Abstract
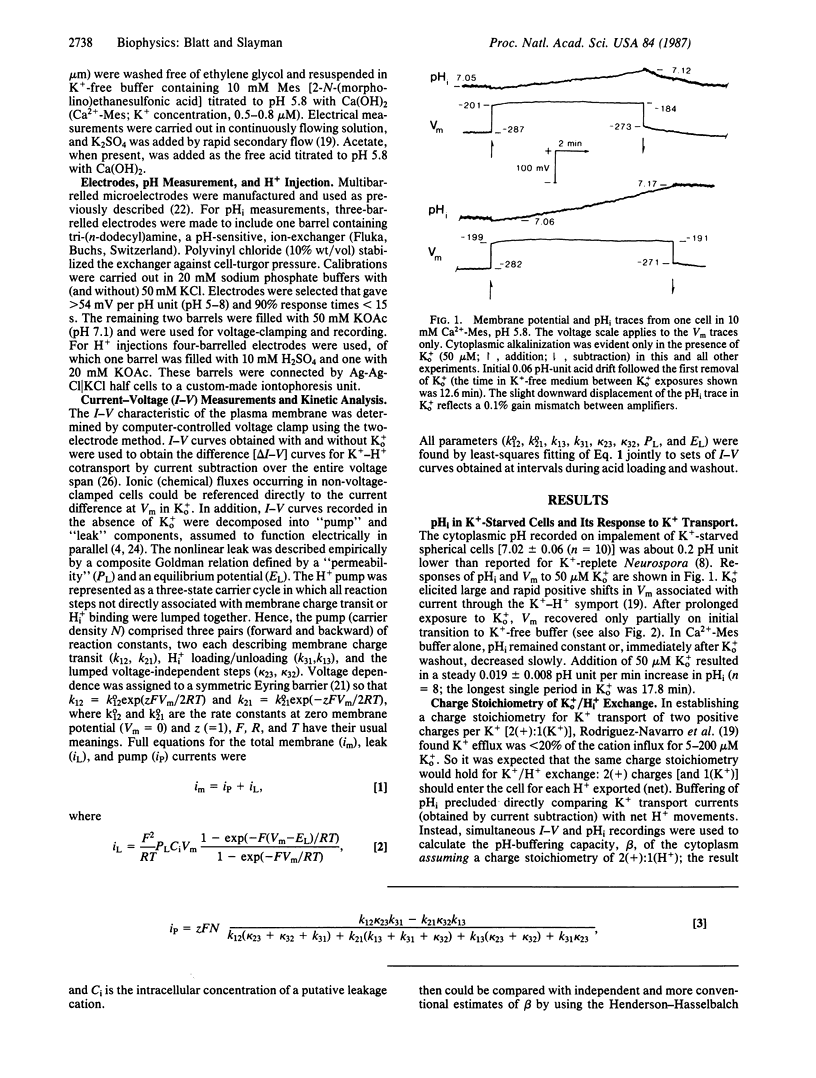
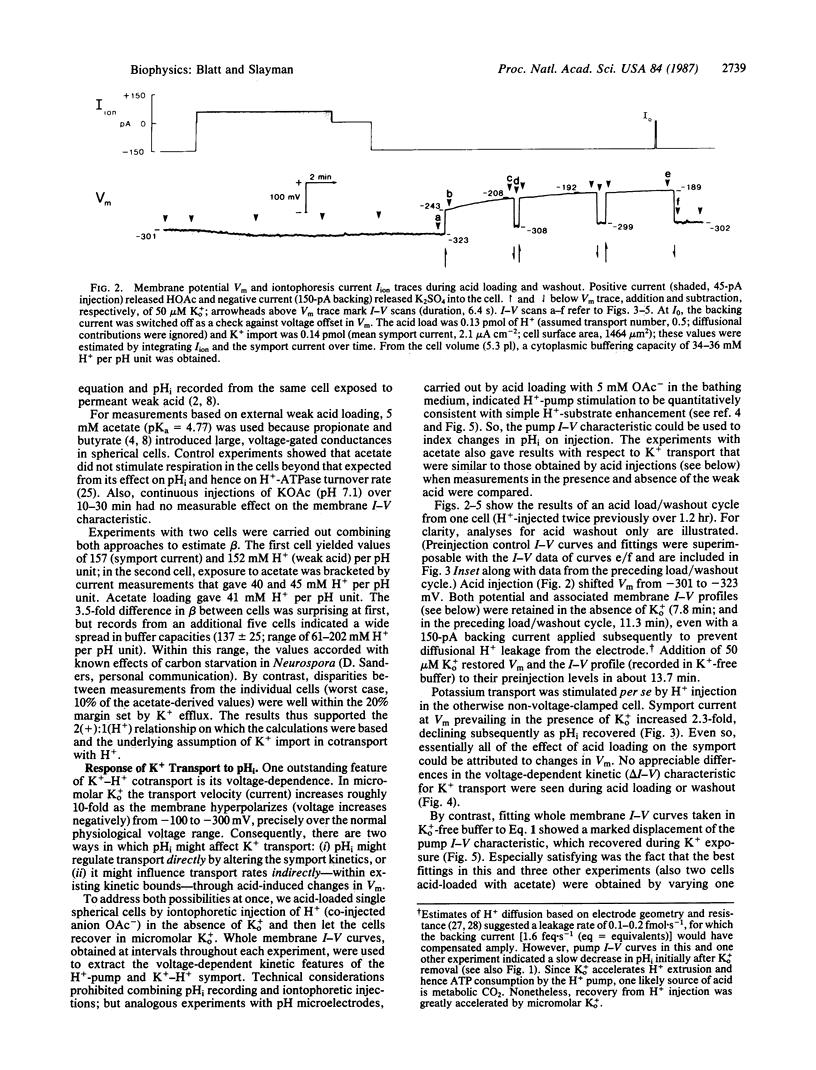
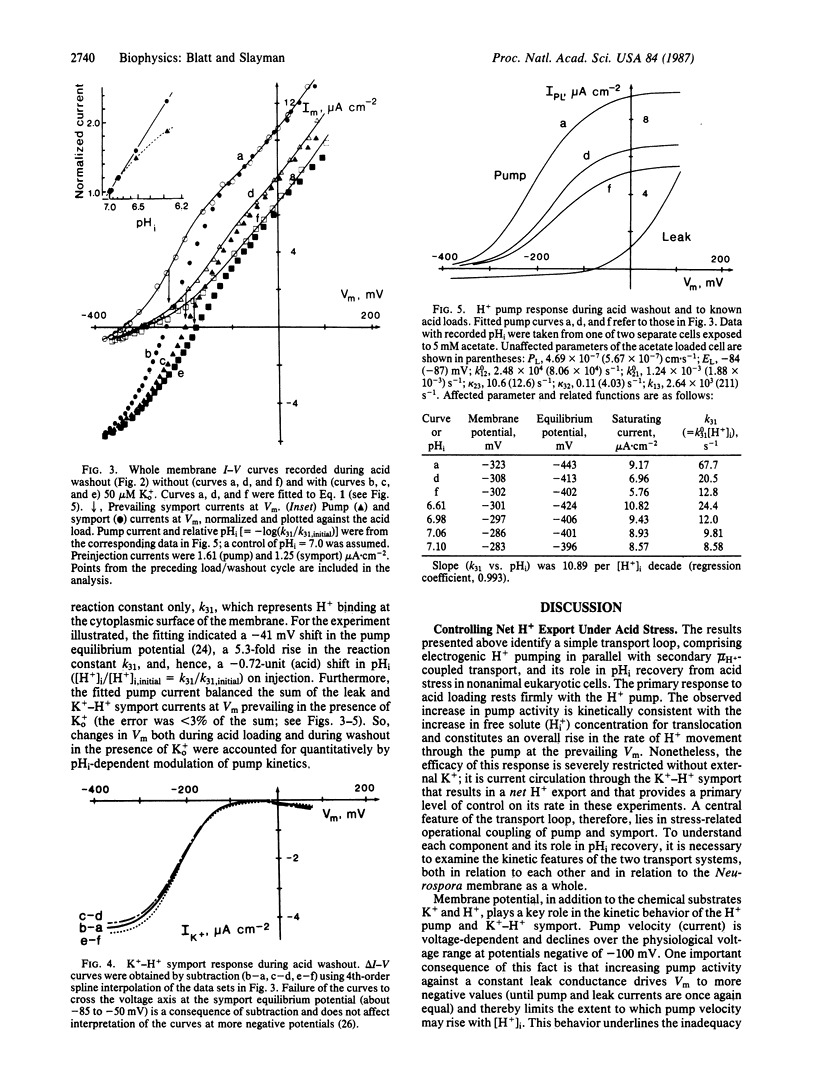
High-affinity potassium uptake in Neurospora occurs by symport with protons [Km (apparent) = 15 microM at pH 5.8], for which a large inward gradient (approximately 400 mV) is generated by the H+-extruding ATPase of the plasma membrane. Operating in parallel, the two transport systems yield a net 1:1 exchange of K+ for cytoplasmic H+. Since this exchange could play a role in cytoplasmic pH (pHi) regulation, the coordinated functioning of the K+-H+ symport and H+ pump has been examined during acid stress. Cytoplasmic acid loads were imposed by injection and by exposure to extracellular permeant weak acid. Multibarrelled microelectrodes were used to monitor membrane potential (Vm), pHi, and the current-voltage (I-V) characteristics of the cells. The behaviors of the H+ pump and K+-H+ symport were resolved, respectively, by fitting whole membrane I-V curves to an explicit kinetic model of the Neurospora membrane and by subtracting I-V curves obtained in the absence from those obtained in the presence of 5-200 microM K+ outside. Proton pumping accelerates nearly in proportion with the cytoplasmic H+ concentration, but pHi recovery from imposed acid loads is dependent on micromolar K+ outside. Potassium import via the symport leads to a measurable alkalinization of the cytoplasm in accordance with stoichiometric (1:1) K+/H+ exchange. Potassium transport is accelerated at low pHi, but in a manner consistent with its inherent voltage sensitivity and changes in Vm resulting from an increased rate of H+ extrusion by the pump. The primary response to acid stress thus rests with the H+ pump, but K+ transport introduces an essential kinetic "valve" that can regulate net H+ export.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P., Harold F. M. Energy coupling to potassium transport in Streptococcus faecalis. Interplay of ATP and the protonmotive force. J Biol Chem. 1980 Jan 25;255(2):433–440. [PubMed] [Google Scholar]
- Blatt M. R. Interpretation of steady-state current-voltage curves: consequences and implications of current subtraction in transport studies. J Membr Biol. 1986;92(1):91–110. doi: 10.1007/BF01869018. [DOI] [PubMed] [Google Scholar]
- Blatt M. R., Slayman C. L. KCl leakage from microelectrodes and its impact on the membrane parameters of a nonexcitable cell. J Membr Biol. 1983;72(3):223–234. doi: 10.1007/BF01870589. [DOI] [PubMed] [Google Scholar]
- Borst-Pauwels G. W. Ion transport in yeast. Biochim Biophys Acta. 1981 Dec;650(2-3):88–127. doi: 10.1016/0304-4157(81)90002-2. [DOI] [PubMed] [Google Scholar]
- CONWAY E. J., DOWNEY M. pH values of the yeast cell. Biochem J. 1950 Sep;47(3):355–360. doi: 10.1042/bj0470355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C. D., Lightfoot E. N., Schmidt F. P., Sy F. Diffusion effects of liquid-filled micropipettes: a pseudobinary analysis of electrolyte leakage. IEEE Trans Biomed Eng. 1972 Sep;19(5):372–375. doi: 10.1109/TBME.1972.324141. [DOI] [PubMed] [Google Scholar]
- Gradmann D., Hansen U. P., Long W. S., Slayman C. L., Warncke J. Current-voltage relationships for the plasma membrane and its principal electrogenic pump in Neurospora crassa: I. Steady-state conditions. J Membr Biol. 1978 Mar 20;39(4):333–367. doi: 10.1007/BF01869898. [DOI] [PubMed] [Google Scholar]
- Hansen U. P., Gradmann D., Sanders D., Slayman C. L. Interpretation of current-voltage relationships for "active" ion transport systems: I. Steady-state reaction-kinetic analysis of class-I mechanisms. J Membr Biol. 1981;63(3):165–190. doi: 10.1007/BF01870979. [DOI] [PubMed] [Google Scholar]
- Läuger P., Stark G. Kinetics of carrier-mediated ion transport across lipid bilayer membranes. Biochim Biophys Acta. 1970 Sep 15;211(3):458–466. doi: 10.1016/0005-2736(70)90251-8. [DOI] [PubMed] [Google Scholar]
- Ogino T., den Hollander J. A., Shulman R. G. 39K, 23Na, and 31P NMR studies of ion transport in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5185–5189. doi: 10.1073/pnas.80.17.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Peña A., Cinco G., Gómez-Puyou A., Tuena M. Effect of the pH of the incubation medium on glycolysis and respiration in Saccharomyces cerevisiae. Arch Biochem Biophys. 1972 Dec;153(2):413–425. doi: 10.1016/0003-9861(72)90359-1. [DOI] [PubMed] [Google Scholar]
- Peña A. Studies on the mechanism of K+ transport in yeast. Arch Biochem Biophys. 1975 Apr;167(2):397–409. doi: 10.1016/0003-9861(75)90480-4. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Wemmer D., Ray P. M., Jardetzky O. Regulation of Cytoplasmic and Vacuolar pH in Maize Root Tips under Different Experimental Conditions. Plant Physiol. 1982 Jun;69(6):1344–1347. doi: 10.1104/pp.69.6.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Navarro A., Blatt M. R., Slayman C. L. A potassium-proton symport in Neurospora crassa. J Gen Physiol. 1986 May;87(5):649–674. doi: 10.1085/jgp.87.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sanders D., Hansen U. P., Slayman C. L. Role of the plasma membrane proton pump in pH regulation in non-animal cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5903–5907. doi: 10.1073/pnas.78.9.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D., Slayman C. L. Control of intracellular pH. Predominant role of oxidative metabolism, not proton transport, in the eukaryotic microorganism Neurospora. J Gen Physiol. 1982 Sep;80(3):377–402. doi: 10.1085/jgp.80.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade A. G., Ward P. J. First impressions of the use of meptazinol in general practice. J Int Med Res. 1981;9(1):74–78. doi: 10.1177/030006058100900113. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Agmon V., Schuldiner S., Padan E. The sodium/proton antiporter is part of the pH homeostasis mechanism in Escherichia coli. J Biol Chem. 1982 Apr 10;257(7):3687–3691. [PubMed] [Google Scholar]



