Abstract
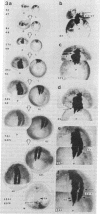
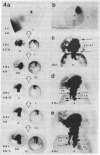
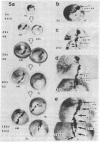
We have examined the process by which small groups of pigmented germinal cells transplanted orthotopically from stage 30-38 donor embryos into stage 28-38 albino hosts contribute new postmitotic cells to the pigmented retinal epithelium of the growing larval eye in Xenopus. In the great majority of chimeric eyes, the transplant healed to occupy a small arc-territory at the intended dorsal or anterior position on the host germinal zone. Over the course of subsequent weeks, the transplanted germinal cells added new mitotically quiescent cells to the distal rim of the pigmented retinal epithelium and so gave rise to an elongating black sector on the growing larval eye. Cellular details at the boundaries of the graft-derived sector were stable over time; the accumulation of such landmarks provided a summary record--in the proximodistal axis of the older eye--of the growth history of the transplant. Case-to-case variation among both groups of transplants suggested a measure of indeterminancy in the details of germinal cell growth.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach D. H., Jacobson M. Patterns of cell proliferation in the retina of the clawed frog during development. J Comp Neurol. 1979 Feb 1;183(3):603–613. doi: 10.1002/cne.901830308. [DOI] [PubMed] [Google Scholar]
- Bodenstein L., Sidman R. L. Cell patterning in vertebrate development: models and model systems. Curr Top Dev Biol. 1987;21:1–29. doi: 10.1016/s0070-2153(08)60131-3. [DOI] [PubMed] [Google Scholar]
- Bryant P. J., Simpson P. Intrinsic and extrinsic control of growth in developing organs. Q Rev Biol. 1984 Dec;59(4):387–415. doi: 10.1086/414040. [DOI] [PubMed] [Google Scholar]
- Conway K., Feiock K., Hunt R. K. Polyclones and patterns in growing Xenopus eye. Curr Top Dev Biol. 1980;15(Pt 1):217–317. doi: 10.1016/s0070-2153(08)60121-0. [DOI] [PubMed] [Google Scholar]
- Grant P., Rubin E., Cima C. Ontogeny of the retina and optic nerve in Xenopus laevis. I. Stages in the early development of the retina. J Comp Neurol. 1980 Feb 15;189(4):593–613. doi: 10.1002/cne.901890403. [DOI] [PubMed] [Google Scholar]
- Hoperskaya O. A. The development of animals homozygous for a mutation causing periodic albinism (ap) in Xenopus laevis. J Embryol Exp Morphol. 1975 Aug;34(1):253–264. [PubMed] [Google Scholar]
- Jacobson M. Cessation of DNA synthesis in retinal ganglion cells correlated with the time of specification of their central conections. Dev Biol. 1968 Feb;17(2):219–232. doi: 10.1016/0012-1606(68)90062-6. [DOI] [PubMed] [Google Scholar]
- Jacobson M. Clonal analysis and cell lineages of the vertebrate central nervous system. Annu Rev Neurosci. 1985;8:71–102. doi: 10.1146/annurev.ne.08.030185.000443. [DOI] [PubMed] [Google Scholar]
- Jacobson M. Histogenesis of retina in the clawed frog with implications for the pattern of development of retinotectal connections. Brain Res. 1976 Feb 27;103(3):541–545. doi: 10.1016/0006-8993(76)90452-2. [DOI] [PubMed] [Google Scholar]
- Kageura H., Yamana K. Pattern formation in 8-cell composite embryos of Xenopus laevis. J Embryol Exp Morphol. 1986 Feb;91:79–100. [PubMed] [Google Scholar]
- Kimmel C. B., Law R. D. Cell lineage of zebrafish blastomeres. III. Clonal analyses of the blastula and gastrula stages. Dev Biol. 1985 Mar;108(1):94–101. doi: 10.1016/0012-1606(85)90012-0. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M. Cell line segregation during peripheral nervous system ontogeny. Science. 1986 Mar 28;231(4745):1515–1522. doi: 10.1126/science.3952494. [DOI] [PubMed] [Google Scholar]
- Stent G. S., Weisblat D. A. Cell lineage in the development of invertebrate nervous systems. Annu Rev Neurosci. 1985;8:45–70. doi: 10.1146/annurev.ne.08.030185.000401. [DOI] [PubMed] [Google Scholar]
- Straznicky K., Gaze R. M. The growth of the retina in Xenopus laevis: an autoradiographic study. J Embryol Exp Morphol. 1971 Aug;26(1):67–79. [PubMed] [Google Scholar]
- Thiébaud C. H. A reliable new cell marker in Xenopus. Dev Biol. 1983 Jul;98(1):245–249. doi: 10.1016/0012-1606(83)90353-6. [DOI] [PubMed] [Google Scholar]
- Till J. E., McCulloch E. A. Hemopoietic stem cell differentiation. Biochim Biophys Acta. 1980 Nov 26;605(4):431–459. doi: 10.1016/0304-419x(80)90009-8. [DOI] [PubMed] [Google Scholar]
- Winklbauer R., Hausen P. Development of the lateral line system in Xenopus laevis. II. Cell multiplication and organ formation in the supraorbital system. J Embryol Exp Morphol. 1983 Aug;76:283–296. [PubMed] [Google Scholar]