Abstract
Matrikines are important components of tumor microenvironments that integrate communication between extracellular matricies and membrane-bound receptors thereby regulating cellular behaviors. One such matrikine that is differentially expressed in cancer microenvironments is the extracellular matrix protein lumican; however its precise role in cancer remains ambiguous. To study the effects of lumican on cancer cells, we created lumican-overexpressing cell lines from murine fibrosarcoma (MCA102) and pancreatic adenocarcinoma (Pan02) cells. Lumican overexpression in Pan02 cells increased invasiveness, decreased soft agar colony size, and increased proliferation. Conversely in MCA102 cells, lumican decreased invasiveness, increased soft agar colony size, but did not influence proliferation. In contrast to these pleiotropic in vitro results, lumican overexpression within the in vivo tumor microenvironment produced uniformly smaller tumors. Importantly, reduced tumor size was correlated with reduced vascular density. Consistent with lumican’s proposed anti-angiogenic activity, lumican increased endothelial cell apoptosis. Importantly, lumican was previously shown to influence Fas expression and our results show that lumican enhanced Fas mediated endothelial cell apoptosis although we were unable to detect any difference in Fas or Fas ligand expression between lumican-overexpressing and control cells. Interestingly, lumican had no effect on MCA102 apoptosis, suggesting that the observed reduction in tumor size is specifically due to endothelial cell apoptosis rather than a direct effect on the cancerous cells themselves. Therefore, this study is the first to demonstrate a causal relationship between tumor reduction and lumican’s effect on angiogenesis as opposed to an effect on the cancerous cells themselves.
Keywords: Lumican, Angiogenesis, Microenvironment, Apoptosis, Cancer, Matrikine
Introduction
The extracellular matrix has garnered much attention in the last two decades, due in large part to the discovery of secreted constituents that can coordinate cell behaviors between structural components of the matrix with membrane-bound receptors on the surface of cells. These “matrikines” can play important roles in regulating multiple cellular functions including growth, differentiation, and homeostasis. Intracellular signaling pathways including those involved in tumorigenesis and angiogenesis are similarly influenced by cell surface interactions with matrikines [1]. Members of the small leucine-rich proteoglycan (SLRP) family have been extensively studied for both their ability to bind collagen and other matrix proteins, and their ability to perform outside-in signaling [2]. The best characterized members of the SLRP family include decorin, fibromodulin, and lumican. The latter of these proteins is our focus, as it is the least understood.
Lumican is a 338 amino acid member of the SLRP family that exists as a 50–100 kDa keratan sulfate proteoglycan in the cornea but exists as a 55–57 kDa glycoprotein in most other tissues [2, 3]. The 37 kDa core protein possesses a middle region containing 11 leucine rich repeats (LRRs), and a C-terminal LRR “ear repeat” arranged in a “banana-shaped” tertiary structure [4]. Lumican was originally identified as a regulator of collagen fibrillogenesis and the concave side of lumican shares a homologous collagen-binding domain on LRR5-7 with its closest relative fibromodulin, another collagen regulator [5]. Indeed, both lum -/- and fmod -/- knockout mice lack appropriate collagen organization [6, 7]. Proper collagen organization is vital to establishing corneal transparency (for which lumican derives its namesake) and scleral thickness. Observations in mice and zebrafish provide confirmatory evidence for this as lum -/- mice exhibit corneal opacity and morpholino knockdown of lumican results in zebrafish scleral thinning [6, 8]. Studies examining SLRPs and their involvement in cancer have mostly focused on decorin, but the role of the SLRP family member lumican in cancer is receiving increased attention [3].
There is substantial work implicating lumican’s involvement in cancer. Immunohistochemical (IHC) analysis of lumican expression has been reported in melanoma, breast, pancreatic, colorectal, cervical, neuroendocrine, and lung carcinomas [9–14]. Similarly, microarray studies have highlighted trends in lumican mRNA expression in various stages of these and other cancer types. At best, these methods provide correlative data for the presence or absence of lumican and the severity of disease, but they do not elucidate the role of lumican in cancer. More information has been gleaned on the role of lumican in cancer from experimental cell biology. For example, lumican-overexpression consistently results in reduced colony formation in anchorage-independent soft agar growth assays [15, 16]. Additionally, melanoma cells exhibit decreased migration, invasion, and metastasis when treated with lumican [15, 17]. Finally, lumican also drives a reduction in subcutaneous tumor volume in mouse models that is associated with reduced vascular density [15, 18].
Multiple lines of evidence support a role for lumican in the regulation of vascular function. For example, lumican is localized to the peripheral blood vessels in adult human lungs and to the thickened intima of the coronary artery and demonstrates binding affinity for αV integrin [3, 19, 20]. Functionally, endothelial cell expression of lumican increases during the resolution phase of angiogenesis in which vascularization ceases and the vessel returns to a state of angiostasis [21]. Additionally, lumican is inversely regulated with endoglin, a marker for angiogenic tissue [22]. Not surprisingly, lum -/- fmod -/- knockout mice exhibit increased vascularization in the myocardium, suggesting an anti-angiogenic role for lumican [7]. Finally, our previous data demonstrate that lumican can reverse the pro-angiogenic affects of basic fibroblast growth factor (bFGF) in Matrigel plug assays, highlighting lumican’s effectiveness as an anti-angiogenic molecule [21].
Our goal in the present study was to expand our understanding of the effects of lumican overexpression on a variety of cancer cells in vitro and in vivo, and the potential mechanism(s) of these effects. Specifically, we test the hypothesis that lumican plays an anti-angiogenic role in the tumor microenvironment. We demonstrate that lumican does not exhibit a consistently positive or negative effect on tumor cells in in vitro murine models for fibrosarcoma (MCA102) and pancreatic adenocarcinoma (Pan02) [23, 24]. Conversely, lumican does appear to consistently reduce tumor volume in vivo by blocking angiogenesis. This likely results from enhanced susceptibility to Fas-induced apoptosis, as lumican increased MB114 endothelial cell susceptibility to Fas-induced apoptosis in vitro. Together, these results support a model in which lumican blocks tumor growth and angiogenesis by enhancing apoptosis of endothelial cells as they invade the tumor stroma.
Materials and Methods
Cell Culture, Plasmids, and Retroviral Infections
The full length murine lumican cDNA (#5707371) (GenBank ID: BQ442885) less the secretory signal was PCR-amplified and cloned into the pSecTag A plasmid via 5′BamHI and 3′ NotI restriction sites using the following oligos. (fwd 5′-GGCGGCGGATCCCAATACTACGATTATGAC-3′) (rev 5′-GGCGGCGCGGCCGCGTTAACGGTGATTTCATT-3′). The resulting Igκ-Lumican-Myc-His6 cassette was PCR-amplified and ligated into the bicistronic retroviral vector pMSCV-Neo via 5′HpaI and 3′ BglII restriction sites using the following oligos. (fwd 5′-CCGGCCGAATTCTTAATACGACTCACTATAGGG-3′) (rev 5′-CCGGCCAGATCTCAACAGATGGCTGGCAACTAG-3′). Retroviral supernatants were produced by EcoPack2 retroviral packaging cells (Clontech, USA) and used to infect the murine fibrosarcoma cell line MCA102, murine pancreatic carcinoma cell line Pan02, and the murine brain microvascular endothelial cell line MB114 as described previously [25]. Polyclonal populations of cells were selected via addition of 400 nM G418 and maintained with 200 nM G418.
Detection of endogenous secreted lumican was performed via TCA 50%/DOC.01% protein precipitation from conditioned serum free media (SFM) and western blotting with rabbit anti-lumican polyclonal antibody. Confirmation of myc-his-tagged secreted lumican overexpression was achieved via Ni-NTA Agarose (Qiagen, Valencia, CA) binding from conditioned serum free media and western blotting with mouse anti-myc monoclonal antibody.
Detection of overexpressed and endogenous intracellular lumican was performed via western blot on collected whole cell lysates using mouse anti-myc monoclonal antibody and rabbit anti-lumican polyclonal antibody, respectively.
In Vitro Cancer Cell Assays
The effect of lumican overexpression on MCA102 and Pan02 cellular invasion was measured using a modified Boyden chamber assay as described previously [26]. Briefly, a porous membrane (8 μm pore, 24-well format; BD Biosciences, San Jose, CA) was coated with 100 μl of a 1:50 dilution of Matrigel (BD Biosciences, San Jose, CA) which was allowed to dry overnight at room temperature. The following day, 100,000 control and lumican-overexpressing cells were cultured on dried membranes in SFM +.1% BSA. Cellular invasion was induced by adding 5% serum to the lower chamber and was allowed to proceed at 37°C for 48 h. Subsequently, Matrigel-invading cells were washed twice with ice-cold PBS and immediately fixed for 10 min with 95% ethanol. Cells remaining in the upper chamber were removed with a cotton swab, whereas those remaining in the lower chamber were stained with crystal violet. Invasion was measured by densitometry utilizing the software ImageJ (NIH, Bethesda, MD).
The ability of lumican to alter the anchorage-independent growth of MCA102 and Pan02 cells was performed as described previously [27]. Briefly, 2 ml of a 1.2% agarose mixture in DMEM were allowed to solidify in 6-well plates. Control or lumican-overexpressing cells (50,000 cells/well) were diluted with an equal amount of DMEM-agarose mixture and allowed to solidify before placing in 37°C for 24 h. Plates were observed and 1 ml DMEM + 10% FBS was added to each well as needed to avoid dehydration. Colony areas were measured after 30 days using the software NIS-Elements D 3.00 SP1 (Build 455) (Nikon, Inc., Melville, NY).
Cell proliferation assays were conducted with WST-1 (Clontech, Mountain View, CA). Briefly, 500 cells were placed in 100 μl complete media in 12 wells of a 96-well plate and allowed to grow at 37°C for 24 h. The following day, the first three wells were replaced with 100 μl complete media containing 10 μl WST-1. Empty wells were also treated to establish a blank baseline. After 4 h, wells were measured at OD 450 nm to determine proliferation. This procedure was followed for each of 4 days to determine the proliferation rates for the control and lumican-overexpressing cells.
In Vivo Tumor Growth Studies
In three independent trials, control and lumican-overexpressing MCA102 and Pan02 cells were resuspended in sterile phosphate buffered saline (PBS) and injected subcutaneously at a density of 1,000,000 cells/100 μl injection between the shoulder blades of 10-week-old male C57BL/6 mice (three mice per condition; Jackson Laboratories, Bar Harbor, ME). Mice were monitored daily and primary tumors were measured externally with calipers between days 9 and 17. Tumor volumes were calculated using the following equation: 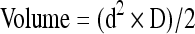 , where D is the long side and d is the short side. After 17 days (or if tumors became necrotic or achieved a size greater than 2,000 mm3) mice were euthanized and their primary tumors were excised and weighed. Animal studies were performed in accordance with the animal protocol procedures approved by the Institutional Animal Care and Use Committee of Indiana State University (protocol #1-19-2008AA).
, where D is the long side and d is the short side. After 17 days (or if tumors became necrotic or achieved a size greater than 2,000 mm3) mice were euthanized and their primary tumors were excised and weighed. Animal studies were performed in accordance with the animal protocol procedures approved by the Institutional Animal Care and Use Committee of Indiana State University (protocol #1-19-2008AA).
Immunohistochemistry
Excised tumors were fixed in 4% paraformaldehyde for 1 h and placed in 70% ethanol before paraffin embedding and sectioning following standard procedure [28]. Sections were prepared via hematoxylin and eosin staining and additional sections were probed with anti-mouse CD31 in the Clarian Pathology Laboratory at Indiana University (Indianapolis, IN).
Colony Forming Survival Assays
The survival ability of lumican-overexpressing endothelial cells was compared to controls in the following manner. Five hundred MB114-Neo and MB114-Lum cells were plated onto 6 cm plates containing 4 ml MB114 media. Cells were allowed to adhere and grow for approximately 1 week until colonies were visible by eye. Media was removed and plates were washed with 1 × PBS. Cells were fixed with 95% ethanol for 5 min and stained with crystal violet stain. Excess stain was removed with water and plates were allowed to dry before counting visible colonies.
Apoptosis Assays
To monitor apoptosis by caspase 3 western blot, 50,000 lumican-overexpressing or control MB114 cells were plated onto 12-well culture plates. Cells were allowed to grow 24 h before washing with PBS and treating with 1 ml of serum free media (SFM) in the presence or absence of the hamster anti-mouse CD95 (Fas) agonizing antibody Jo-2 (final concentration 1 μg/ml) (554254; BD Pharmingen, San Jose, CA). Afterward, live and dead cells were collected and whole cell lysates were analyzed by western blot analysis.
To monitor apoptosis by Caspase-3/7 activity, 5,000 MB114-Neo or MB114-Lum cells were each plated in four wells of a 96-well culture plate. Alternatively, to examine the effect of lumican conditioned media on endothelial cells, media isolated from lumican-overexpressing or control MCA102 or Pan02 cells was filtered and buffered with 10 mM pH 7.3 HEPES buffer. MB114 control cells were grown in the presence of each conditioned media. In both cases, MB114 cells were incubated in the presence or absence of Jo-2 for 24 h before detection of caspase 3/7 with the caspase-Glo 3/7 assay as described by the manufacturer (Promega). Luminescence was monitored on a Glomax luminometer.
Western Blot and Antibodies Used
Western blotting was performed as described previously [26]. Antibodies utilized in the experiments include the following: mouse anti-c-Myc (1:1000) (9E10, MMS-150R) (Covance, Inc., Princeton, NJ); mouse anti-β-actin (1:1000) (sc-47778), rabbit anti-Fas-L (1:500) (C-178, sc-6237), rabbit anti-Fas (1:500) (A-20, sc-1023) (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit anti-caspase-3 (1:500) (#9662) (Cell Signaling Technology, Inc., Danvers, MA); rabbit anti-lumican (1:100) (kindly provided by Dr. S. Chakravarti, Johns Hopkins University, Baltimore, MA); and sheep anti-mouse (1:5000) (NA931-1ML), donkey anti-rabbit (1:5000) (NA934-1ML) (GE Healthcare, Piscataway, NJ).
Results
Lumican Overexpression Exhibits Pleiotropic Effects on MCA102 Murine Fibrosarcoma Cells and Pan02 Murine Pancreatic Adenocarcinoma Cells in Vitro
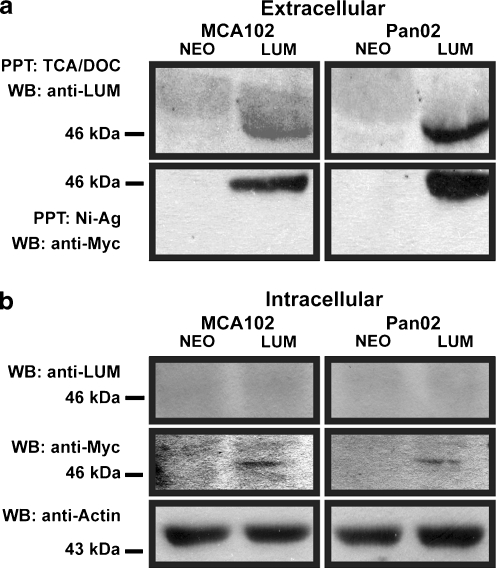
To examine the effect of lumican overexpression on a wider variety of cell types, we created lumican-overexpressing fibrosarcoma and pancreatic adenocarcinoma cell lines. The murine MCA102 fibrosarcoma and Pan02 pancreatic adenocarcinoma cell lines were transduced with retroviral constructs encoding either murine lumican cDNA to which a C-terminal Myc-epitope had been appended or an empty control vector. Selection with G418 was subsequently used to establish stable polyclonal cell lines. Conditioned media from these cell lines was precipitated and western blotting with the anti-Myc antibody was used to confirm expression of secreted lumican transgenes in the stably selected cell lines (Fig. 1a). Stripping and reblotting with polyclonal anti-lumican antibodies confirmed that the Myc-tagged protein was lumican. Importantly, the fact that anti-lumican antibodies failed to detect secretion of endogenous lumican in either MCA102 or Pan02-neo cells confirmed that overexpression of lumican in our stable cell lines was achieved. Since some small leucine-rich proteoglycans have intracellular roles it was also important to examine the relative abundance of intracellular lumican in the cell lines. To accomplish this, whole cell lysates from the cell lines were collected and western blotted with the anti-Myc and anti-lumican antibodies to detect intracellular lumican (Fig. 1b). Although anti-Myc western blotting revealed a light but detectable band in the overexpressing cell lines, western blotting with anti-lumican failed to detect intracellularily expressed lumican. This indicated that in both MCA102 and Pan02 cells, overexpressed lumican is almost entirely secreted to the extracelluar environment.
Fig. 1.
Confirmation of lumican overexpression and efficient secretion (a) Confirmation of secreted lumican transgene expression was performed on conditioned media from the MCA102 fibrosarcoma and Pan02 pancreatic adenocarcinoma cell lines. TCA/DOC precipitation and detection with polyclonal anti-lumican antibodies revealed little or no endogenous lumican expression. Ni-Ag precipitation and detection with anti-cMyc antibody confirmed overexpression of Myc-tagged lumican. (b) Detection of intracellular lumican was performed on whole cell lysates from the MCA102 and Pan02 cell lines using polyclonal anti-lumican antibodies. Overexpressed lumican was detected using anti-cMyc antibody. Stripping and reblotting with β-actin antibodies served as a loading control
Due to the deficit of direct evidence regarding the role of lumican in cancer, we subjected the lumican-overexpressing MCA102 and Pan02 cells (MCA102-Lum and Pan02-Lum) and their control counterparts (MCA102-Neo and Pan02-Neo) to a variety of in vitro experiments designed to mimic several aspects of tumor behavior.
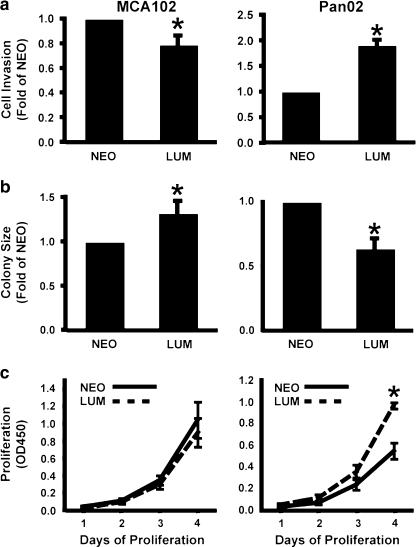
Tumor cell invasion is one of the deadliest aspects of cancer as the ability to enzymatically degrade a proteinaceous matrix is correlated with metastatic potential [29]. Thusly, Boyden chamber invasion assays were performed to assess the ability of lumican to affect invasion through a Matrigel matrix. Lumican overexpression resulted in a 22% reduction in invasion in the MCA102 cells, but enhanced invasion in the Pan02 cells by 91% (Fig. 2a).
Fig. 2.
Lumican overexpression exhibits pleiotropic effects in vitro (a) Lumican-overexpressing cell lines and their corresponding control cell lines were induced to invade through Matrigel coated Boyden chambers. Lumican overexpression increased invasion in PanO2 cells but decreased invasion in MCA102 cells. Data is the average (+/-SEM) of at least four independent experiments. (b) The anchorage independent growth of lumican-overexpressing and control cells was examined by culturing cells in soft-agar. The volumes of the resulting colonies were calculated from two dimensional measurements of colony diameter. Lumican increased MCA102 colony size but decreased PanO2 colony size. Data is presented as the average of three independent experiments (+/-SEM). (c) Lumican-overexpressing PanO2 and MCA102 cells were cultured for 1–4 days. Each day, the relative number of cells was measured with WST-1 cell proliferation reagent. Lumican increased PanO2 proliferation, but did not affect MCA102 proliferation. Data is presented as the average of four independent experiments (+/-SEM). (* indicates p < .05, Student’s T-Test)
Cancer cells have the unique ability to form colonies in soft agar, as they do not require anchorage via an extracellular substrate to grow. As this is an excellent in vitro analog for tumor formation, we performed soft agar assays to determine if lumican overexpression affects anchorage-independent growth of cancer cells. In contrast to the results obtained in the invasion assays, lumican overexpression increased the average colony size of MCA102 cells by 38%, but in Pan02 cells the average colony size was reduced by 36% (Fig. 2b).
A hallmark of cancer cells is their rapid rate of proliferation. To monitor the effect of lumican on cancer proliferation, we used WST-1 proliferation assays to determine if lumican overexpression influences the growth rate of cancer cells. Similar to the results of the invasion assay, lumican overexpression significantly increased the proliferation of Pan02 cells but had no detectable effect on MCA102 cell proliferation (Fig. 2c).
Collectively, these in vitro data indicated that the overexpression of lumican in these tumor cell lines resulted in no consistent pattern of effects on the various tumor cell activities of invasion, proliferation, or anchorage-independent growth between the two cell lines. From this data we were unable to arrive at a singular conclusive effect of lumican on cancer cells. However, in vitro data is removed from the complex microenvironment of the host organism and may not reflect the behavior of the cancer in vivo. In light of this, we next sought to determine what effect lumican would have in an animal model of tumor growth.
Lumican Overexpression Consistently Reduces MCA102 Murine Fibrosarcoma and Pan02 Pancreatic Adenocarcinoma Tumor Volume in Syngenic Mice
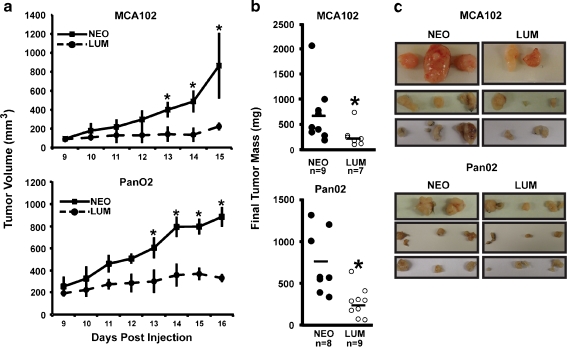
Understanding the effect of lumican in cancer requires consideration of the microenvironment established by complex interactions between the host and the cancer cells. To determine how lumican-overexpressing cancer cells would interact with a syngenic host, we injected male C57BL/6 mice subcutaneously with equal numbers of syngenic MCA102-Lum, MCA102-Neo, Pan02-Lum, or Pan02-Neo cells and monitored both tumor growth and final tumor mass. Despite significant variation between individual tumor growth curves and final tumor masses, both measures indicated that lumican overexpression caused an approximately 60% reduction in mean tumor size for both MCA102 and Pan02 cells (Fig. 3). The reason for this variability in tumor growth is unknown but may be related to the random subcutaneous placement of tumor inoculations. Importantly, these results show a consistent effect on tumor growth in vivo and are in stark contrast to our in vitro data which demonstrated cell-specific effects on invasion, proliferation, and soft agar colony formation. Previously, we have demonstrated that lumican negatively impacts endothelial cell behaviors and can reduce blood vessel growth into Matrigel plugs [21]. Such a reduction in angiogenesis might explain the reason for the smaller average tumor volume. We hypothesized that reduced growth in lumican overexpressing tumors was due to insufficient angiogenesis and therefore examined blood vessel density within the extracted tumors.
Fig. 3.
Lumican consistently reduces tumor growth in vivo Equal numbers of lumican-overexpressing MCA102 fibrosarcoma and Pan02 pancreatic adenocarcinoma cells or their corresponding control counterparts were injected in triplicate into syngenic C57BL/6 mice. (a) Tumor volumes were calculated daily and are reported as the average for each day relative to the first day after inoculation that tumors appeared. Data is the average (+/-SEM) of three independent experiments. (* indicates p < .05, Mann–Whitney rank sum test). (b) Tumor masses were recorded at time of removal. Data is the final tumor mass (mg) for each tumor. Midlines represent mean mass. (* indicates p < .05, Mann–Whitney rank sum test). (c) Photos depicting the actual tumors from each of three independent experiment are shown
Tumors Overexpressing Lumican Possess Reduced Vasculature
Vascular density in lumican-overexpressing and control tumors was determined by measuring vascular area in tumor sections subjected to immunohistochemistry with CD31 expression. As shown in Fig. 4a, tumors derived from lumican overexpressing MCA102 and PanO2 had consistently less CD31 staining compared to tumor derived from their control counterpart cells. Lumican is well known for its collagen organizing activities [6, 30]. Therefore, we also performed Masson’s trichrome staining of tumor sections derived from control or lumican overexpressing cells. As shown in Fig. 4a, there was no obvious difference in collagen staining (blue) in tumors derived from lumican overexpressing cells. Overall, lumican overexpression provoked a 58% decrease in vascular density in MCA102 cells and a 44% decrease in vascular density in PanO2 cells. Collectively, these results suggest that reduced tumor volume is not a consequence of a direct effect on the tumor cells or a consequence of significantly altered collagen deposition within the tumor microenvironment, but rather the reduced growth is coupled to a reduction in angiogenesis.
Fig. 4.
Lumican overexpression reduces tumor vasculature (a) Tumors derived from lumican overexpressing and control cell lines were sectioned and stained with Masson’s trichrome or by immunohistochemistry with anti-CD31 antibodies to visualize tumor vasculature. Shown are representative images collected from three independently performed tumor studies performed in triplicate. (b) Vascular area in lumican overexpressing and control tumors was determined by measuring the amount of CD31 staining present in 5 representative fields from each tumor using Image J densitometry software. Lumican reduced vascular density by ~58% in tumors derived from MCA102 cells and by ~44% in tumors derived from PanO2 cells. Data is presented as fold CD31 staining (+/-SEM) of each tumor compared to control. (* indicates p < .05, Student’s T-Test)
Previous research suggests that lumican may mediate Fas-Fas-L interactions, contributing to induction of apoptosis [31, 32]. Such an effect on endothelial cells could provide a basis for the observed reduction in vasculature. Based on these previous findings, we sought to determine what effect, if any, lumican has on the induction of apoptosis in endothelial cells.
Lumican Increases Apoptosis in Endothelial Cells
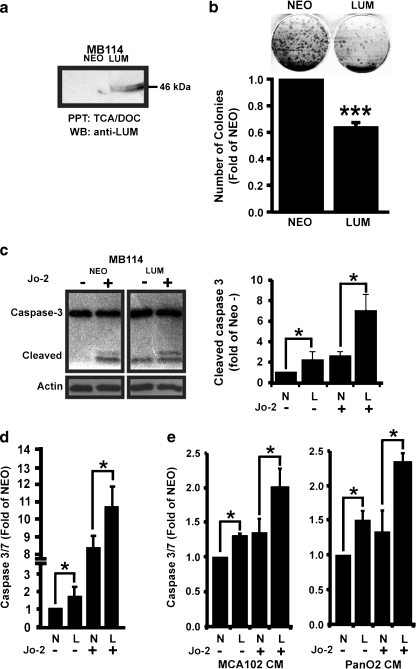
To determine if lumican could facilitate apoptosis in endothelial cells, we first established lumican-overexpressing and control MB114 endothelial cell lines and confirmed overexpression via western blot (Fig. 5a). Once again, western blot analysis with the anti-lumican antibody failed to detect endogenous lumican expression in control cells but readily detected lumican in the conditioned media of MB114-Lum cells. Intracellular lumican, as detected by western blotting with anti-Myc and anti-lumican antibodies, was determined to be in low abundance and roughly equivalent (data not shown). As an initial measurement of endothelial cell survival, we examined the ability of single MB114-Lum or control cells to form colonies in cell culture. Five hundred cells were cultured into 6 cm plates and allowed to form colonies for approximately 1 week. Consistent with a role in promoting apoptosis, 33% fewer MB114-Lum cells survived to form colonies when plated at a low density as compared to MB114-Neo cells (Fig. 5b). This result could be explained by decreased cell proliferation; however the size of MB114-Lum colonies was not significantly reduced compared to control colonies and we have not previously noted an effect on proliferation associated with lumican in endothelial cells [21]. This finding supported our hypothesis that lumican promotes endothelial cell apoptosis.
Fig. 5.
Lumican enhances Fas-mediated apoptosis (a) Lumican overexpression in MB114 cells was confirmed by western blot analysis of conditioned media from lumican overexpressing or control (NEO) MB114 cells. Polyclonal anti-lumican antibodies revealed little or no endogenous expression of lumican in control MB114 cells and confirmed expression of lumican in transduced cells. (b) (Top) Representative colonies formed after plating 500 MB114-lumican or MB114-NEO cells into 6 cm plates. (Bottom) Average number of colonies present in control and lumican overexpressing colony formation assays. Data is presented as the average fold of colonies formed by MB114-Neo (+/-SEM) of four independent experiments. (c) Control or Lumican-overexpressing MB114 cells were cultured in the presence or absence of Fas-cross-linking Jo-2 antibodies. Whole cell lysates were probed with anti-caspase-3 antibodies to detect cleaved caspase-3 and with anti β-actin antibodies to control for protein loading. Shown is a representative experiment that was independently performed four times. Resulting western blots were quantified by densitometry with ImageJ software and the resulting data is presented as the average fold increase of cleaved caspase-3 (+/-SEM) compared to unstimulated control cells. * denotes p < .05, student’s t-test. (d) Stably expressing MB114-Lum and MB114-Neo cells were incubated with Caspase-Glo 3/7 reagent after 48 h in the presence or absence of Jo-2. Data is presented as the average fold increase (+/-SEM) of caspase-3/7 activity compared to untreated MB114-Neo cells from three independent experiments. (e) MB114 endothelial cells were cultured for 24 h in conditioned media from control or lumican overexpressing MCA102 or PanO2 tumor cells and for an additional 24 h in the presence or absence of Fas activating Jo-2 antibodies. Caspase 3/7 activity was monitored by luminometry with the Caspase-Glo 3/7 reagent and normalized to control cells incubated in Neo conditioned media alone. Data is presented as the fold caspase 3/7 activity (+/-SEM) of three independent experiments. In all figures, * denotes p < .05, *** denotes p < .01, student’s t-test
Previously, lumican has been associated with the expression of Fas receptors and with enhancing Fas mediated apoptosis in corneal fibroblasts [31, 32]. Therefore to more precisely define the mechanistic basis for lumican’s pro-apoptotic effect, MB114-Lum and MB114-Neo cells were cultured in the presence or absence of the Fas-cross-linking antibody Jo-2 under serum free conditions. Live and dead cells were collected and apoptosis was assessed via immunoblotting for cleaved caspase-3. Blots were subsequently stripped and reblotted with anti-β-actin to monitor protein loading. Lumican-overexpressing MB114 cells demonstrated a greater amount of cleaved caspase-3 than control cells in both untreated conditions and upon treatment with Jo-2 (Fig. 5c left panel). Densitometry of the resulting western blots demonstrated a significant increase in cleaved caspase 3 present in lumican overexpressing MB114 cells both before and after Jo2 treatment (Fig. 5c right panel).
To further quantify the pro-apoptotic effects of lumican, MB114-Lum and MB114-Neo cells were again cultured in the presence or absence of the Fas-cross-linking Jo-2 antibody and subsequently incubated with the Caspase-Glo 3/7 Reagent. Caspase 3/7 activity was monitored by luminometry. As shown in Fig. 5d, MB114-Lum cells demonstrated significantly more caspase 3/7 activity in both unstimulated and Jo-2 stimulated conditions. To mimic the conditions of the animal study, MB114 control cells were also treated with conditioned media from control or lumican overexpressing MCA102 or PanO2 cells. Prior to applying the conditioned media, we confirmed that MCA102-Lum and Pan02-Lum media expressed lumican while their control media did not (data not shown). MB114 cells treated with either MCA102 or PanO2 lumican conditioned media in the presence or absence of Jo-2 demonstrated more cleaved caspase-3/7 activity than those treated with control conditioned media (Fig. 5e). Despite this increase in apoptosis and sensitivity to Fas-mediated cell death, we were unable to detect any difference in expression of Fas receptors or Fas-ligand in the presence or absence of lumican (data not shown)
We also examined the effect of lumican on apoptosis in Pan02 and MCA102 cells. Interestingly, while lumican overexpression did appear to increase apoptosis in Pan02 cells in the presence of Jo-2, it lacked this effect in MCA102 cells (data not shown). As was the case in MB114 cells, western blotting for Fas-receptor and Fas-ligand failed to detect any difference in expression between control and lumican overexpressing cells (data not shown). These results are particularly interesting as our in vitro data demonstrated consistently greater proliferation and invasion in the Pan02-Lum cell line. As no increase in apoptosis was observed in the MCA102-Lum cell line, a general increase in total cellular apoptosis susceptibility is an unlikely explanation for the reduction in tumor size observed in the mice.
Discussion
Much of our current understanding regarding the role of lumican in cancer is derived from non-hypothesis-driven immunohistochemical correlations of the relative abundance of the protein in the stroma of cancerous and non-cancerous tissues. Our hypothesis-driven approach illuminates a functional role of lumican in tumor growth. In this study, we demonstrated that overexpression of the extracellular matrix protein lumican has differential effects on cancer cell proliferation, invasion, and anchorage independent growth in the fibrosarcoma cell line MCA102 and the pancreatic adenocarcinoma cell line Pan02. Despite the pleiotropic in vitro effects, lumican overexpression consistently reduced tumor size and blood vessel density in vivo. Furthermore, we provide evidence that this reduction in blood vessel density is due to a pro-apoptotic effect of lumican on the endothelial cells invading the tumor stroma, although we have not pinpointed the precise mechanism by which this is achieved.
To study the affect of lumican on tumor cell behaviors, we overexpressed lumican in two murine cell lines syngenic to the C57BL/6 host. The results of our in vitro analyses on MCA102 fibrosarcoma cells and Pan02 adenocarcinoma cells overexpressing lumican demonstrate the cell-specific effects of lumican on several deadly aspects of cancer in isolation from the host microenvironment. More specifically, Pan02 cells overexpressing lumican were more invasive in Matrigel-coated Boyden chamber invasion assays than their control counterparts. However, the opposite effect was observed in MCA102 cells, which exhibited a marked reduction in invasion. Soft agar assays were performed to assess lumican’s effect on anchorage independent growth. In these experiments lumican overexpression resulted in smaller average colony size in MCA102 cells. Past experiments on a variety of cell lines support the hypothesis that lumican reduces the size of soft agar colonies [15, 16, 18]. However, this pattern was not observed in Pan02, in which lumican overexpression resulted in increased colony size. Finally, we assessed the effect lumican overexpression has on cellular proliferation. In MCA102 cells, lumican overexpression did not affect proliferation. In Pan02 cells however, lumican overexpression produced a significant increase in proliferation. This result is also in direct contrast with previous reports of lumican’s effect on proliferation in melanoma, osteosarcoma, murine embryonic fibroblasts, and HEK 293T cells [15, 31, 33, 34]. Collectively, these results demonstrated that lumican has pleiotropic effects on cancer cell biology.
We currently do not understand how lumican elicits these pleiotropic effects on various tumor cells. Lumican was previously shown to have dose dependent effects on osteosarcoma cells in vitro [33] and as shown in Fig. 1, lumican was not equally overexpressed in MCA102 and PanO2 cells. Therefore, it is possible that our results showing plieotropic effects of lumican on cancer cell biology may be due to differential expression of lumican instead of a bona-fide pleiotropic cell type specific activity. Alternatively, these divergent observations could be explained by the presence of multiple high and low affinity lumican receptors present in various cancer cells. In support of this, lumican has been shown to interact with several integrins including those containing the β1 and α2, 3, 4, 5, and v subunits [20]. Future research will clearly need to address this point by correlating lumican receptor expression with the biological impact of lumican on cell behaviors.
Although there is no consensus between lumican’s in vitro effects on MCA102 and Pan02 cells, we have demonstrated that lumican consistently has negative effects on tumors produced from these cell lines in vivo. Moreover, since the in vitro effects of lumican on these cell lines are pleiotropic, the reduction in tumor size is unlikely due to direct effects on the tumor cells. The observed reduction in tumor size is in accordance with previous reports using induced oncogenic fibroblasts and melanoma cell lines [15, 18]. The consistency of effects in vivo highlights the importance of the host microenvironment in cancer progression. Earlier reports have demonstrated that lumican consistently has a negative effect on melanoma cells in vitro, making it difficult to fully appreciate or isolate the in vivo effects of lumican on the host microenvironment [15, 17, 35]. Additionally, our previous findings suggested that lumican’s anti-angiogenic potential could be implicated in the reduced tumor volume [21].
CD31 localization of the extracted tumor sections revealed a reduced vessel density in lumican-overexpressing MCA102 and Pan02 cells. This finding supports the hypothesis that lumican performs an anti-angiogenic role in the tumor microenvironment and is in agreement with earlier studies which have found that myocardial vascularization increases in lum -/- fmod -/- knockout mice [7]. Similarly, Matrigel plug experiments have demonstrated that lumican can reduce vascularization induced by bFGF [21]. Recent reports of endothelial cells plated on lumican reveals reduced pseudotube formation [17] and previous research on tubulating endothelial cells has demonstrated expression of lumican increases during the resolution phase of angiogenesis in which vascularization ceases and the vessel returns to a state of angiostasis [21]. This angiostatic state can be observed in large resting vessels where expression of lumican is high [22].
Several ECM proteins including angiostatin, canstatin, thrombospondin-1, and decorin block angiogenesis by promoting endothelial cell apoptosis [36–40]. Similarly, our results now show that lumican promotes apoptosis in endothelial cells. Upon analysis of endothelial colony-forming assays as well as caspase-3/7 cleavage, we report that lumican consistently increases susceptibility to Fas-induced apoptosis in lumican-overexpressing endothelial cells; and endothelial cells cultured in conditioned media from lumican-overexpressing tumor cells. Previous reports have demonstrated that lumican increases Fas-receptor expression, preferentially binds to soluble Fas-L, and increases apoptosis in corneal fibroblasts [31, 32]. We identified the presence of Fas-receptor and Fas-ligand in equal abundance in MB114, MCA102, and Pan02 cells without regard to lumican overexpression (data not shown) suggesting that increased endothelial cell apoptosis is not due to increased expression of these pro-apoptotic proteins. Thus, lumican increases the susceptibility of endothelial cells to Fas-mediated apoptosis by a mechanism independent of Fas or Fas-L upregulation. This is slightly different from the proposed anti-angiogenic mechanisms of canstatin and thrombospondin-1 which involve increased endothelial Fas-ligand expression followed by Fas-mediated apoptosis [40, 41].
In addition to Fas-mediated apoptosis, it is possible that lumican may block angiogenesis by manipulating additional molecular mechanisms not explored in this study. For example, expression of lumican is inversely correlated with the expression of endoglin, a pro-angiogenic co-receptor of TGF-β in endothelial cells [22, 42]. Endoglin mediates it’s pro-angiogenic activities by emphasizing activation of pro-angiogenic SMADs 1/5/8 and deemphasizing activation of anti-angiogenic SMADs 2/3 [43, 44]. Importantly, lumican has been linked to TGF-β signaling in a variety of cell systems [33, 45] suggesting that lumican may also regulate angiogenesis by interacting with TGF-β signaling mechanisms. For instance, in the absence of endoglin, lumican may promote TGF-β mediated SMAD2/3 signaling and angiostasis.
Collectively, our findings expand our understanding of how matricellular proteins impact angiogenesis via apoptotic mechanisms and challenge earlier reports that lumican does not induce endothelial cell apoptosis [17].
Conclusion
We have shown that lumican overexpression consistently reduces tumor growth in vivo regardless of its pleiotropic effects on tumor cells in vitro. Furthermore, this reduction in tumor growth is associated with reduced vascular density. Finally, we have provided evidence to support a Fas-specific pro-apoptotic role for the SLRP lumican in the endothelium.
Acknowledgements
We thank Dr. Shukti Chakravarti (Johns Hopkins University, School of Medicine) for the rabbit anti-lumican antibody. Madison Updike is thanked for her contributions to this research. Members of the Albig lab are thanked for their critical review of this manuscript. This work was supported by grants from the National Institutes of Health (3R15CA13829-01A1S1) and Indiana State University URC (University Research Council) (UNR286) to AR Albig.
Abbreviations
- MCA102
murine fibrosarcoma cell line
- Pan02
murine pancreatic adenocarcinoma cell line
- MB114
murine brain microvascular endothelial cell line
- Fas
CD95
- Fas-L
Fas ligand
- SLRP
small leucine-rich proteoglycan
- LRR
leucine-rich repeat
- Lum
lumican
- FMOD
fibromodulin
References
- 1.Maquart FX, et al. An introduction to matrikines: extracellular matrix-derived peptides which regulate cell activity. Implication in tumor invasion. Crit Rev Oncol Hematol. 2004;49(3):199–202. doi: 10.1016/j.critrevonc.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998;17(1):1–19. doi: 10.1016/S0945-053X(98)90121-4. [DOI] [PubMed] [Google Scholar]
- 3.Naito Z. Role of the small leucine-rich proteoglycan (SLRP) family in pathological lesions and cancer cell growth. J Nippon Med Sch. 2005;72(3):137–145. doi: 10.1272/jnms.72.137. [DOI] [PubMed] [Google Scholar]
- 4.McEwan PA, et al. Structural correlations in the family of small leucine-rich repeat proteins and proteoglycans. J Struct Biol. 2006;155(2):294–305. doi: 10.1016/j.jsb.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Kalamajski S, Oldberg A. Homologous sequence in lumican and fibromodulin leucine-rich repeat 5–7 competes for collagen binding. J Biol Chem. 2009;284(1):534–539. doi: 10.1074/jbc.M805721200. [DOI] [PubMed] [Google Scholar]
- 6.Chakravarti S, et al. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141(5):1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jepsen KJ, et al. A syndrome of joint laxity and impaired tendon integrity in lumican- and fibromodulin-deficient mice. J Biol Chem. 2002;277(38):35532–35540. doi: 10.1074/jbc.M205398200. [DOI] [PubMed] [Google Scholar]
- 8.Yeh LK et al Knockdown of zebrafish lumican gene (zlum) causes scleral thinning and increased size of scleral coats. J Biol Chem 285(36):28141–28155 [DOI] [PMC free article] [PubMed]
- 9.Koninger J, et al. Pancreatic tumor cells influence the composition of the extracellular matrix. Biochem Biophys Res Commun. 2004;322(3):943–949. doi: 10.1016/j.bbrc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Shinji S, et al. Different expression levels of lumican in human carcinoid tumor and neuroendocrine cell carcinoma. Int J Oncol. 2005;26(4):873–880. [PubMed] [Google Scholar]
- 11.Garber ME, et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci USA. 2001;98(24):13784–13789. doi: 10.1073/pnas.241500798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brezillon S, et al. Expression of lumican, a small leucine-rich proteoglycan with antitumour activity, in human malignant melanoma. Clin Exp Dermatol. 2007;32(4):405–416. doi: 10.1111/j.1365-2230.2007.02437.x. [DOI] [PubMed] [Google Scholar]
- 13.Lu YP, et al. Expression of lumican in human colorectal cancer cells. Pathol Int. 2002;52(8):519–526. doi: 10.1046/j.1440-1827.2002.01384.x. [DOI] [PubMed] [Google Scholar]
- 14.Leygue E, et al. Expression of lumican in human breast carcinoma. Cancer Res. 1998;58(7):1348–1352. [PubMed] [Google Scholar]
- 15.Vuillermoz B, et al. The small leucine-rich proteoglycan lumican inhibits melanoma progression. Exp Cell Res. 2004;296(2):294–306. doi: 10.1016/j.yexcr.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, et al. Cleavage of lumican by membrane-type matrix metalloproteinase-1 abrogates this proteoglycan-mediated suppression of tumor cell colony formation in soft agar. Cancer Res. 2004;64(19):7058–7064. doi: 10.1158/0008-5472.CAN-04-1038. [DOI] [PubMed] [Google Scholar]
- 17.Brezillon S et al (2009) Lumican core protein inhibits melanoma cell migration via alterations of focal adhesion complexes. Cancer Lett [DOI] [PubMed]
- 18.Yoshioka N, et al. Isolation of transformation suppressor genes by cDNA subtraction: lumican suppresses transformation induced by v-src and v-K-ras. J Virol. 2000;74(2):1008–1013. doi: 10.1128/JVI.74.2.1008-1013.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolhnikoff M, et al. Expression of lumican in human lungs. Am J Respir Cell Mol Biol. 1998;19(4):582–587. doi: 10.1165/ajrcmb.19.4.2979. [DOI] [PubMed] [Google Scholar]
- 20.D’Onofrio MF, et al. Identification of beta1 integrin as mediator of melanoma cell adhesion to lumican. Biochem Biophys Res Commun. 2008;365(2):266–272. doi: 10.1016/j.bbrc.2007.10.155. [DOI] [PubMed] [Google Scholar]
- 21.Albig AR, et al. Transcriptome analysis of endothelial cell gene expression induced by growth on matrigel matrices: identification and characterization of MAGP-2 and lumican as novel regulators of angiogenesis. Angiogenesis. 2007;10(3):197–216. doi: 10.1007/s10456-007-9075-z. [DOI] [PubMed] [Google Scholar]
- 22.Botella LM, et al. Lumican is down-regulated in cells expressing endoglin. Evidence for an inverse correlationship between Endoglin and Lumican expression. Matrix Biol. 2004;22(7):561–572. doi: 10.1016/j.matbio.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Shu SY, Chou T, Sakai K. Lymphocytes generated by in vivo priming and in vitro sensitization demonstrate therapeutic efficacy against a murine tumor that lacks apparent immunogenicity. J Immunol. 1989;143(2):740–748. [PubMed] [Google Scholar]
- 24.Corbett TH, et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 1984;44(2):717–726. [PubMed] [Google Scholar]
- 25.Albig AR, Schiemann WP. Fibulin-5 antagonizes vascular endothelial growth factor (VEGF) signaling and angiogenic sprouting by endothelial cells. DNA Cell Biol. 2004;23(6):367–379. doi: 10.1089/104454904323145254. [DOI] [PubMed] [Google Scholar]
- 26.Albig AR, Schiemann WP. Identification and characterization of regulator of G protein signaling 4 (RGS4) as a novel inhibitor of tubulogenesis: RGS4 inhibits mitogen-activated protein kinases and vascular endothelial growth factor signaling. Mol Biol Cell. 2005;16(2):609–625. doi: 10.1091/mbc.E04-06-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokol JP, et al. The use of cystatin C to inhibit epithelial-mesenchymal transition and morphological transformation stimulated by transforming growth factor-beta. Breast Cancer Res. 2005;7(5):R844–R853. doi: 10.1186/bcr1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clapp NK, et al. Effect of various biological factors on spontaneous marmoset and tamarin colitis. A retrospective histopathologic study. Dig Dis Sci. 1988;33(8):1013–1019. doi: 10.1007/BF01535999. [DOI] [PubMed] [Google Scholar]
- 29.Liotta LA, et al. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284(5751):67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 30.Chakravarti S. Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconj J. 2002;19(4–5):287–293. doi: 10.1023/A:1025348417078. [DOI] [PubMed] [Google Scholar]
- 31.Vij N, et al. Lumican suppresses cell proliferation and aids Fas-Fas ligand mediated apoptosis: implications in the cornea. Exp Eye Res. 2004;78(5):957–971. doi: 10.1016/j.exer.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Vij N, et al. Lumican regulates corneal inflammatory responses by modulating Fas-Fas ligand signaling. Invest Ophthalmol Vis Sci. 2005;46(1):88–95. doi: 10.1167/iovs.04-0833. [DOI] [PubMed] [Google Scholar]
- 33.Nikitovic D, et al. Lumican expression is positively correlated with the differentiation and negatively with the growth of human osteosarcoma cells. FEBS J. 2008;275(2):350–361. doi: 10.1111/j.1742-4658.2007.06205.x. [DOI] [PubMed] [Google Scholar]
- 34.Ishiwata T, et al. Effect of morpholino antisense oligonucleotide against lumican mRNA in human embryonic kidney (HEK) 293 cells. Pathol Int. 2004;54(2):77–81. doi: 10.1111/j.1440-1827.2004.01593.x. [DOI] [PubMed] [Google Scholar]
- 35.Radwanska A, et al. Lumican affects actin cytoskeletal organization in human melanoma A375 cells. Life Sci. 2008;83(19–20):651–660. doi: 10.1016/j.lfs.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Sulochana KN, et al. Peptides derived from human decorin leucine-rich repeat 5 inhibit angiogenesis. J Biol Chem. 2005;280(30):27935–27948. doi: 10.1074/jbc.M414320200. [DOI] [PubMed] [Google Scholar]
- 37.Lucas R, et al. Multiple forms of angiostatin induce apoptosis in endothelial cells. Blood. 1998;92(12):4730–4741. [PubMed] [Google Scholar]
- 38.Kamphaus GD, et al. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem. 2000;275(2):1209–1215. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- 39.Rege TA, et al. Thrombospondin-1-induced apoptosis of brain microvascular endothelial cells can be mediated by TNF-R1. J Cell Physiol. 2009;218(1):94–103. doi: 10.1002/jcp.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volpert OV, et al. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat Med. 2002;8(4):349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- 41.Panka DJ, Mier JW. Canstatin inhibits Akt activation and induces Fas-dependent apoptosis in endothelial cells. J Biol Chem. 2003;278(39):37632–37636. doi: 10.1074/jbc.M307339200. [DOI] [PubMed] [Google Scholar]
- 42.Fonsatti E, et al. Endoglin: an accessory component of the TGF-beta-binding receptor-complex with diagnostic, prognostic, and bioimmunotherapeutic potential in human malignancies. J Cell Physiol. 2001;188(1):1–7. doi: 10.1002/jcp.1095. [DOI] [PubMed] [Google Scholar]
- 43.Lee NY, et al. Endoglin promotes transforming growth factor beta-mediated Smad 1/5/8 signaling and inhibits endothelial cell migration through its association with GIPC. J Biol Chem. 2008;283(47):32527–32533. doi: 10.1074/jbc.M803059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lebrin F, et al. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004;23(20):4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honardoust D, et al. Localization of small leucine-rich proteoglycans and transforming growth factor-beta in human oral mucosal wound healing. Wound Repair Regen. 2008;16(6):814–823. doi: 10.1111/j.1524-475X.2008.00435.x. [DOI] [PubMed] [Google Scholar]