Abstract
A unique property of lymphocytes among all body tissues is their capacity for rapid proliferation in the context of responding to infectious challenges. Lymphocyte proliferation involves a transition from a quiescent metabolic state adjusted to maintain cellular energy homeostasis, to a proliferative metabolic state in which aerobic glycolysis is used to generate energy and biosynthetic precursors necessary for the accumulation of cell mass. Here we show that modulation of TRPM7 channel function in tumor B lymphocytes directly induces quiescent/proliferative metabolic transitions. As TRPM7 is widely expressed outside of the immune system, our results suggest that TRPM7 may play an active role in regulating metabolic transitions associated with rapid cellular proliferation and malignancy.
Key words: aerobic glycolysis, lymphocyte, metabolism, quiescence, TRPM7
Introduction
Lymphocyte expansion is regulated through proliferative signals received through multiple types of receptors. When the “ensemble” signal a lymphocyte receives reaches a threshold sufficient to drive proliferation, lymphocytes radically shift their metabolism from a state of quiescence adjusted to support cellular energy homeostasis, to a state of anabolism that supports a rapid accumulation of cell mass and completion of one or more cell cycles. When the “ensemble signal” falls below the proliferative threshold, metabolism returns to its original quiescent state, mass accumulation ceases and the lymphocyte exits the cell cycle. A key regulator of these quiescent/proliferative transitions is the PI3K/Akt/mTOR pathway, which directly controls cellular energy metabolism and catabolism/anabolism balance in response to the availability of nutrients and myriad types of growth-promoting hormones.
The dual function ion channel-protein kinase-transient receptor potential cation channel, subfamily M, member 7 (TRPM7) has recently been shown to support sustained phosphoinositide 3-kinase (PI3K) signaling in proliferating lymphocytes,1 positioning TRPM7 alongside PI3K as an important component in the processes which control lymphocyte metabolism. TRPM7's influence on lymphocyte metabolism is linked to its role in regulation of cellular Mg2+ uptake: TRPM7-deficient cells downregulate PI3K/Akt/mTOR signaling and undergo proliferative arrest when transitioned to standard tissue culture media; this phenotype is suppressible either by provision of supplemental extracellular Mg2+ or heterologous expression of a Mg2+ transporter.2,3
A striking aspect of the proliferative arrest exhibited by TRPM7-deficient cells is that a high percentage of arrested cells are positioned at the beginning of the cell cycle as indicated by flow cytometric analysis of DNA content and cell size.1 Similar DNA content and size parameters are observed in primary lymphocytes positioned in quiescence/G0,4,5 suggesting the potential involvement of TRPM7 in processes required to transition out of quiescence/G0. In this study, we examined the biological events that occur when TRPM7-deficient lymphocytes are proliferation-arrested in standard tissue culture media and observed that these cells exhibit multiple signatures associated with quiescence. Provision of either supplemental Mg2+ or TRPM7-mediated Mg2+ entry fully reversed the key features associated with quiescence, implicating TRPM7-mediated Mg2+ uptake as a key mechanism required for cell cycle re-entry from G0.
Results
TRPM7-deficient B cells reversibly exit cell cycle and enter quiescence.
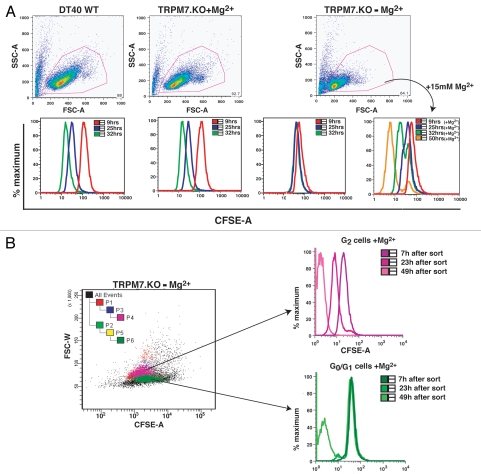
We have previously shown that TRPM7-deficient cells stop accumulating mass and cease to proliferate when moved from Mg2+ supplemented media to regular media.1 In order to better understand the nature of the metabolic transition that occurs when these cells are shifted from supplemental to physiological external Mg2+, we compared WT cells cultured in standard tissue culture/regular media, TRPM7-KO (or TRPM7-deficient) cells in growth-supporting media (media with 15 mM Mg2+; +media) and TRPM7-deficient cells transitioned to regular media for 24–48 hrs, which were labeled with CFSE (Fig. 1). When a CFSE labeled cell enters the cell cycle, each daughter cell undergoes a 50% decrease in CFSE fluorescence as the dye is partitioned between daughter cells at each cell division; if all cells of a population are able to continue rapid division, a progressive decrease in population CFSE fluorescence is observed.6 Our analyses show that both WT cells growing in regular media and TRPM7-KO cells in growth-supporting media continue their rapid proliferation and exhibit a progressive loss of CFSE fluorescence over time in culture. In contrast, TRPM7-KO cells transitioned to regular media exhibit nearly completely stable CFSE staining (Fig. 1A), consistent with their proliferative arrest in a viable state. That the proliferation-arrested TRPM7-deficient cells are viable is evidenced by their ability to both maintain a constant level of CFSE staining, and their retention of normal membrane integrity as assessed by propidium iodide staining (Suppl. Fig. 2). Provision of 15 mM supplemental Mg2+ to TRPM7-KO cells after 24 hrs supported cell cycle re-entry as indicated by a progressive loss of CFSE fluorescence in the TRPM7-KO population, and sorting based on cell size demonstrated that cells with either 2n or 4n DNA content are able to resume cell division (Fig. 1B, (4n cells are distinctly larger than those which have arrested with 2n DNA content;7 Suppl. Fig. 1)). Taken together with our previous analyses of DNA content and cell size,1 these results indicate that TRPM7-deficient cells placed in regular media have reversibly exited the cell cycle to a state of quiescence. The smaller cells with 2n DNA content would be predicted to have exited after mitosis, suggesting they have entered a state of G0, while the larger cells arrested with 4n DNA content appear to have exited somewhere in G2, as has been previously reported to occur in other eukaryotic systems.8,9
Figure 1.
proliferation arrested TRPM7-KO cells exhibit stable CFSE staining upon transition to regular media. (A) TRPM7-deficient cells transitioned to regular media for 24 hours undergo proliferative arrest and show stable CFSE staining. Top part: Forward scatter vs. side scatter analysis displaying cell size distribution of WT cells in regular media, TRPM7-deficient cells in growth-supporting media with supplemental Mg2+ and TRPM7-KO cells transitioned to regular media for 24 hours without and with re-addition of 15 mM Mg2+. All cell lines were labeled with CSFE 24 hrs post transition of TRPM7-KO cells to regular media. Data was acquired on BD LSRII flow cytometer and analyzed by Flowjo (Tree Star, Inc.; Ashland, Oregon). Bottom part: CFSE-labeled cells were acquired at 9, 24 and 32 hours and analyzed by Flowjo. By 25 hours (time not including 24 hours post transition), TRPM7-deficient cells stop undergoing further rounds of proliferation in regular media (-media) without 15 mM supplemental Mg2+ and display substantially stable CFSE staining (3rd bottom part). Provision of supplemental Mg2+ at 24 hrs post transition allowed recovery of only a small proportion of cells by 32 hours (4th bottom part). (B) Proliferation-arrested TRPM7-KO cells resume cell division in presence of supplemental Mg2+. TRPM7-deficient cells cultured in regular media for 24 hours were labeled with CFSE and sorted on the basis of their cell size (smaller G0/G1 and larger G2 cells; see Suppl. Fig. 1) followed by provision of 15 mM supplemental Mg2+ to both populations. Further reacquisition of these two populations at 7, 23 and 49 hours indicated that both G1 and G2 cells are able to resume cell division.
p27kip1 is upregulated in quiescent TRPM7-deficient lymphocytes.
To further define the properties of proliferation-arrested TRPM7-deficient cells, we evaluated the expression level of a key cell cycle regulator-p27kip1.10 The CDK inhibitor p27kip1 (hereafter p27/p27kip1) is thought to provide a crucial block for the transition of cells from G0 through G1 into S phase in diverse type of cells, including lymphocytes (reviewed in ref. 11). A growing body of literature suggests that one of the important signatures of cellular quiescence is an elevation in p27 levels, since its abundance has been observed to precipitously decrease as quiescent primary lymphocytes progress out of G0 into G1 upon mitogen/growth-factor stimulation.12,13
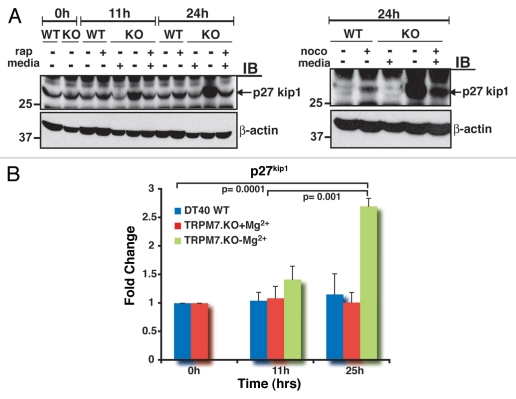
To evaluate the p27kip1 status of proliferation-arrested TRPM7-deficient cells, TRPM7-deficient cells were analyzed for p27kip1 expression at 11 and 24 hrs after transition to regular media with physiologic levels of Mg2+, and compared to both WT DT40 cells in regular media and TRPM7-KO cells in growth-supporting media with 15 mM Mg2+. Comparative analysis showed that p27 expression was significantly elevated in TRPM7-deficient cells growing in regular media by 11 hrs, followed by an even further augmentation at 24 hrs (Fig. 2A, left part). These levels were prominently higher than those observed in TRPM7-KO and WT cells treated with rapamycin, an inhibitor of mTOR that induces G1 arrest. Treatment of WT and TRPM7-KO cells with nocodazole, a microtubule inhibitor that induces G1 and G2 arrest,14 also resulted in expression of p27 at 24 hrs, but again not to the degree observed in TRPM7-KO cells cultured in regular media (Fig. 2A, right part). Quantitative fold change analysis showed that relative to proliferating WT cells in regular media and TRPM7-KO cells in growth-supporting media, TRPM7-deficient cells in regular media exhibited a 2.7-fold increase in p27 expression at 25 hrs, which was found to be statistically significant (Fig. 2B). In conjunction with previous observations on the time course of PI3K-Akt signaling downregulation that occurs in TRPM7-deficient cells,1 these results suggest that accumulation of p27 follows the downregulation of PI3K-Akt growth signaling as TRPM7-deficient cells exit the cell cycle, a correlation also observed in primary lymphocytes transitioning from proliferation to quiescence (reviewed in ref. 15, 16). Taken together, these data indicate that proliferation-arrested TRPM7-deficient lymphocytes exhibit a key molecular signature of cell cycle exit to quiescence-accumulation of high levels of p27Kip1.
Figure 2.
A signature protein of cellular quiescence, p27kip1, is upregulated in proliferation-arrested TRPM7-KO cells. (A) Levels of p27kip1 are significantly elevated in non-proliferating TRPM7-KO cells. Left part: WT, TRPM7-KO cells and TRPM7-KO cells transitioned to regular media were analyzed for p27 expression at 11 and 24 hours post transition. At 11 hours, levels of p27 were elevated in TRPM7-deficient cells transitioned to regular media and were even significantly high at 24 hours post transition compared with WT cells growing in regular media and TRPM7-KO cells in growth-supporting media either with or without rapamycin, a mTO R inhibitor. Right part: Similar to the experiment in the left part, WT, proliferating TRPM7-deficient cells and non-proliferating TRPM7-KO cells were treated with a microtubule inhibitor, nocodazole for 24 hours. Exposure of cells to nocodazole resulted in p27 expression in both WT as well as proliferating TRPM7-KO cells but the levels were considerably lower than those observed in non-proliferating TRPM7-deficient cells. (B) Analysis of the fold change in p27kip1 from three independent experiments is shown. The graph shows averages plus standard errors of the means (error bars) and p values for the fold change were calculated using Student's t test. A 2.7-fold increase in p27 expression was observed by 25 hours in TRPM7-KO cells transitioned to regular media and found to be statistically significant compared to WT cells in regular media at 0 and 11 hours (p = 0.0001 and p = 0.0006) as well as TRPM7-KO cells cultured in growth-supporting media with 15 mM Mg2+ at similar time points (p = 0.0001 and p = 0.001).
Reduced RNA, store-operated calcium entry (SOCE) and altered energy processes characterize quiescent TRPM7-deficient cells.
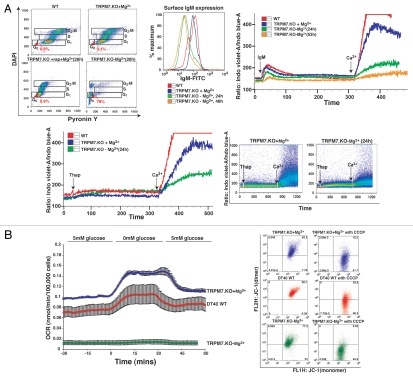
A defining characteristic of quiescent cells is the presence of extremely low levels of RNA compared to cycling cells in G1, S or G2-M phase. Cellular RNA levels can be defined in relation to their cell cycle position by sequential staining of cells with DAPI (binds to DNA), followed by Pyronin Y (PY) staining (binds to RNA only, after DNA binding sites are saturated with DAPI).17 In order to assess RNA levels and cell cycle status of TRPM7-KO lymphocytes transitioned to regular media for 26 hrs, we compared them with sequentially labeled WT cells in regular media, TRPM7-KO cells in growth-supporting media and TRPM7-KO cells in growth-supporting media treated with rapamycin for 26 hrs. We observed a significant proportion of PYlow cells in TRPM7-deficient cells in regular media (79%), suggesting that the majority of cycling cells had become quiescent (Fig. 3A, top left part; reviewed in ref. 18).
Figure 3.
Non-proliferating TRPM7-deficient cells display low RNA content, significantly downregulated store-operated Ca2+ entry (SOCE) and oxygen consumption rate analogous to primary lymphocytes. (A) TRPM7-deficient cells transitioned to regular media exhibit reduced RNA and SOCE. Top left part: Flow cytometric analysis of WT cells in regular media, TRPM7-KO cells in growth-supporting media with/without rapamycin (26 hrs) and TRPM7-KO cells transitioned to regular media for 26 hours stained with DAPI for DNA (Y-axis) and Pyronin Y for RNA (X-axis) content. TRPM7-deficient cells in regular media exhibited a high proportion of Pyronin Y negative cells that are in G0 compared to proliferating WT and TRPM7-KO cells as well as TRPM7-KO treated with rapamycin. The analysis was performed on the same day. Top middle part: TRPM7-KO cells were transitioned to regular media for 24 and 48 hours and their surface IgM expression was analyzed with an anti-IgM antibody directly conjugated to FITC (Bethyl laboratories). WT cells in regular media and TRPM7-KO cells in Mg2+-supplemented media displayed higher surface IgM expression compared to TRPM7-deficient cells transitioned to regular media, consistent with their smaller cell size. Top right part: Store-operated calcium entry was evaluated in WT, TRPM7-KO cells in growth-supporting media with 15 mM Mg2+ and TRPM7-KO cells induced into a non-proliferative state upon transition to regular media for 24 and 52 hours. Cells were labeled with Ca2+-binding dye, indo-1 and acquired on BD LSRII cytometer. Flow kinetic profiles are shown comparing the mean indo-1 ratio (violet/blue) as a function of time before and after stimulation with M4 followed by addition of 1 mM Ca2+. A significant reduction in SOCE was observed in TRPM7-deficient cells cultured in regular media for 24 and 52 hours. Lower left part: WT cells growing in regular media, TRPM7-KO cells in Mg2+-supplemented media and proliferation-arrested TRPM7-deficient cells (24 hours) were analyzed for SOCE upon treatment with thapsigargin. Flow kinetic profiles obtained were similar to what was observed with M4 stimulation (top right part). Lower right part: Changes in intracellular calcium over time for TRPM7-KO cells in media with supplemental Mg2+ and in regular media without 15 mM Mg2+. The response from the whole population of cells from one representative experiment is shown. Upon addition of 1 mM Ca2+, the ion flux in the proliferation-arrested TRPM7-deficient cells (right dot plot) is significantly reduced as compared to the cells in growth-supporting media (left dot plot). Live/dead cell differentiation was done by propidium iodide (PI) staining in all experiments. (B) Proliferation-arrested TRPM7-deficient cells lack Crabtree effect despite functional mitochondria. Left part: A flow cell approach was used for measuring the oxygen consumption rate (OCR) in proliferation-arrested TRPM7-deficient cells compared to WT DT40 cells cultured in regular media and TRPM7-deficient cells in growth-supporting media. Non-proliferating TRPM7-KO cells showed a considerable drop in the OCR, closely resembling the oxygen consumption rate of quiescent primary lymphocytes, when compared to WT and TRPM7-KO cells in Mg2+-supplemented media. Right part: Mitochondrial function was evaluated by determination of mitochondrial membrane potential in WT, TRPM7-deficient cells in growth-supporting media and non-proliferating TRPM7-KO cells transitioned to regular media for 24 hours. While cells labeled with MitoProbe JC-1 displayed mitochondria that retained their normal membrane potential, treatment with CCCP for 5 minutes led to a complete disruption of their mitochondrial electrochemical gradient, which was observed as the shift of JC-1 to its monomeric form (FL-1).
To further define the nature of the quiescent state that TRPM7-deficient cells had entered, we examined the magnitude of store-operated calcium entry in TRPM7-KO cells transitioned to regular media, which has been shown to be diminished in quiescent lymphocytes,19 and cellular energy utilization, which is also reduced and dominated by aerobic/mitochondrial ATP production in quiescence (reviewed in ref. 20).
Engagement of antigen receptors on lymphocytes is followed by calcium-release from endoplasmic reticulum (ER) stores into the cytosol, resulting in the activation of store-operated calcium entry via calcium-release-activated calcium (CRAC) channels on the plasma membrane (reviewed in ref. 21, 22). The magnitude of store-operated Ca2+ entry in primary lymphocytes driven to actively proliferate has recently been shown to be markedly enhanced relative to those that are quiescent.19 To evaluate store-operated calcium entry in proliferation-arrested TRPM7-deficient lymphocytes, we compared WT DT40 cells in regular media with TRPM7-deficient cells maintained either in Mg2+-supplemented media or those that had been transitioned to regular media for 24 hours to induce a state of non-proliferation. Each cell type was activated with M4, an IgM monoclonal antibody that activates the BCR or thapsigargin, an inhibitor of the SERCA (sarco endoplasmic reticulum Ca2+ ATPase) pump responsible for Ca2+ transport into the ER.23,24 Surface expression of IgM was confirmed with a specific anti-IgM antibody (Fig. 3A, top middle part) and release of calcium from intracellular stores followed by entry of extracellular Ca2+ was monitored using the Ca2+-binding dye, indo-1.25 We observed a considerable reduction in anti-IgM evoked intracellular Ca2+ depletion from ER stores and extracellular Ca2+ entry in TRPM7-deficient B-lymphocytes transitioned to regular media for 24 hrs, as compared with WT cells growing in regular media and TRPM7-KO cells cultured in growth-supporting media (Fig. 3A, top right part). Similar results were obtained upon depleting the intracellular Ca2+ stores with thapsigargin. Together, these results demonstrate that proliferation-arrested TRPM7-deficient cells exhibit downregulated store-operated Ca2+ entry, which is both qualitatively and quantitatively similar to that observed in quiescent primary lymphocytes (Fig. 3A, lower part and reviewed in ref. 19).
An additional fundamental property of cellular quiescence is the nature of cellular energy metabolism. In lymphocytes, energy metabolism in quiescence is dominated by respiration, and thus quiescent lymphocytes exhibit low rates of glucose utilization and oxygen consumption (reviewed in ref. 26, 27). In contrast, rapidly proliferating lymphocytes exhibit a high rate of energy utilization that is associated with a shift in carbon flux through glycolysis into metabolic pathways, which generate biosynthetic building blocks required to add cell mass. The shift of carbon flux into glycolytic metabolism results in a significant fraction of energy production via glycolysis in addition to a high rate of ongoing respiration, and is thus known as aerobic glycolysis. A sine-qua-non of ongoing aerobic glycolysis is a paradoxical increase in oxygen consumption upon glucose restriction, as restriction of cellular energy production via glycolysis is made up for via a shift of energy metabolism towards respiration, a phenomenon known as the Crabtree effect.28 Thus, quiescent cells exhibit a low rate of oxygen consumption and lack a “Crabtree effect” upon glucose restriction.29
To evaluate the metabolism of proliferation-arrested TRPM7-deficient cells, we used a flow cell with continuous on line monitoring of oxygen consumption through detection of the phosphorescence lifetime of an oxygen-sensitive dye (platinum tetrapentafluorophenyl porphyrin).30 Using this approach, the oxygen consumption rate (OCR) was measured as environmental glucose was varied from 0 to 5 mM glucose (Fig. 3B, left part). Oxygen consumption rates of proliferating WT cells in regular media and TRPM7-deficient cells in growth-supporting media were approximately 0.7 nmol/105 cell/minute, and markedly increased upon transition to glucose deprivation conditions (Fig. 3B, left part), consistent with their cell metabolism existing in a state of aerobic glycolysis required to support continuous rapid proliferation. In contrast, the OCR of proliferation-arrested TRPM7-KO cells decreased to an absolute rate approaching what has previously been reported for quiescent primary thy- mocytes,26,31 and essentially no change in OCR was observed upon glucose deprivation. These observations indicate that the proliferation-arrested TRPM7-deficient cells have transitioned to a metabolic state in which they have low energy needs, and those needs are met primarily through mitochondrial respiration. 20 Mitochondrial function was also directly evaluated in the proliferation-arrested TRPM7-deficient cells by assessment of the mitochondrial membrane potential using MitoProbe JC-1, which reports high mitochondrial membrane potential as fluorescent J-aggregates (red/FL-2), and loss of membrane potential as a shift of the dye to its monomeric form (green/FL-1).32 Significant mitochondrial membrane potentials were apparent in WT DT40 cells cultured in regular media, TRPM7-deficient cells maintained in Mg2+ supplemented media and proliferation-arrested TRPM7-deficient cells maintained in regular media (Fig. 3B, right part), as can be seen through the sizeable collapse of the membrane potential (and shift to green/FL-1 fluorescence) resulting from treatment with the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP) in each type of cell (Fig. 3B, right part).
Proliferation-arrested, quiescent TRPM7-deficient lymphocytes can resume cell cycle.
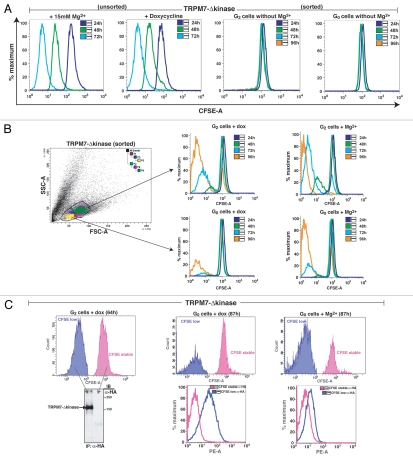
The cumulative results above indicate that proliferation-arrested TRPM7-deficient cells acquire every major reported quiescence-associated molecular and physiological signature. To determine whether TRPM7 channel function is sufficient to reverse an established quiescent state, we made use of a previously described TRPM7-deficient cell line which expresses a TRPM7 kinase domain deletion mutant under the control of a doxycycline-regulated promoter (TRPM7-Δ-kinase, reviewed in ref. 3). This cell line was chosen over TRPM7-KO cells complemented with full-length TRPM7 (cWT), as we have previously shown that cWT cells continue to cycle even when uninduced, apparently due to low basal expression level of the transfected protein driven by a leaky promoter.3 In contrast, when cultured in standard tissue culture media in the absence of doxycycline, TRPM7-Δ-kinase overexpressing cells undergo proliferative arrest indistinguishable from that of uncomplemented TRPM7-deficient cells; when doxycycline is added to the tissue culture media, these cells regain the ability to proliferate in standard tissue culture media, albeit at a reduced rate relative to growth in Mg2+ supplemented media.3 Analogous to earlier experiments with TRPM7-deficient cells, untreated TRPM7-Δ-kinase cells cultured in media with supplemental Mg2+ were transitioned to regular media for 24 hours, resulting in their proliferative arrest. Arrested cells were sorted on the basis of their forward scatter into small (putative 2n/G0) and large (putative 4n/G2-quiescent) populations, labeled with CFSE and were then either kept in regular media, transitioned back to growth-supporting media with supplemental Mg2+ or TRPM7-Δ-kinase expression was induced with doxycycline. Both the sorted putative G0 and G2-quiescent populations kept in standard media displayed stable CFSE staining identical to that observed in proliferation-arrested TRPM7-KO cells (Fig. 4A). Upon provision of 15 mM Mg2+, a proportion of cells in both putative G0 and G2-quiescent populations were able to re-enter the cell cycle (Fig. 4B). This result is identical to that observed for TRPM7-KO cells, and confirms that Mg2+ uptake is necessary and sufficient for resumption of proliferation from a state of quiescence for TRPM7-Δ-kinase cells. Induction of TRPM7-Δ-kinase expression via doxycycline was also able to support a proportion of cells in both G0/G1 and G2 populations to re-enter the cell cycle. To determine whether TRPM7-Δ-kinase expression correlated with cell cycle re-entry, we analyzed TRPM7-Δ-kinase expression in uninduced and induced cells maintained in the various conditions (Fig. 4C). Consistent with TRPM7-dependent Mg2+ uptake playing an essential role in supporting cell cycle re-entry of quiescent cells, the induced cells re-entering the cell cycle show substantial expression of TRPM7-Δ-kinase, while those unable to re-enter the cell cycle lack TRPM7-Δ-kinase expression (Fig. 4C).
Figure 4.
Induction of TRPM7 by doxycycline or provision of supplemental Mg2+ allows proliferation-arrested TRPM7-deficient cells to re-enter the cell cycle. (A) TRPM7-Δ-kinase cells transitioned to regular media display proliferation arrest in both G0/G1 and G2 populations. TRPM7-Δ-kinase cells cultured in growth-supporting media were transitioned to regular media for 24 hours following which they were sorted on the basis of their size scatter (as shown in B) into G0/G1 and G2 populations, commensurate to TRPM7-KO cells. Cells were labeled with CFSE along with positive controls-proliferating TRPM7-Δ-kinase cells in growth supporting media and TRPM7-Δ-kinase cells induced with doxycycline. Comparative analysis showed that while the positive controls proliferated and led to subsequent partitioning of the dye, proliferation arrested and sorted cells in G0/G1 and G2 transitioned to regular media, displayed stable CFSE staining and did not undergo any further cell divisions. (B) Proliferation-arrested TRPM7-Δ-kinase cells are able to recommence proliferation upon doxycycline induction or provision of 15 mM Mg2+. CFSE-labeled sorted G0/G1 and G2 populations were either induced with doxycycline (dox; 1 µg/ml) for TRPM7-Δ-kinase expression or replenished with supplemental Mg2+. By 72 hours, a sizable magnitude of cells were able to exit cell cycle arrest in both G0/G1 as well as G2 populations and resume cell division. However, TRPM7-Δ-kinase cells provided with doxycycline exhibited a lag in resumption of cell division as compared to the cells provided with supplemental Mg2+, which could be attributed to the time required by the dox-induced cells to initiate protein synthesis. (C) TRPM7-Δ-kinase expression drives both G0/G1 and G2 proliferation-arrested cells to reenter cell cycle. TRPM7-Δ-kinase cells transitioned to regular media for 24 hours were sorted into G0/G1 and G2 populations and labeled with CFSE. Left part: Post-sort, cells were transitioned to growth-supporting media or induced with doxycycline. Non-proliferating, stable CFSE labeled cells and proliferating, CFSE-low populations from doxycycline-induced G2 cells were further sorted at 64 hours, lysed and immunoprecipitated with anti-HA antibody. The immunoprecipitates were run on 8% SDS-PAGE and immunoblotted with anti-HA antibody for expression analysis of HA-tagged TRPM7-Δ-kinase. Right part: Similar CFSE-labeled populations, as mentioned in the left part, were sorted at 87 hours for doxycycline induced G0/G1 cells (stable CFSE labeled and CFSE low populations) expressing TRPM7-Δ-kinase and uninduced cells provided with 15 mM supplemental Mg2+. Cells were fixed, permeabilized and labeled with anti-HA/anti-mouse PE antibodies for detection of TRPM7-Δ-kinase by flow cytometry on BD LSRII. A dramatic increase in fluorescence was observed in proliferating CFSE-low, doxycycline-induced G0/G1 population as compared to the non-proliferating, stable CFSE-labeled cells. A small shift in fluorescence was also observed in the uninduced CFSE-low G0/G1 cells, which could be attributed to a leaky promoter.
Discussion
Overall, our data suggests that culture of TRPM7-deficient cells in standard tissue culture media results in their entering a state of quiescence: they reversibly cease proliferation, upregulate expression of p27kip1, display low RNA content, downregulate store-operated Ca2+ entry, and adopt a metabolic state dominated by mitochondrial respiration. As either supplemental Mg2+ or induction of TRPM7 expression are able to initiate cell cycle re-entry after proliferation arrest, uptake of extracellular Mg2+ via TRPM7 is identified as an essential biochemical event regulating transitions from quiescent to proliferative metabolism in lymphocytes. While the conditions used to induce quiescence also result in a significant fraction of TRPM7-deficient cells irreversibly exiting the cell cycle, this is attributable to those cells experiencing a prolonged period of quiescence, which is frequently observed to lead to irreversible cell cycle exit and senescence.33,34
An intriguing question raised by our results is the nature of the role TRPM7-dependent Mg2+ uptake has in supporting the transition from quiescent to proliferative physiology. Previous models have posited that glucose uptake is a key event driving the transition to aerobic glycolysis upon lymphocyte activation (reviewed in ref. 35). Because both respiration and aerobic glycolysis utilize glucose and other fuel substrates, it is difficult to see how uptake of fuel substrates alone might lead to such a marked metabolic alteration. However, the respective requirements of respiration and aerobic glycolysis for external Mg2+ uptake are entirely divergent: respiration recycles MgADP to MgATP, and thus does not affect the size of the cellular adenine nucleotide pool. In contrast, aerobic glycolysis is associated with massive de novo expansion of the adenine nucleotide pool, resulting in generation of new molecules of ADP, each of which requires a new Mg2+ ion to be taken up from the extracellular milieu. Thus, enhanced Mg2+ uptake is only required when a cell must expand its adenine nucleotide pool—precisely the situation which arises when a cell is activated to rapidly proliferate and must adjust its metabolism accordingly. If TRPM7-dependent Mg2+ uptake is generally required to initiate metabolic transitions associated with rapid proliferation, such as those that occur during malignant transformation, then targeting of Mg2+ uptake via TRPM7 could provide a novel strategy for inhibition of the growth of diverse types of malignant cells.
Materials and Methods
Cell culture.
DT40 B-lymphocytes were maintained in Roswell Park Memorial Institute (RPMI 1640) (Mediatech) with 10% fetal bovine serum (FBS), 1% chicken serum, 10 U/ml penicillin/streptomycin, 2 mM glutamine and 50 µg/ml blasticidin. For cell division experiments, cells were stained with CFSE according to the manufacturer's instructions (Invitrogen). CFSE-labeled cells were cultured in complete RPMI with 10% FBS, 1% chicken serum, 10 U/ml penicillin/streptomycin and 2 mM glutamine. In some experiments supplemental Mg2+, rapamycin, nocodazole and staurosporine were added at final concentrations of 15 mM, 100 nM, 3.3 µM and 1 µM, respectively.
Measurement of intracellular Ca2+ and Ca2+ flux.
For measurement of [Ca2+]i, cells were washed and resuspended in PBS containing 5% FBS at 106–107 cells/ml. Cell permeant indo-1-acetoxymethyl (Invitrogen) was added at a final concentration of 7 µM, and incubation was performed for 30 min at 37°C. The indo-1 fluorescence ratio (indo-1 was excited at 325 nm and emission detected using a 530/30 nm filter for Ca2+ free and a 405/20 nm filter for Ca2+ bound) of the cells was acquired as a function of time using a flow cytometer (BD-LSR II; Becton Dickinson). For each experiment, collection of 1-minute baseline measurement was followed by an initial stimulation with either 10 µg/ml anti-chicken IgM (Bethyl laboratories) or 1 µM thapsigargin (Biomol) with subsequent addition of 1 mM Ca2+ after 10–20 minutes, as indicated. For the purposes of direct comparison, the data presented in the figures are from single experiments performed in parallel though experiments were repeated two to three times with similar results. The kinetics of the data was analyzed with FlowJo software (TreeStar).
Immunoprecipitation, electrophoresis and western blotting.
Untreated or treated cells were washed once with PBS and whole cell lysates were prepared by lysing the cells in ice-cold lysis buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA] and complete mini-protease-inhibitor cocktail without EDTA (Roche), used according to manufacturer's instructions. The lysates were rotated for 45 min at 4°C and cell debris was removed by centrifugation at 13,000 rpm for 15 min at 4°C. Protein concentration of lysates was determined by Bicinchoninic acid (BCA; Pierce) assay using the manufacturer's specifications and anti-HA antibody (Cell Signaling) was used for immunoprecipitation of HA-tagged TRPM7-Δ-kinase. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out according to the method of Laemmli. Aliquots of supernatants/immunoprecipitates were separated on SDS-PAGE gels (10–12% for p27 and 6% for TRPM7-Δ-kinase) and analyzed by immunoblotting (IB). The proteins were transferred to 0.45 µm pore size polyvinylidene fluoride membranes (PVDF; Millipore) in transfer buffer (39 mM glycine, 48 mM tris base and 20% methanol) for 1–2 hours at 4°C. Membranes were blocked in 5% blocking buffer (5% w/v nonfat dry milk in TBS-0.1% Tween-20) for 1 hr at room temperature. Primary-antibody incubations were done overnight at the dilutions specified by the vendor. Incubations with the secondary antibody were performed with peroxidase-coupled anti-rabbit/anti-mouse immunoglobulin in 5% blocking buffer and the bound antibody was detected by ECL Chemiluminescence Detection System (Amersham).
Measurement of oxygen consumption rate (OCR).
OCR was measured in a perifusion system that allows for continuous measurement and flow rate was set to 80 microl/min; chamber volume was 400 microL. Cells (5–20 million) were loaded into the chamber with Cytodex beads (Amersham Biosciences) and sandwiched between two layers of Cytopore beads (Amersham Biosciences). OCR was calculated as the flow rate times the difference between inflow and outflow oxygen tension, which was measured by detecting the phosphorescence lifetime of an oxygen-sensitive dye that was painted on the inside of the perifusion chamber. Phosphorescent lifetimes were monitored using an MFPF-100 multifrequency phase fluorometer lifetime measurement system made by TauTheta Instruments (Boulder, CO) where the end of the excitation light guide (2 mm fiber optic patch cord; TauTheta part no. SFO-026) was illuminated by a 405 nm light-emitting diode that was just touching the outside of the glass opposite where the dye was painted and the detecting light guide was positioned at a 90° angle.
Measurement of mitochondrial membrane potential.
Mitochondrial membrane potential was measured by MitoProbe™ JC-1 Assay Kit for Flow Cytometry (M34152; Invitrogen). Briefly, cells were suspended in RPMI at approximately 1 × 106 cells/ml and 10 µl of 200 µM JC-1 was added. Cells were incubated at 37°C, 5% CO2 for 20 minutes and for the control sample, 1 µl of 50 mM mitochondrial membrane potential disruptor, CCCP (carbonyl cyanide 3-chlorophenylhydrazone) was added and cells were further incubated at 37°C for 5 minutes. After a wash with PBS, cells were resuspended in 500 µl of PBS by gentle flicking of the tube and analyzed by flow cytometry on BD FACSCalibur.
Flow cytometric analysis of DNA and RNA content.
DNA content and cell cycle analyses were carried out after fixation of cells and staining them with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI). Cells were acquired on LSRII flow cytometer (BD Biosciences) and analyzed by FlowJo (Ashland, OR). Briefly, 1–2 × 106 cells were spun and washed once with PBS. Cells were then fixed by adding 1 ml of ice-cold 70% ethanol and were stored at 4°C for at least 1 hr, followed by two washes with PBS. DAPI (1 mg/ml) dissolved in PBS, 0.1% BSA and 0.1% Triton X-100 was added to the cells and mixed well. Cells were kept at 4oC for 30 min and analyzed on the LSRII flow cytometer (BD Biosciences) with ultraviolet excitation and DAPI emission collected at >450 nm. For RNA content analysis, 1.5 µg/ml Pyronin Y was added to the samples just before acquisition.
Acknowledgements
We would like to thank Dr. Sarah Andrews and Mr. Ashok Bandaranayake (University of Washington and Seattle Children's Research Institute, Seattle, WA) for their helpful suggestions. This work was supported in part by NIH grants to A.M.S. and I.R.S. [DK17047 (the DERC Islet Core)]. We gratefully acknowledge the expert assistance of the Diabetes and Obesity Center of Excellence, University of Washington.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/12798
Supplementary Material
References
- 1.Sahni J, Scharenberg AM. TRPM7 ion channels are required for sustained phosphoinositide 3-kinase signaling in lymphocytes. Cell Metab. 2008;8:84–93. doi: 10.1016/j.cmet.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahni J, Nelson B, Scharenberg AM. SLC41A2 encodes a plasma-membrane Mg2+ transporter. Biochem J. 2007;401:505–513. doi: 10.1042/BJ20060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf I, Fruman DA. Regulation of quiescence in lymphocytes. Trends Immunol. 2003;24:380–386. doi: 10.1016/s1471-4906(03)00141-8. [DOI] [PubMed] [Google Scholar]
- 5.Giaretti W, Abmayr W, Dormer P, Santi L. The G0 in equilibrium G1 transitions of human lymphocytes as monitored by quantitative 14C-uridine autoradiography and high-resolution image analysis. Cytometry. 1985;6:219–225. doi: 10.1002/cyto.990060308. [DOI] [PubMed] [Google Scholar]
- 6.Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147–154. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 7.Gerson DF, Kiefer H. Intracellular pH and the cell cycle of mitogen-stimulated murine lymphocytes. J Cell Physiol. 1983;114:132–136. doi: 10.1002/jcp.1041140121. [DOI] [PubMed] [Google Scholar]
- 8.Negre N, Ghysen A, Martinez AM. Mitotic G2-arrest is required for neural cell fate determination in Drosophila. Mech Dev. 2003;120:253–265. doi: 10.1016/s0925-4773(02)00419-7. [DOI] [PubMed] [Google Scholar]
- 9.Wei W, Nurse P, Broek D. Yeast cells can enter a quiescent state through G1, S, G2 or M phase of the cell cycle. Cancer Res. 1993;53:1867–1870. [PubMed] [Google Scholar]
- 10.Kaldis P. Another piece of the p27kip1 puzzle. Cell. 2007;128:241–244. doi: 10.1016/j.cell.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 12.Coats S, Flanagan WM, Nourse J, Roberts JM. Requirement of p27kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- 13.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Blajeski AL, Phan VA, Kottke TJ, Kaufmann SH. G(1) and G(2) cell cycle arrest following microtubule depolymerization in human breast cancer cells. J Clin Invest. 2002;110:91–99. doi: 10.1172/JCI13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kops GJ, Medema RH, Glassford J, Essers MA, Dijkers PF, Coffer PJ, et al. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol Cell Biol. 2002;22:2025–2036. doi: 10.1128/MCB.22.7.2025-2036.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzachanis D, Lafuente EM, Li L, Boussiotis VA. Intrinsic and extrinsic regulation of T lymphocyte quiescence. Leuk Lymphoma. 2004;45:1959–1967. doi: 10.1080/1042819042000219494. [DOI] [PubMed] [Google Scholar]
- 17.Deng X, Ewton DZ, Friedman E. Mirk/Dyrk1B maintains the viability of quiescent pancreatic cancer cells by reducing levels of reactive oxygen species. Cancer Res. 2009;69:3317–3324. doi: 10.1158/0008-5472.CAN-08-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darzynkiewicz Z, Traganos F, Melamed MR. New cell cycle compartments identified by multiparameter flow cytometry. Cytometry. 1980;1:98–108. doi: 10.1002/cyto.990010203. [DOI] [PubMed] [Google Scholar]
- 19.Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, et al. Orai1 and STIM1 move to the immunological synapse and are upregulated during T cell activation. Proc Natl Acad Sci USA. 2008;105:2011–2016. doi: 10.1073/pnas.0706122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 21.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 22.Cahalan MD, Zhang SL, Yeromin AV, Ohlsen K, Roos J, Stauderman KA. Molecular basis of the CRAC channel. Cell Calcium. 2007;42:133–144. doi: 10.1016/j.ceca.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lievremont JP, Bird GS, Putney JW., Jr Mechanism of inhibition of TRPC cation channels by 2-aminoethoxydiphenylborane. Mol Pharmacol. 2005;68:758–762. doi: 10.1124/mol.105.012856. [DOI] [PubMed] [Google Scholar]
- 24.Pogue SL, Kurosaki T, Bolen J, Herbst R. B cell antigen receptor-induced activation of Akt promotes B cell survival and is dependent on Syk kinase. J Immunol. 2000;165:1300–1306. doi: 10.4049/jimmunol.165.3.1300. [DOI] [PubMed] [Google Scholar]
- 25.June CH, Rabinovitch PS. Flow cytometric measurement of intracellular ionized calcium in single cells with indo-1 and fluo-3. Methods Cell Biol. 1990;33:37–58. doi: 10.1016/s0091-679x(08)60510-5. [DOI] [PubMed] [Google Scholar]
- 26.Buttgereit F, Burmester GR, Brand MD. Therapeutically targeting lymphocyte energy metabolism by high-dose glucocorticoids. Biochem Pharmacol. 2000;59:597–603. doi: 10.1016/s0006-2952(99)00273-7. [DOI] [PubMed] [Google Scholar]
- 27.Krauss S, Brand MD, Buttgereit F. Signaling takes a breath—new quantitative perspectives on bioenergetics and signal transduction. Immunity. 2001;15:497–502. doi: 10.1016/s1074-7613(01)00205-9. [DOI] [PubMed] [Google Scholar]
- 28.Ibsen KH. The Crabtree effect: a review. Cancer Res. 1961;21:829–841. [PubMed] [Google Scholar]
- 29.Guppy M, Greiner E, Brand K. The role of the Crabtree effect and an endogenous fuel in the energy metabolism of resting and proliferating thymocytes. Eur J Biochem. 1993;212:95–99. doi: 10.1111/j.1432-1033.1993.tb17637.x. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert M, Jung SR, Reed BJ, Sweet IR. Islet oxygen consumption and insulin secretion tightly coupled to calcium derived from L-type calcium channels but not from the endoplasmic reticulum. J Biol Chem. 2008;283:24334–24342. doi: 10.1074/jbc.M802097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herst PM, Berridge MV. Cell surface oxygen consumption: a major contributor to cellular oxygen consumption in glycolytic cancer cell lines. Biochim Biophys Acta. 2007;1767:170–177. doi: 10.1016/j.bbabio.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 34.Toledo LI, Murga M, Gutierrez-Martinez P, Soria R, Fernandez-Capetillo O. ATR signaling can drive cells into senescence in the absence of DNA breaks. Genes Dev. 2008;22:297–302. doi: 10.1101/gad.452308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol. 2008;84:949–957. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.