Abstract
Deposition of proteins of aberrant conformation is the hallmark of many neurodegenerative diseases. Misfolding of the normally globular mutant superoxide dismutase-1 (SOD1) is a central, early, but poorly understood event in the pathogenic cascade leading to familial forms of ALS. Here we report that aggregates composed of an ALS-causing SOD1 mutant penetrate inside cells by macropinocytosis and rapidly exit the macropinocytic compartment to nucleate aggregation of the cytosolic, otherwise soluble, mutant SOD1 protein. Once initiated, mutant SOD1 aggregation is self-perpetuating. Mutant SOD1 aggregates transfer from cell to cell with remarkable efficiency, a process that does not require contacts between cells but depends on the extracellular release of aggregates. This study reveals that SOD1 aggregates, propagate in a prion-like manner in neuronal cells and sheds light on the mechanisms underlying aggregate uptake and cell-to-cell transfer.
Keywords: amyotrophic lateral sclerosis, amyloid, transmission, protein folding, endocytosis
In many neurodegenerative diseases, such as Alzheimer’s disease, Parkinson disease, prion disorders, ALS, and polyglutamine (polyQ) expansion disorder, a specific protein converts from an otherwise soluble and benign conformation into a misfolded one that assembles into an amyloid or amyloid-like state, defined by a common cross-β structure (1, 2). Aggregation has been well studied in vitro using diverse disease-associated recombinant proteins and proceeds by nucleated growth polymerization, a process that can be accelerated by addition of preformed aggregates or seeds (2). It is not clear whether this phenomenon occurs naturally in neurodegenerative diseases. In the case of prion disorders, the conformational switch of the cellular protein PrPc into the misfolded conformation PrPsc is unique because it confers infectious properties to the protein. PrPsc infects cells and propagates misfolding by converting PrPc into the pathogenic conformation (3). Curiously, recent studies have indicated that aggregation of Aβ42, polyQ peptides, tau, and α-synuclein can be induced experimentally by exogenous seeds (4–10).
The superoxide dismutase-1 (SOD1) is a highly structured protein, and mutations in the SOD1 gene cause some dominantly inherited familial forms of ALS (fALS), defined by the presence of SOD1 inclusions in affected neurons (11, 12). ALS has an adult onset and is rapidly progressive, and these properties are recapitulated in mouse models where both the deposition of SOD1 aggregates and the symptoms are progressive (13). It is now well established that ALS with SOD1 mutations is caused by a gain of toxic properties associated with the propensity of mutant SOD1 to misfold (11, 12), but what elicits the progressive aggregation of mutant SOD1 in ALS is unknown. The onset of motor symptoms is variable, but always focal, and subsequently spreads to contiguous areas (14). Although primarily cytosolic, SOD1 can be secreted (15, 16) and has been detected in cerebrospinal fluid of ALS patients (17). The recent success of SOD1 immunotherapy in transgenic mice clearly establishes the pathophysiological importance of extracellular SOD1 in ALS (18). These features are compatible with the hypothesis that SOD1-misfolding pathology might spread from the site of disease onset to neighboring cells.
It was recently reported that exogenous amyloids composed of the natively unstructured polypeptides Aβ42, polyQ, tau, and α-synuclein can induce misfolding in cells or in tissues (4–10) but the underlying mechanisms remain unknown. Here we found that exogenous aggregates composed of the normally folded mutant SOD1, which are not pure amyloids (19, 20), penetrate in cells, induce misfolding of the soluble mutant protein and efficiently transfer from cell to cell. Furthermore, we found that mutant SOD1 misfolding induced by exogenous seeds is self-propagating. This study reveals that mutant SOD1 aggregates exhibit properties reminiscent of prions and provide insights into some of the underlying mechanisms.
Results
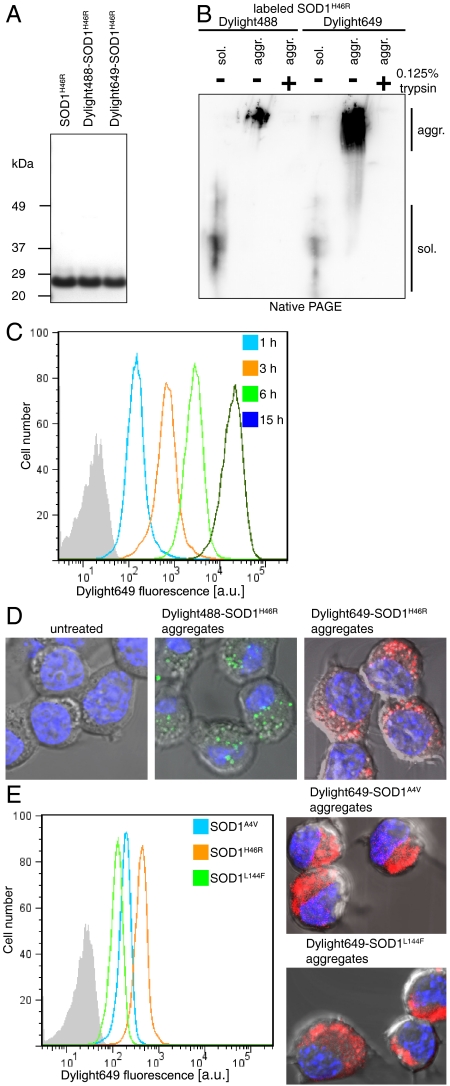
The common fALS-causing SOD1 mutant H46R was expressed in insect cells, purified to homogeneity (Fig. 1A), as previously described (21), and labeled with Dylight dyes, a modification that did not significantly alter the electrophoretic mobility of the protein (Fig. 1A). Mutant SOD1 was exposed to trifluoroethanol (TFE), a treatment provoking the formation of aggregates containing both fibrillar and granular components, reminiscent of the granule-coated fibrils found in fALS patients with SOD1 mutations (19), as we have shown before (21). Like aggregates composed of the protein tau (7), the mutant SOD1 aggregates were completely digested by a 1-min treatment with 0.125% trypsin (Fig. 1B). This property enabled us to use flow cytometry analysis to determine whether mutant SOD1 aggregates could directly penetrate inside cells. Neuro-2a cells were inoculated with mutant SOD1 aggregates for different periods of time, treated with 0.25% trypsin for 2 min, and analyzed by flow cytometry. We found that 60 min after the addition of Dylight649-labeled mutant SOD1 aggregates, at a concentration of 0.2 μM monomer equivalent, to the culture media, the vast majority of cells were strongly fluorescent and the fluorescence intensity gradually increased with time (Fig. 1C). This finding strongly suggested that extracellular mutant SOD1 aggregates penetrated inside the cells. Observation by confocal microscopy of cells inoculated with Dylight649-labeled mutant SOD1 aggregates for 15 min confirmed that these aggregates rapidly and efficiently penetrated inside cells (Fig. 1D). Having found in a previous study that the structurally diverse ALS-causing SOD1 mutants share a common feature consisting in an increased surface hydrophobicity (21), it seemed likely that penetration of mutant SOD1 aggregates inside cells may also be a property shared by the diverse SOD1 mutants. We next examined whether aggregates composed of the common fALS-causing wild-type-like mutant, A4V, harboring a mutation in the dimer interface, exhibited properties similar to the metal-binding region mutant SOD1H46R. SOD1A4V, labeled with Dylight649 and aggregated with TFE (21), penetrated efficiently inside cells (Fig. 1E). Similarly, aggregates composed of SOD1L144F, a β-barrel mutant, also penetrated efficiently in cells (Fig. 1E).
Fig. 1.
Mutant SOD1 aggregates penetrate inside neuronal cells. (A) Purified SOD1H46R (1.2 μg), unlabeled or labeled with Dylight dyes, analyzed by NuPAGE and stained with Coomassie brilliant blue. (B) Soluble (sol.) or aggregated (aggr.) SOD1H46R labeled with Dylight dyes, digested with 0.125% trypsin for 1 min where indicated, prior to native PAGE followed by immunoblot analysis with SOD1 polyclonal antibodies. (C) Neuro-2a take up labeled SOD1H46R aggregates in a time-dependent manner. Flow cytometry analysis of Neuro-2a cells inoculated with Dylight649-SOD1H46R aggregates (0.2 μM monomer equivalent); a.u., arbitrary units. (D) Confocal micrographs of cells untreated or inoculated with labeled SOD1H46R aggregates 15 min before fixing the cells. Nuclei were stained with H33258. Aggregates were estimated to be 2–4 μm inside the cell, not at the surface. Representative results of at least three independent experiments are shown. (E) Flow cytometry and confocal analysis of cells left untreated for 3 h after inoculation with Dylight649-labeled SOD1A4V, SOD1H46R, or SOD1L144F aggregates.
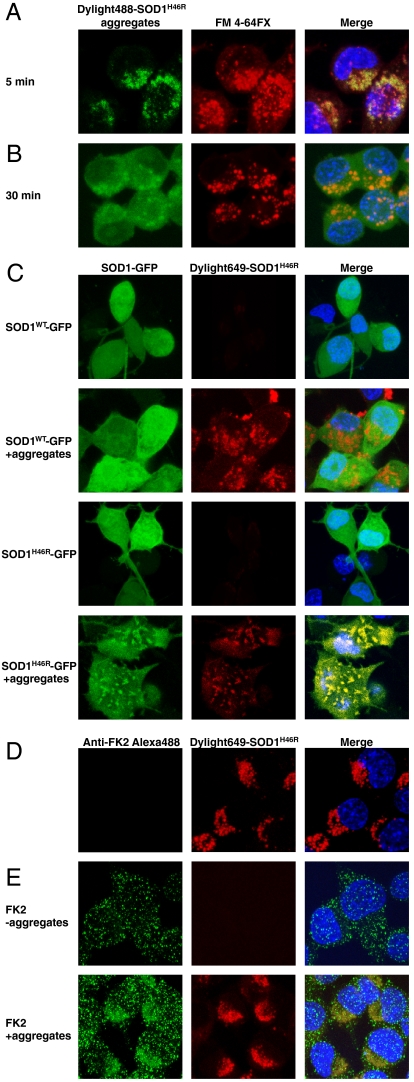
Having shown that aggregates composed of diverse SOD1 mutants efficiently penetrate inside cells, we explored this phenomenon further, focusing on SOD1H46R. To determine whether aggregates were internalized by endocytosis, cells were concomitantly labeled with the fixable membrane FM 4-64FX dye that reveals endocytic vesicles (22) and inoculated with mutant SOD1 aggregates for various periods of time. Five minutes after inoculation, aggregates were clearly colocalized with the endocytic membranes (Fig. 2A). In contrast, 30 min after the addition of exogenous aggregates to cells, the vast majority of the internalized aggregates no longer colocalized with the FM 4-64FX dye but rather exhibited a diffused, cytosolic localization (Fig. 2B). Penetration of extracellular mutant SOD1 aggregates into cells could potentially serve as a seed to trigger aggregation of the endogenous soluble protein, as is the case for prions and a few other proteins (23–25). Endogenous mutant SOD1 was mostly soluble in cells expressing the fALS-causing protein (Fig. 2C), as previously reported (26). Transient exposure of mutant SOD1 expressing cells to exogenous mutant SOD1 aggregates strongly increased the amount of insoluble mutant SOD1, as revealed by biochemical fractionation of cell lysates (Fig. S1). It is important to note that the extracellular aggregates were digested before cell lysis. In contrast, exogenous mutant SOD1 aggregates did not seed aggregation of wild-type SOD1 (Fig. S1). The fluorescence of unseeded cells expressing GFP-tagged wild-type or mutant SOD1 proteins was diffusely distributed and remained diffused in cells expressing wild-type SOD1, even after treatment with extracellular mutant SOD1 aggregates (Fig. 2C). In contrast, addition of exogenous mutant SOD1 aggregates converted the diffused fluorescence of cells expressing GFP-tagged mutant SOD1 into puncta that colocalized with the seeds (Fig. 2C). This finding reveals that exogenous aggregates access the cytosolic compartment of mammalian cells to seed aggregation of cytosolic, otherwise soluble, mutant SOD1.
Fig. 2.
Mutant SOD1 aggregates are internalized by vesicles and escape the endocytic compartment to seed aggregation of the endogenous mutant protein. (A) SOD1H46R aggregates localize in membrane-enclosed vesicles. Confocal micrographs of cells after inoculation with Dylight488-SOD1H46R aggregates (green) for 5 min together with the fixable membrane dye FM 4-64FX (red). Nuclei were stained with H33258 dye. (B) Aggregates rapidly exit the FM 4-64FX marked vesicles. Same as in A except that cells were fixed 30 min after addition of aggregates and the FM 4-64FX dye. (C) Confocal micrographs of cells transiently expressing SOD1-GFP wild-type or SOD1H46R-GFP and inoculated with 0.2 μM (monomer equivalent) Dylight649-SOD1H46R aggregates, where indicated, for 15 h before fixing the cells. (D and E) Cells were exposed to Dylight649-SOD1H46R for 15 h, trypsinized, and cultured for 2 d before fixing, labeling with the polyubiquitin antibody FK2 where indicated, and confocal microscopy. Representative results of at least three independent experiments are shown.
We noted that internalized aggregates changed morphology over time. Aggregates seemed punctate when in the endocytic vesicles (Fig. 2A), but 30 min later, appeared diffused in the cytosol but excluded from the nucleus (Fig. 2B), indicating that they are too big to diffuse through nuclear pore, in contrast to the soluble endogenous protein (Fig. 2C). Thus, the diffused fluorescence is likely to be contributed by aggregated species, possibly oligomers; such species cannot be resolved by confocal microscopy. Fifteen hours after uptake, the diffused aggregates coalesced in puncta, whether or not mutant SOD1 was expressed in the recipient cells (Fig. 2 C and D). We next tested whether the internalized aggregates were ubiquitinated. Two days after their inoculation, internalized aggregates were labeled with the polyubiquitin antibody confirming that they are in the cytosol (Fig. 2E).
The remarkable efficiency by which mutant SOD1 aggregates penetrated inside the cells provided us with an experimental system to dissect the uptake mechanism. The colocalization of the aggregates with the FM 4-64FX dye, in the first 5 min after their inoculation, clearly established that the vast majority of aggregates entered cells via an endocytic pathway. We then systematically examined which of the various endocytic pathways contributed to the uptake of mutant SOD1 aggregates. To determine whether mutant SOD1 aggregates entered by a clathrin-dependent pathway, we examined whether mutant SOD1 aggregates colocalized with transferrin, a well-established marker of the coated pit pathway (27). Transferrin and mutant SOD1 aggregates exhibited a distinct localization, indicating that mutant SOD1 aggregates enter the cells by a clathrin-independent pathway (Fig. 3A).
Fig. 3.
Mutant SOD1 aggregates enter cells by macropinocytosis. (A) Confocal micrographs of Neuro-2a cells inoculated with 0.2 μM (monomer equivalent) Dylight649-SOD1H46R aggregates (red) and Alexa488-transferrin (green) for 5 min before fixing the cells. (B) The effect of inhibitors of endocytosis on SOD1H46R aggregate uptake. Cells either treated with the indicated drugs or expressing dominant-negative (DN) mutants of components of endocytic pathways, or lacking key components of endocytic pathways, were inoculated with 0.2 μM (monomer equivalent) Dylight649-SOD1H46R aggregates for 30 min. Aggregate uptake was monitored by flow cytometry. Data are means ± SD (n≥3) normalized to that of either untreated cells, mock transfected cells, or to the matching wild-type control cells when analyzing uptake in genetically modified cells. Conditions inhibiting macropinocytosis are highlighted in green. (C) Table recapitulating the effects of the different treatments used in B.
To determine whether aggregates penetrated inside the cells via an active process, cells were exposed to sodium azide and 2-deoxyglucose to deplete cellular ATP prior to the exposure to aggregates. This treatment markedly reduced uptake (Fig. S2A). Aggregate uptake was also reduced in cells treated with the actin-polymerization inhibitors cytochalasin A and D (Fig. S2B and Fig. 3B). In agreement with the distinct localization of transferrin and mutant SOD1 aggregates (Fig. 3A), we found that neither chlorpromazine, nor the dominant-negative mutants of clathrin-coated-pit adaptor proteins eps15 (28) or AP180 (29) blocked mutant SOD1 aggregate uptake (Fig. 3B), confirming that their penetration does not require the clathrin-dependent pathway. Both eps15 and AP180 dominant-negative mutants actually increased mutant SOD1 aggregate uptake, suggesting that aggregates are taken up by an alternative pathway, which tends to be up-regulated when clathrin-mediated endocytosis is down-regulated. The dynamin inhibitor dynasore (30) and the dynamin dominant-negative mutant, GTPase inactive, dynamin K44A (31), did not prevent mutant SOD1 aggregate uptake, demonstrating that dynamin is not essential for this process (Fig. 3B). These findings establish that uptake of mutant SOD1 aggregates is clathrin- and dynamin-independent.
We then probed the requirement for various clathrin-independent endocytic pathways in mutant SOD1 aggregate uptake (Fig. 3 B and C). Calveolin was not required for mutant SOD1 aggregate uptake because this process was undistinguishable in cells containing or lacking Caveolin-1 (32) and unperturbed by genistein, a tyrosine kinase inhibitor that inhibits caveolin-dependent endocytosis (Fig. 3B). Similarly, we found that penetration of mutant SOD1 aggregates was undistinguishable in cells containing or lacking flotillin-1 (33). In contrast, we found that 5-N-ethyl-N-isopropyl-amiloride, as well as wortmannin, an inhibitor of phosphoinositide 3-kinase, and inhibitor targeting P21-activated kinase activation, strongly diminished aggregate uptake, indicating that aggregates used macropinocytosis to enter the cells (Fig. 3B). Similar to aggregates made of tau or PrPsc (7, 34), we observed that mutant SOD1 aggregates transiently colocalize with fluorescent dextran (Mr ∼ 10,000), a marker of fluid phase uptake (Fig. S3). Estimation of the size of the endocytic vesicles containing mutant SOD1 aggregates by confocal microscopy suggested that they have a diameter of about 1 μm, a size consistent with macropinosomes and significantly larger than other endocytic vesicles. The cholesterol depleting agent methyl-β-cyclodextrin (MβCD), disrupting lipid rafts, dramatically reduced aggregate entry (Fig. 3B). The thorough analysis of the different endocytic pathways revealed that extracellular mutant SOD1 aggregates use lipid raft-dependent macropinocytosis to enter cells (Fig. 3C).
We next investigated whether mutant SOD1 aggregation, induced by extracellular aggregates, can persist and propagate, like PrPsc or the heterologous yeast prion domain Sup35NM in mammalian cells (35). Mutant SOD1 aggregation was elicited in Neuro-2a cells stably expressing SOD1H46R-GFP (Fig. 4A) by transient exposure to extracellular mutant SOD1 aggregates (Fig. 4B and Fig. S4). One day after seeding, extracellular aggregates had entered the cells and provoked the coalescence of the otherwise diffused SOD1H46R-GFP fluorescence into puncta (Fig. 4 A and B). Over time, while the cells continued to divide, the internalized mutant SOD1 aggregates gradually disappeared (Fig. 4 B and C). Although the internalized aggregates were no longer detectable 8 d after their inoculation, SOD1H46R-GFP fluorescence remained punctate in nearly all cells, 1 mo after the induction of aggregation (Fig. 4 B and C and Fig. S4). In contrast, the fluorescence remained diffused in the cells that had not been exposed to aggregates (Fig. 4 A and C). This finding reveals that mutant SOD1 aggregation, induced by uptake of extracellular aggregates, is a persistent and heritable phenotype.
Fig. 4.
Induced mutant SOD1 aggregation is persistent and heritable. (A) Confocal micrographs of Neuro-2a cells stably expressing SOD1H46R-GFP left untreated or (B) transiently inoculated for 15 h with 0.2 μM (monomer equivalent) Dylight649-SOD1H46R aggregates (red). Neuro-2a cells were passaged 3 times per week and plated on coverslips 12 h before fixing at the indicated time after seeding. Nuclei were stained with H33258. Representative results of least three independent experiments are shown. (C) Cells (approximately 130 per condition) treated as in B were blindly scored for the presence of internalized or induced aggregates.
Having found that mutant SOD1 aggregates can efficiently penetrate inside cells, we next investigated whether internalized mutant SOD1 aggregates could transfer from cell to cell. Neuro-2a cells were separately inoculated with mutant SOD1 aggregates labeled with either Dylight488 or Dylight649. Extracellular aggregates were removed by trypsin and an equal number of Neuro-2a cells that had internalized Dylight488-labeled SOD1H46R aggregates were mixed with Neuro-2a cells containing Dylight649-labeled SOD1H46R aggregates. Flow cytometry analysis performed on trypsinized cells, 20 h after mixing the two cell populations, revealed that the vast majority of Dylight488-positive cells were also Dylight649-positive, suggesting that aggregates transfer from cell to cell (Fig. 5A). The Dylight649-label taken up by Dylight488 positive cells did not originate from the inoculum because the mixed population of cells appeared unlabeled when the donor cells had been trypsinized just after inoculation with the exogenous aggregates (Fig. S5). Cell-to-cell transfer of aggregates was time-dependent because no transfer was detected when cells were mixed just prior to flow cytometry analysis and only 16% of cells had the two labels 4 h after transfer. The transfer was found as efficient when the inoculum had been removed 6 h before mixing the two cell populations (Fig. 5B). This experiment suggests that the cytosolic pool of aggregates can transfer, because most of the aggregates access the cytosol 30 min after their inoculation on cells (Fig. 2B). Observations by confocal fluorescence microscopy confirmed the presence of both Dylight488- and Dylight649-labeled SOD1H46R aggregates in cells (Fig. 5C).
Fig. 5.
Transfer of aggregates from cell to cell is dependent on their release to the extracellular space. (A) Cells were inoculated separately with either Dylight488- or Dylight649-SOD1H46R aggregates, trypsinized to remove the inoculum, mixed, and cocultured for 15 h before flow cytometry analysis. (B) As in A, except that cells were left for 6 h after digestion of the inoculum with trypsin before they were cocultured. (C) Confocal micrographs of cells treated as in A. (D) Cells inoculated separately with either Dylight488 or Dylight649-SOD1H46R aggregates were cocultured in a 24 multiwell dish for 15 h, with increasing volumes of medium and analyzed by flow cytometry. Transfer was scored as the number of Dylight488 and Dylight649 double-positive cells. (E) Flow cytometry analysis of Neuro-2a cells containing Dylight488-SOD1H46R aggregates inoculated with conditioned media from Neuro-2a cells with internalized Dylight648-SOD1H46R aggregates. (F) Same as in A except that the two cell populations were cocultured in a chamber separated by a 0.4-μm filter; a.u., arbitrary units. Representative results of at least three independent experiments are shown.
These results establish that mutant SOD1 aggregates transfer from cell to cell with a very high efficiency, thereby defining an experimental system that can be exploited to identify the underlying mechanism. Using the procedure defined in Fig. 5A, we observed that the efficiency of aggregate transfer between cocultured cells dramatically decreased when increasing the volume of the cell culture media (Fig. 5D), revealing that the cell-to-cell transfer of aggregates depends on the release of aggregates in the extracellular space, rather than cell-to-cell contacts. We next investigated whether aggregates could be transferred from conditioned media obtained from the culture of Neuro-2a cells containing labeled aggregates. Cells cultured in conditioned media acquired the fluorescence of the donor cells (Fig. 5E). Furthermore, we found that this transfer occurred efficiently between two cocultured cell populations separated by a 0.4-μm filter (Fig. 5F). These experiments establish that mutant SOD1 aggregates efficiently transfer between cells and this transfer does not depend on cell-to-cell contacts but on the release of aggregates to the extracellular space.
Discussion
This study reveals that aggregates composed of the normally globular, fALS-causing SOD1 mutant penetrate inside cells and trigger the self-perpetuating aggregation of the endogenous protein. Mutant SOD1 aggregates selectively use macropinocytosis to enter the cells and seed aggregation of the endogenous protein. Like SOD1 aggregates, possibly PrPsc and tau aggregates (7, 34) and perhaps other prion-like aggregates, several viruses also hijack macropinocytosis to penetrate into the cytosol of host cells (36). Once in the cytosol, mutant SOD1 aggregates efficiently transmit misfolding to the endogenous mutant protein. Like ALS, prion diseases are mostly sporadic and occasionally inherited (3). Studies in mouse models have established that inherited forms of prion diseases are governed both by a genetic and an infectious component (37, 38). Likewise, we found that the endogenous mutant protein is required for perpetuating mutant SOD1 misfolding. The transcellular spread of SOD1 aggregates does not require cell-to-cell contacts but depends on their extracellular release. This finding has important implications as it provides a rationale for the development of immunotherapies targeting the extracellular misfolded agent.
Aggregation of mutant SOD1 induced by extracellular aggregates is a highly penetrant and heritable phenotype that stably persists long after the disappearance of the seeds, demonstrating that mutant SOD1 misfolding is self-perpetuating. PolyQ aggregates induced by exogenous delivery of seeds can also persist, but only in a small fraction of cells (approximately 4%) (6). Efficient propagation of prions depends on the fragmentation of aggregates into smaller seeds that can be transmitted to daughter cells (24). In contrast to polyQ aggregates that tend to be segregated in large and often unique foci (6), mutant SOD1 aggregates were abundant in each cell (Fig. 4B), suggesting that mutant SOD1 aggregates may be more efficiently fragmented in mammalian cells than polyQ aggregates, a property that could explain their ability to propagate efficiently. Our model provides a cellular system to dissect the mechanisms underlying the efficient propagation of protein misfolding.
This study establishes that aggregates composed of normally tightly folded mutant SOD1 penetrate inside mammalian cells, induce a heritably propagating misfolding pathology in recipient cells, and transfer misfolding from cell to cell with a remarkable efficiency. These findings establish that ALS-causing mutant SOD1 exhibits prion-like properties and that propagation of protein misfolding is a robust phenomenon.
Materials and Methods
Purification of SOD1H46R, SOD1A4V, SOD1L144F, labeling, preparation, and proteolysis of aggregates.
SOD1H46R, SOD1A4V, SOD1L144F were purified as described (21) and labeled with Dylight649 or Dylight488 NHS Ester (Thermo Scientific). The non-cross-linked label was removed by dialysis. Aggregates were prepared in 20% TFE as described (21).
Cell culture, electrophoresis, fractionations, immunoblottings, and immunocytochemistry are described in SI Materials and Methods.
Uptake of Extracellular Mutant SOD1 Aggregates.
Neuro-2a cells were grown on multiwell plates or glass coverslips for confocal analysis (described SI Material and Methods) and inoculated with 0.2 μM monomer equivalent of labeled aggregates. Before analysis, the remaining inoculum was digested with 0.25% trypsin for 2 min as indicated and the cells were collected for analysis by flow cytometry (described SI Material and Methods).
Seeding and Propagation of Mutant SOD1 Misfolding.
Neuro-2a cells either transfected or stably expressing with SOD1 or SOD1H46R, HA, or GFP-tagged were inoculated with 0.2 μM monomer equivalent of Dylight649-SOD1H46R aggregates for the indicated time before analysis.
Perturbation of Endocytosis.
Reagents and procedures and are described in SI Materials and Methods.
Cell-to-Cell Transfer.
Neuro-2a cells were separately inoculated with SOD1H46R aggregates labeled with either Dylight488 or Dylight649 for 24 h before cocultured for the indicated time and analysis by flow cytometry or confocal microscopy. The preparation of conditioned media and subsequent uptake is described in SI Materials and Methods Where indicated, cells were cocultured in the chambers of 0.4-μm polycarbonate membrane filters (Nunc), incubated overnight, and analyzed by flow cytometry.
Supplementary Material
Acknowledgments.
We are grateful to Ben Nichols, Harvey McMahon, Emmanuel Boucrot, Kirsi Riento, Rachel Angers, and Nigel Unwin for discussions, reagents, and comments on the manuscript. The Medical Research Council supported this work.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017275108/-/DCSupplemental.
References
- 1.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 2.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer-Luehmann M, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 5.Yang W, Dunlap JR, Andrews RB, Wetzel R. Aggregated polyglutamine peptides delivered to nuclei are toxic to mammalian cells. Hum Mol Genet. 2002;11:2905–217. doi: 10.1093/hmg/11.23.2905. [DOI] [PubMed] [Google Scholar]
- 6.Ren PH, et al. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clavaguera F, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li JY, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 10.Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 12.Valentine JS, Doucette PA, Zittin Potter S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, et al. Progressive aggregation despite chaperone associations of a mutant SOD1-YFP in transgenic mice that develop ALS. Proc Natl Acad Sci USA. 2009;106:1392–1397. doi: 10.1073/pnas.0813045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caroscio JT, Mulvihill MN, Sterling R, Abrams B. Amyotrophic lateral sclerosis. Its natural history. Neurol Clin. 1987;5:1–8. [PubMed] [Google Scholar]
- 15.Mondola P, et al. The Cu,Zn superoxide dismutase in neuroblastoma SK-N-BE cells is exported by a microvesicles dependent pathway. Mol Brain Res. 2003;110:45–51. doi: 10.1016/s0169-328x(02)00583-1. [DOI] [PubMed] [Google Scholar]
- 16.Urushitani M, et al. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2006;9:108–118. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsson J, Jonsson PA, Andersen PM, Forsgren L, Marklund SL. Superoxide dismutase in CSF from amyotrophic lateral sclerosis patients with and without CuZn-superoxide dismutase mutations. Brain. 2001;124:1461–1466. doi: 10.1093/brain/124.7.1461. [DOI] [PubMed] [Google Scholar]
- 18.Urushitani M, Ezzi SA, Julien JP. Therapeutic effects of immunization with mutant superoxide dismutase in mice models of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2007;104:2495–2500. doi: 10.1073/pnas.0606201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato S, et al. Pathological characterization of astrocytic hyaline inclusions in familial amyotrophic lateral sclerosis. Am J Pathol. 1997;151:611–620. [PMC free article] [PubMed] [Google Scholar]
- 20.Kerman A, et al. Amyotrophic lateral sclerosis is a non-amyloid disease in which extensive misfolding of SOD1 is unique to the familial form. Acta Neuropathol. 2010;119:335–344. doi: 10.1007/s00401-010-0646-5. [DOI] [PubMed] [Google Scholar]
- 21.Munch C, Bertolotti A. Exposure of hydrophobic surfaces initiates aggregation of diverse ALS-causing superoxide dismutase-1 mutants. J Mol Biol. 2010;399:512–525. doi: 10.1016/j.jmb.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaffield MA, Betz WJ. Imaging synaptic vesicle exocytosis and endocytosis with FM dyes. Nat Protoc. 2007;1:2916–2921. doi: 10.1038/nprot.2006.476. [DOI] [PubMed] [Google Scholar]
- 23.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11:155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krammer C, Schatzl HM, Vorberg I. Prion-like propagation of cytosolic protein aggregates: Insights from cell culture models. Prion. 2009;3:206–212. doi: 10.4161/pri.3.4.10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–307. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston JA, Dalton MJ, Gurney ME, Kopito RR. Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2000;97:12571–12576. doi: 10.1073/pnas.220417997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 28.Benmerah A, et al. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J Cell Biol. 1998;140:1055–1062. doi: 10.1083/jcb.140.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford MG, et al. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- 30.Macia E, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Cao H, Thompson HM, Krueger EW, McNiven MA. Disruption of Golgi structure and function in mammalian cells expressing a mutant dynamin. J Cell Sci. 2000;113:1993–2002. doi: 10.1242/jcs.113.11.1993. [DOI] [PubMed] [Google Scholar]
- 32.Razani B, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 33.Ludwig A, et al. Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J Cell Biol. 2010;191:771–781. doi: 10.1083/jcb.201005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magalhaes AC, et al. Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells. J Neurosci. 2005;25:5207–5216. doi: 10.1523/JNEUROSCI.0653-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krammer C, et al. The yeast Sup35NM domain propagates as a prion in mammalian cells. Proc Natl Acad Sci USA. 2009;106:462–467. doi: 10.1073/pnas.0811571106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercer J, Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 37.Nazor KE, et al. Immunodetection of disease-associated mutant PrP, which accelerates disease in GSS transgenic mice. EMBO J. 2005;24:2472–2480. doi: 10.1038/sj.emboj.7600717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsiao KK, et al. Serial transmission in rodents of neurodegeneration from transgenic mice expressing mutant prion protein. Proc Natl Acad Sci USA. 1994;91:9126–9130. doi: 10.1073/pnas.91.19.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.