666–5673. [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Ion extrusion systems in Escherichia coli. Methods Enzymol. 1986;125:328–336. doi: 10.1016/s0076-6879(86)25028-4. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Fishkes H. Sodium-proton antiport in isolated membrane vesicles of Escherichia coli. Biochemistry. 1978 Feb 21;17(4):706–711. doi: 10.1021/bi00597a023. [DOI] [PubMed] [Google Scholar]
- Shiota S., Yazyu H., Tsuchiya T. Escherichia coli mutants with altered cation recognition by the melibiose carrier. J Bacteriol. 1984 Oct;160(1):445–447. doi: 10.1128/jb.160.1.445-447.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Lopilato J., Wilson T. H. Effect of lithium ion on melibiose transport in Escherichia coli. J Membr Biol. 1978 Jul 21;42(1):45–59. doi: 10.1007/BF01870393. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Raven J., Wilson T. H. Co-transport of Na+ and methul-beta-D-thiogalactopyranoside mediated by the melibiose transport system of Escherichia coli. Biochem Biophys Res Commun. 1977 May 9;76(1):26–31. doi: 10.1016/0006-291x(77)91663-1. [DOI] [PubMed] [Google Scholar]
- West I. C., Mitchell P. Proton/sodium ion antiport in Escherichia coli. Biochem J. 1974 Oct;144(1):87–90. doi: 10.1042/bj1440087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Padan E., Schuldiner S. A single locus in Escherichia coli governs growth in alkaline pH and on carbon sources whose transport is sodium dependent. FEBS Lett. 1980 Jul 28;116(2):177–180. doi: 10.1016/0014-5793(80)80637-5. [DOI] [PubMed] [Google Scholar]



