Abstract
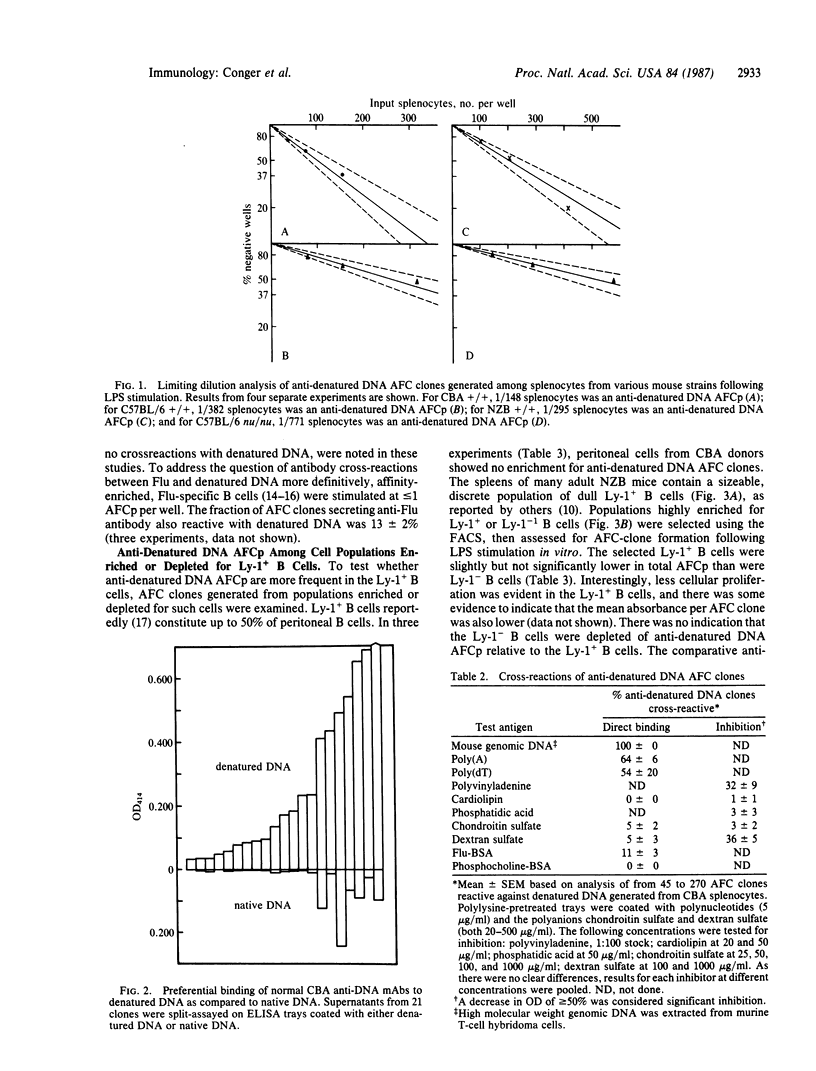
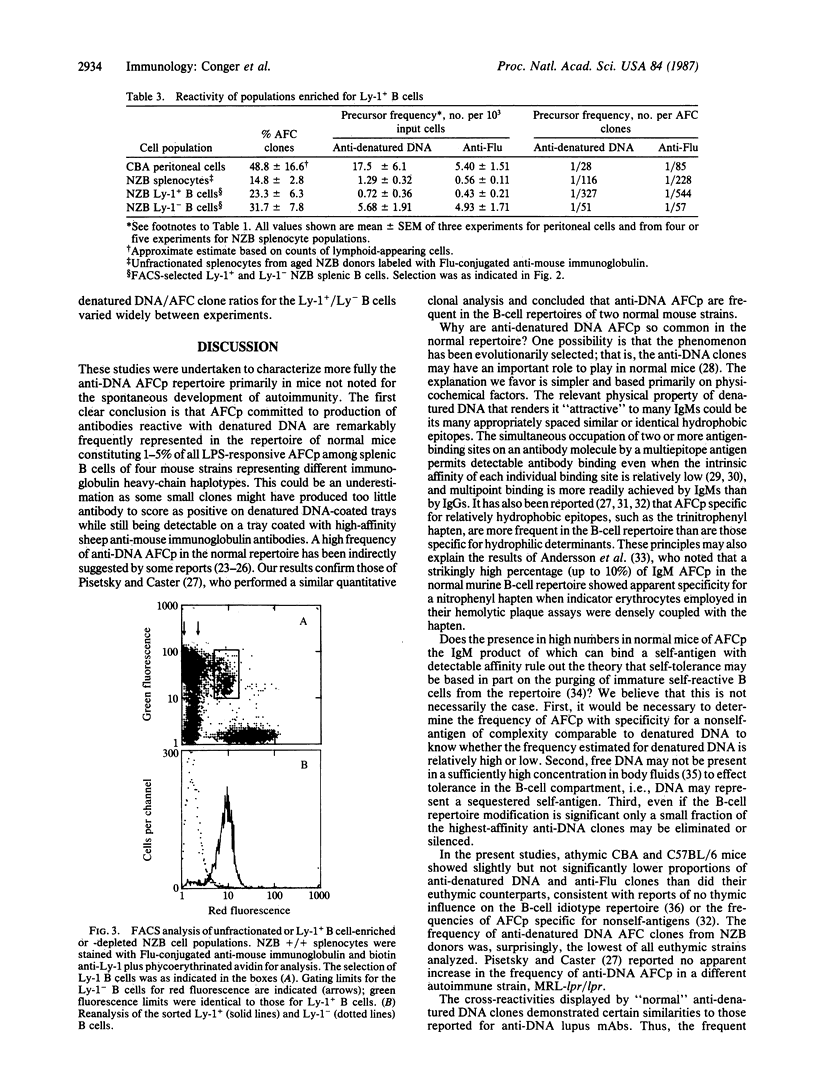
The present studies characterize at the clonal level the repertoire of lipopolysaccharide-responsive murine B lymphocytes committed to the production of antibodies reactive with denatured DNA. This repertoire is vast in normal mice as 1-5% of total mitogen-induced antibody-forming cell clones secreted denatured DNA-reactive antibodies when the splenocyte donors were CBA (Ighj), BALB/c (Igha), C57BL/6 (Ighb), CBA nu/nu, and C57BL/6 nu/nu athymic mice. The autoimmune NZB (Ighe) strain did not display elevated proportions of anti-denatured DNA antibody-forming cell precursors. Cross-reactions shown by CBA anti-denatured DNA antibodies suggest that many antibodies might derive significant binding energy from interaction with the bases or similar hydrophobic moieties. Cross-reactions with other tested polynucleotides were frequent, but cross-reactions with phospholipids and phosphocholine were undetectable. Most anti-DNA antibodies bound preferentially or exclusively to single-stranded denatured DNA as compared to double-stranded native DNA. The frequency of anti-denatured DNA antibody-forming cell precursors among CBA peritoneal cells was not elevated. Fluorescence-activated cell sorter-selected Ly-1-positive NZB splenic B cells were not enriched, and Ly-1 negative B cells were not depleted of anti-DNA antibody-forming cell precursors. These results show that antibody-forming cell precursors specific for denatured DNA are not restricted to the Ly-1 positive B-cell subset.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Coutinho A., Melchers F. Frequencies of mitogen-reactive B cells in the mouse. II. Frequencies of B cells producing antibodies which lyse sheep or horse erythrocytes, and trinitrophenylated or nitroiodophenylated sheep erythrocytes. J Exp Med. 1977 Jun 1;145(6):1520–1530. doi: 10.1084/jem.145.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns E., Block J., Bell D. A. Anti-DNA autoantibody-producing hybridomas of normal human lymphoid cell origin. J Clin Invest. 1984 Sep;74(3):880–887. doi: 10.1172/JCI111505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Mazié J. C., Rouyre S., Butler-Browne G. S., Whalen R. G., Avrameas S. Murine hybridomas secreting natural monoclonal antibodies reacting with self antigens. J Immunol. 1983 Nov;131(5):2267–2272. [PubMed] [Google Scholar]
- Eilat D. Monoclonal autoantibodies: an approach to studying autoimmune disease. Mol Immunol. 1982 Jul;19(7):943–955. doi: 10.1016/0161-5890(82)90360-1. [DOI] [PubMed] [Google Scholar]
- Faaber P., Capel P. J., Rijke G. P., Vierwinden G., van de Putte L. B., Koene R. A. Cross-reactivity of anti-DNA antibodies with proteoglycans. Clin Exp Immunol. 1984 Mar;55(3):502–508. [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur J Immunol. 1986 Apr;16(4):450–456. doi: 10.1002/eji.1830160423. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch S., Schwaber J. Specificity analysis of human anti-DNA antibodies. J Immunol. 1986 Feb 1;136(3):892–897. [PubMed] [Google Scholar]
- Izui S., Kobayakawa T., Zryd M. J., Louis J., Lambert P. H. Mechanism for induction of anti-DNA antibodies by bacterial lipopolysaccharides in mice; II. Correlation between anti-DNA induction and polyclonal antibody formation by various polyclonal B lymphocyte activators. J Immunol. 1977 Dec;119(6):2157–2162. [PubMed] [Google Scholar]
- Juy D., Primi D., Sanchez P., Cazenave P. A. The selection and maintenance of the V region determinant repertoire is germ-line encoded and T cell-independent. Eur J Immunol. 1983 Apr;13(4):326–331. doi: 10.1002/eji.1830130410. [DOI] [PubMed] [Google Scholar]
- Kelsoe G., Stout J. T. Cloning of mitogen- and antigen-reactive B lymphocytes on filter paper discs. II. Paratope frequencies within the mitogen-selected repertoire. Cell Immunol. 1986 Apr 1;98(2):506–516. doi: 10.1016/0008-8749(86)90309-6. [DOI] [PubMed] [Google Scholar]
- Klinman N. R., Sigal N. H., Metcalf E. S., Pierce S. K., Gearhart P. J. The interplay of evolution and environment in B-cell diversification. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):165–173. doi: 10.1101/sqb.1977.041.01.022. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Rauch J., Andrzejewski C., Jr, Mudd D., Furie B., Furie B., Schwartz R. S., Stollar B. D. Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. J Exp Med. 1981 Apr 1;153(4):897–909. doi: 10.1084/jem.153.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J. Bone marrow pre-B cells and the clonal anergy theory of immunologic tolerance. Int Rev Immunol. 1987 Mar;2(3):321–338. doi: 10.3109/08830188709044760. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Improved procedure for the fraction and in vitro stimulation of hapten-specific B lymphocytes. J Immunol. 1978 Jan;120(1):145–150. [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Single cell studies on the antibody-forming potential of fractionated, hapten-specific B lymphocytes. Immunology. 1976 Feb;30(2):189–202. [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Nossal G. J. A high-efficiency cloning system for single hapten-specific B lymphocytes that is suitable for assay of putative growth and differentiation factors. Proc Natl Acad Sci U S A. 1985 May;82(10):3395–3399. doi: 10.1073/pnas.82.10.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Nossal G. J. A reappraisal of "T-independent" antigens. I. Effect of lymphokines on the response of single adult hapten-specific B lymphocytes. J Immunol. 1984 Apr;132(4):1687–1695. [PubMed] [Google Scholar]
- Pisetsky D. S., Caster S. A. The B-cell repertoire for autoantibodies: frequency of precursor cells for anti-DNA antibodies. Cell Immunol. 1982 Sep 15;72(2):294–305. doi: 10.1016/0008-8749(82)90477-4. [DOI] [PubMed] [Google Scholar]
- Pisetsky D. S. Hybridoma SLE autoantibodies: insights for the pathogenesis of autoimmune disease. Clin Immunol Rev. 1984;3(2):169–234. [PubMed] [Google Scholar]
- Prabhakar B. S., Saegusa J., Onodera T., Notkins A. L. Lymphocytes capable of making monoclonal autoantibodies that react with multiple organs are a common feature of the normal B cell repertoire. J Immunol. 1984 Dec;133(6):2815–2817. [PubMed] [Google Scholar]
- Reynolds F., Grunberger D., Pitha J., Pitha P. M. Inhibition of cell-free protein synthesis by poly(9-vinyladenine), poly(1-vinyluracil), and the corresponding vinyl copolymer. Biochemistry. 1972 Aug 15;11(17):3261–3266. doi: 10.1021/bi00767a021. [DOI] [PubMed] [Google Scholar]
- Sawada S., Pillarisetty R. J., Michalski J. P., Palmer D. W., Talal N. Lymphocytes binding polyriboadenylic acid and synthesizing antibodies to nucleic acids in autoimmune and normal mice. J Immunol. 1977 Jul;119(1):355–360. [PubMed] [Google Scholar]
- Schwartz R. S., Stollar B. D. Origins of anti-DNA autoantibodies. J Clin Invest. 1985 Feb;75(2):321–327. doi: 10.1172/JCI111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serban D., Rordorf-Adam C., Sun Y. Z., Gordon J. Murine polyspecific antibodies. I. Monoclonal and serum anti-DNA antibodies cross-reactive with 2,4,6-trinitrophenyl derivatives. J Immunol. 1985 Nov;135(5):3122–3127. [PubMed] [Google Scholar]
- Shoenfeld Y., Rauch J., Massicotte H., Datta S. K., André-Schwartz J., Stollar B. D., Schwartz R. S. Polyspecificity of monoclonal lupus autoantibodies produced by human-human hybridomas. N Engl J Med. 1983 Feb 24;308(8):414–420. doi: 10.1056/NEJM198302243080802. [DOI] [PubMed] [Google Scholar]
- Smith H. R., Steinberg A. D. Autoimmunity--a perspective. Annu Rev Immunol. 1983;1:175–210. doi: 10.1146/annurev.iy.01.040183.001135. [DOI] [PubMed] [Google Scholar]
- Steele E. J., Cunningham A. J. High proportion of Ig-producing cells making autoantibody in normal mice. Nature. 1978 Aug 3;274(5670):483–484. doi: 10.1038/274483a0. [DOI] [PubMed] [Google Scholar]
- Steinman C. R. Free DNA in serum and plasma from normal adults. J Clin Invest. 1975 Aug;56(2):512–515. doi: 10.1172/JCI108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward M. W., Lew A. M. The importance of antibody affinity in the performance of immunoassays for antibody. J Immunol Methods. 1985 Apr 22;78(2):173–190. doi: 10.1016/0022-1759(85)90074-2. [DOI] [PubMed] [Google Scholar]
- Suter M., Pike B. L., Nossal G. J. An ELISA assay efficiently detects clonal antibody formation by single, hapten-specific B lymphocytes. J Immunol Methods. 1985 Nov 28;84(1-2):327–341. doi: 10.1016/0022-1759(85)90440-5. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Underwood J. R., Pedersen J. S., Chalmers P. J., Toh B. H. Hybrids from normal, germ free, nude and neonatal mice produce monoclonal autoantibodies to eight different intracellular structures. Clin Exp Immunol. 1985 May;60(2):417–426. [PMC free article] [PubMed] [Google Scholar]


