Abstract
The mitogen-activated protein kinases (MAPKs) pathways are highly organized signaling systems that transduce extracellular signals into a variety of intracellular responses. In this context, it is currently poorly understood how kinases constituting these signaling cascades are assembled and activated in response to receptor stimulation to generate specific cellular responses. Here, we show that AKAP-Lbc, an A-kinase anchoring protein (AKAP) with an intrinsic Rho-specific guanine nucleotide exchange factor activity, is critically involved in the activation of the p38α MAPK downstream of α1b-adrenergic receptors (α1b-ARs). Our results indicate that AKAP-Lbc can assemble a novel transduction complex containing the RhoA effector PKNα, MLTK, MKK3, and p38α, which integrates signals from α1b-ARs to promote RhoA-dependent activation of p38α. In particular, silencing of AKAP-Lbc expression or disrupting the formation of the AKAP-Lbc·p38α signaling complex specifically reduces α1-AR-mediated p38α activation without affecting receptor-mediated activation of other MAPK pathways. These findings provide a novel mechanistic hypothesis explaining how assembly of macromolecular complexes can specify MAPK signaling downstream of α1-ARs.
Keywords: Adaptor Proteins, Adrenergic Receptor, G Protein-coupled Receptors (GPCR), p38 MAPK, Protein Kinase A (PKA), Signal Transduction
Introduction
α1-Adrenergic receptors (α1-AR)2 are seven-transmembrane domain receptors coupled to heterotrimeric G proteins of the Gq and G12/G13 family (1, 2). Evidence accumulated over the last years indicate that these receptors, besides their well known implication in controlling vascular contractility, glucose metabolism, genitourinary functions, and behavioral responses (3), are also crucially involved in the regulation of various pathological cardiovascular remodeling processes including vascular smooth muscle cell hypertrophy, proliferation, and migration in response to injury (4, 5) as well as cardiac hypertrophy (6–8). It is now evident that mitogen-activated protein kinases (MAPKs) signaling pathways play a central role in mediating many of these pathological responses (1, 9–11).
MAPKs are proline-directed serine/threonine kinases that induce the majority of their physiological effects through phosphorylation and activation of transcription factors and the regulation of the expression of specific sets of genes (12). Mammalian MAPKs can be subdivided into five families including ERK1/2, JNK, p38, ERK3/4, and ERK5, which display different biological functions (12). MAPK signaling cascades are organized into functional signaling modules of three kinases in which a MAP kinase kinase kinase (MAPKKK) phosphorylates and activates a MAP kinase kinase (MAPKK) that, in turn, phosphorylates and activates a MAPK (13). The modular organization of the pathway is controlled by scaffolding proteins that can bind each of the kinases (13). Although the implication of MAPK pathways in the pathophysiological responses induced by α1-ARs has been extensively studied it is currently unknown how MAPK signaling modules are assembled and activated in response to α1-AR stimulation to generate specific cellular responses.
Several evidences indicates that small molecular weight GTPase RhoA plays a central role in mediating the activation of MAPK pathways downstream of α1-ARs (1, 10, 14). Rho-GTPases are molecular switches that cycle between an active GTP-bound state, which is able to bind effector proteins, and an inactive GDP-bound state. In vivo, the cycling between the GDP- and GTP-bound forms is regulated by guanine nucleotide exchange factors (GEFs) that stimulate the exchange of GDP with GTP. All guanine nucleotide exchange factors exhibiting exchange activity toward Rho GTPases share a Dbl homology domain and an adjacent pleckstrin homology domain (15, 16). The Dbl homology domain is responsible for the guanine nucleotide exchange activity, whereas the pleckstrin homology domain determines the subcellular localization of the exchange factor or contributes to the binding pocket for Rho-GTPases (16).
We identified an exchange factor, termed AKAP-Lbc, which functions as GEF for RhoA as well as an A-kinase anchoring protein (AKAP) (17, 18). Interestingly, AKAP-Lbc is a key mediator of α1-AR-induced activation of RhoA (2). α1-ARs enhance AKAP-Lbc Rho-GEF activity through a signaling pathway that requires the α subunit of the heterotrimeric G protein G12 (17). On the other hand, AKAP-Lbc inactivation occurs through a mechanism that requires recruitment of the regulatory protein 14-3-3 (19).
Our present results indicate that AKAP-Lbc organizes a p38 MAPK complex composed of RhoA effectors PKNα, MLTK, MKK3, and p38α that specifically promotes RhoA-dependent activation of p38 in response to α1-AR stimulation. They also provide evidence that the ability of AKAP-Lbc to assemble and activate this p38 activation complex is inhibited following the recruitment of the regulatory protein 14-3-3. Overall, these findings provide a novel mechanistic hypothesis explaining how MAPK signaling pathways can be selectively activated by α1-ARs.
EXPERIMENTAL PROCEDURES
Expression Constructs
AKAP-Lbc fragments encoding amino acids 1585–1922, 1625–1922, 1655–1922, 1715–1922, 1765–1922, 1388–1585, 1388–1655, 1388–1715, and 1388–1765 were PCR-amplified from the AKAP-Lbc pEGFPN1 vector (17) and subcloned at EcoRI/SalI into the pFLAG-CMV6 vector to generate protein fragments fused with the FLAG epitope. A region encoding residues 1388–1922 of AKAP-Lbc was PCR amplified from the AKAP-Lbc pEGFPN1 vector and subcloned at SalI/NotI into pET30a vector to generate protein fragments fused with the histidine tag. The AKAP-Lbc fragment encoding residues 1585–1715 and corresponding to the PKNα binding domain of AKAP-Lbc was PCR amplified from the AKAP-Lbc pEGFPN1 vector and subcloned at EcoRI/SalI into pFLAG-CMV6 and pEGFPN vectors to generate protein fragments fused with the FLAG epitope and GFP, respectively. The FLAG-tagged AKAP-Lbc mutant missing the PKNα binding domain was generated by deleting the region encoding amino acids 1585–1715 by standard PCR-directed mutagenesis using the FLAG-AKAP-Lbc vector (19) as a template.
Double-stranded hairpin (sh) oligonucleotides based upon the human AKAP-Lbc mRNA sequence (GI: 15986728, bases 6688–6706 and 228–246) were cloned into the HindIII and BglII sites in the pSUPER vector. The oligonucleotide sequences used were: human AKAP-Lbc shRNA1 (sense strand), 5′-GTGCGTCTCAATGAGATTT-3′, and human AKAP-Lbc shRNA2 (sense strand), 5′-GGTCAGTTCTGATACATTG-3′.
To generate lentiviral transfer vectors encoding AKAP-Lbc shRNAs, cDNA fragments containing the H1 RNA polymerase III promoter as well as sequences encoding shRNAs were excised using BamHI/SalI from the pSUPER vector and subcloned into the pAB286.1 transfer vector. Mission® lentiviral transfer vectors encoding PKNα shRNAs or a control non-target shRNA were purchased from Sigma. These vectors contain a puromycin cassette that allows the selection of infected cells. The lentiviral packaging vectors pCMVDR8.91 and pMD2. VSVG encode the viral capsid and the vesicular stomatitis virus-G envelope protein, respectively (20).
The full-length cDNA encoding human p38α was PCR amplified from a human heart cDNA library and subcloned at NotI-BamHI into pFLAG-CMV6, BamHI-XhoI into HA-pRK5, or BamHI-Not1 into pGEX4T1 to generate proteins fused to the FLAG and HA epitopes or GST, respectively. Similarly, the full-length cDNA encoding human MLTKβ was PCR amplified from a human heart cDNA library and subcloned at NotI-BamHI into pFLAG-CMV6, BamHI-SalI into HA-pRK5, or BamHI-XhoI into pGEX4T1. Fragments 1–305 and 305–942 of PKNα were amplified from Myc-PKNα (generous gift from Dr. S. Gutkind, NIH, Bethesda, MD) and subcloned at NotI-SalI and NotI/XhoI into pET30a, respectively, to generate fusion proteins with the histidine tag. HA-tagged JNK1, MKK3, MKK6, MEK1, and MEKK1 as well as FLAG-tagged JNK1 constructs were generous gifts from Dr. C. Widmann (Department of Morphology and Cell Biology, University of Lausanne). GFP-ERK1 was obtained from Addgene. Plasmids encoding HA-MLK3 and HA-TAK1 were generous gifts from Dr. L. B. Holzman (University of Michigan Medical School) and Dr. J. Ninomiya-Tsuji (North Carolina State University), respectively. Vectors encoding FLAG-AKAP-Lbc S1565A, AKAP-Lbc S1565A-GFP, and FLAG-tagged AKAP-Lbc fragments encoding residues 1–503, 504–1000, 1001–1387, 1388–1922, 1923–2336, and 2337–2817, as well as 14-3-3β-GFP, were described previously (19).
Expression and Purification of Recombinant Proteins in Bacteria
GST fusion proteins of AKAP-Lbc, p38α, MKK3, and MLTK were expressed using the bacterial expression vector pGEX-4T1 in the BL21(DE3) strain of Escherichia coli and purified. Exponentially growing bacterial cultures were incubated 16 h at 16 °C with 1 mm isopropyl 1-thio-β-d-galactopyranoside, and subsequently subjected to centrifugation. Pelleted bacteria were lysed in buffer D (20 mm Tris, pH 7.4, 150 mm NaCl, 5 mm MgCl2, 1% (w/v) Triton X-100, 1 μg/ml of aprotinin, 2 μg/ml of leupeptin, 2 μg/ml of pepstatin, and 0.1 mm PMSF), sonicated, and centrifuged at 38,000 × g for 30 min at 4 °C. After incubating the supernatants with glutathione-Sepharose beads (GE Healthcare) for 2 h at 4 °C, the resin was washed five times with 10 bed volumes of buffer D and stored at 4 °C.
His6-tagged fusion proteins of PKNα and AKAP-Lbc were expressed using the bacterial expression vector pET30 in BL21(DE3) bacteria and purified. Bacterial extracts containing His6-tagged fusion proteins were prepared in buffer E (20 mm Hepes, pH 7.8, 500 mm NaCl, 10 mm imidazole, 1 mm benzamidine, 2 μg/ml of leupeptin, 2 μg/ml of pepstatin). After a 1-min sonication, the lysates were centrifuged at 38,000 × g for 30 min at 4 °C. The His6-tagged fusion proteins were purified by incubating the supernatant with nickel-nitrilotriacetic acid chelating resin (Amersham Biosciences) for 1 h at 4 °C. The resin was then washed 5 times with 10 bed volumes of buffer E and stored at 4 °C. His6-tagged fusion proteins were eluted from the resin with 20 mm Hepes, pH 7.8, 500 mm NaCl, 300 mm imidazole, 1 mm benzamidine, 2 μg/ml of leupeptin, 2 μg/ml of pepstatin for 1 h at room temperature, dialyzed, and stored at −20 °C. The protein content of the eluates was assessed by Coomassie staining of SDS-PAGE gels.
Production of Lentiviruses
Vesicular stomatitis virus-G (VSVG) pseudotyped lentiviruses were produced by cotransfecting 293-T cells with 20 μg of pAB286.1 vector (21), pAB286.1-AKAP-Lbc shRNA vectors (2), Mission® Non-target shRNA control vector (Sigma) or Mission PKNα shRNA vectors (Sigma), 15 μg of pCMVDR8.91, and 5 μg of pMD2.VSVG (20) using the calcium phosphate method. Culture medium was replaced by serum-free DMEM at 12 h after transfection. Cell supernatants were collected 48 h later, filtered through a 0.45-mm filter unit, and concentrated using Centricon-Plus-70 MW 100,000 columns (Millipore). Virus titers were determined by infecting 293-T cells using serial dilutions of the viral stocks and by scoring the number of puromycin-resistant clones (at 6 days after infection). Titers determined using these methods were between 5 × 108 and 1.0 × 109 transducing units/ml for viruses generated from pAB286.1 vectors and between 4 × 108 and 8 × 108 transducing units/ml for viruses generated from Mission vectors.
Lentiviral Infection
HEK-293 cells were infected at 60% confluence using pAB286.1-based lentiviruses encoding wild type or mutated AKAP-Lbc shRNAs at a multiplicity of infection of 20 in the presence of 8 μg/ml of Polybrene. Two days after infection puromycin was added to the culture medium at a final concentration of 2 μg/ml. After 4 days of selection, puromycin-resistant cells were collected and amplified in selective medium containing puromycin at a final concentration of 2 μg/ml.
Cell Culture and Transfections
HEK-293 were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum and gentamycin (100 μg/ml) and transfected at 50–60% confluence in 100-mm dishes using the calcium-phosphate method. For the overexpression of constructs containing the full-length AKAP-Lbc, HEK-293 cells were transfected at 80% confluence in 100- or 35-mm dishes using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After transfection, cells were grown for 48 h in DMEM supplemented with 10% fetal calf serum before harvesting. The total amount of transfected DNA was 10–24 μg/100-mm dish and 1–4 μg/35-mm dish.
In Vitro GST Pulldown Experiments
For in vitro GST pulldowns, 100 nm bacterially purified His6-tagged fragments encompassing PKNα residues 1–305 and 305–942 as well as AKAP-Lbc residues 1388–1922 were incubated with glutathione-Sepharose beads (Amersham Biosciences) coupled to GST, or to GST fusion proteins of p38α, MKK3, MLTK, or the AKAP-Lbc fragment encompassing residues 1388–1922 in 0.5 ml of buffer A (20 mm Tris, pH 7.4, 150 mm NaCl, 1% (w/v) Triton X-100, 5 μg/ml of aprotinin, 10 μg/ml of leupeptin, and 1 mm PMSF) for 4 h at 4 °C. The beads were then washed five times with buffer A containing 300 mm NaCl, resuspended in SDS-PAGE sample buffer (65 mm Tris, 2% SDS, 5% glycerol, 5% β-mercaptoethanol, pH 6.8) and boiled for 3 min at 95 °C. Eluted proteins were analyzed by SDS-PAGE and Western blotting.
Immunoprecipitation Experiments
For immunoprecipitation experiments, HEK-293 cells grown in 100-mm dishes and expressing various constructs were lysed in 1 ml of buffer A. Cell lysates were incubated 1 h at 4 °C on a rotating wheel. The solubilized material was centrifuged at 100,000 × g for 30 min at 4 °C and the supernatants were incubated 4 h at 4 °C with 20 μl of anti-FLAG M2 affinity resin (Sigma) to immunoprecipitate overexpressed FLAG-tagged proteins. Following a brief centrifugation on a bench-top centrifuge, the pelleted beads were washed five times with buffer C, twice with PBS, and proteins were eluted in SDS-PAGE sample buffer by boiling samples for 3 min at 95 °C. Eluted proteins were analyzed by SDS-PAGE and Western blotting. For immunoprecipitation of endogenous AKAP-Lbc complexes, HEK-293 cells were lysed in 1 ml of buffer B (20 mm Hepes, pH 7.4, 150 mm NaCl, 1% Triton X-100, and 1 mm PMSF). Soluble proteins were isolated by centrifugation as indicated above and incubated with or without 0.25 mm dithiobis(succinimidyl propionate) for 1 h at 4 °C. Cross-linking reactions were blocked by adding Tris, pH 7.4, to the lysate to a final concentration of 50 mm. Immunoprecipitations were performed as indicated previously (19) by incubating 3 mg of lysate with 4 μg of affinity-purified rabbit polyclonal anti-AKAP-Lbc antibodies (Covance).
p38α, JNK, and MLTK Activity Assays
Transfected HEK-293 cells grown in 100-mm dishes were lysed in 1 ml of buffer C (20 mm Tris, pH 7.4, 150 mm NaCl, 1% (w/v) Triton X-100, 10 mm NaF, 10 mm sodium pyrophosphate, 1 mm sodium orthovanadate, 1 mm glycerophophate, 5 μg/ml of aprotinin, 10 μg/ml of leupeptin, and 1 mm PMSF). Cell lysates were incubated 10 min at 4 °C on a rotating wheel. The solubilized material was centrifuged at 100,000 × g for 30 min at 4 °C. 200 μl of supernatant was incubated either with 2 μl of mouse monoclonal anti-p38α antibodies (Cell Signaling Technology) and 20 μl of protein A-Sepharose beads (Amersham Biosciences) for 2 h at 4 °C to immunoprecipitate endogenous p38α or with 20 μl of anti-FLAG M2 affinity resin (Sigma) for 1 h at 4 °C to immunoprecipitate overexpressed FLAG-tagged JNK1 or MLTK. Following centrifugation on a bench-top centrifuge, the pelleted beads were washed three times with buffer C and twice with a buffer containing 50 mm Tris, pH 7.4, and 5 mm MgCl2. Immunoprecipitates containing p38α or JNK1 were incubated with 1 μg of purified GST-ATF2 (Cell Signaling Technology), whereas those containing FLAG-tagged MLTK were incubated with 1 μg of purified GST-MKK3. Reactions were carried out in 50 mm Tris, pH 7.4, 5 mm MgCl2, and 1 mm ATP-Na2 for 30 min at 30 °C and ended by the addition of SDS-PAGE sample buffer and loaded on SDS-PAGE gels.
SDS-PAGE and Western Blotting
Samples denatured in SDS-PAGE sample buffer were separated on acrylamide gels and electroblotted onto nitrocellulose membranes. The blots were incubated with primary antibodies and horseradish-conjugated secondary antibodies (Amersham Biosciences) as previously indicated (17). The following affinity purified primary antibodies were used for immunoblotting: affinity purified rabbit polyclonal anti-AKAP-Lbc (Covance, 0.1 mg/ml, 1:1000 dilution), mouse monoclonal anti-FLAG (Sigma, 4.9 mg/ml, 1:2000 dilution), mouse monoclonal anti-GFP (Roche Applied Science, 400 μg/ml, 1:500 dilution), rabbit polyclonal anti-GFP (Roche Applied Science, 400 μg/ml, 1:1000 dilution), rabbit polyclonal anti-HA (Sigma, 1:1000 dilution), mouse monoclonal anti-HA (Sigma, 1:5000 dilution), rabbit polyclonal anti-phospho-p38α (threonine 180 and tyrosine 182) (Cell Signaling Technologies, 1:1000 dilution), rabbit polyclonal and mouse monoclonal anti-p38α (Cell Signaling Technologies, 1:1000 dilution), rabbit polyclonal anti-phospho-ATF2 (threonine 71) (Cell Signaling Technologies, 1:1000 dilution), rabbit polyclonal anti-ATF2 (Cell Signaling Technologies, 1:1000 dilution), mouse monoclonal anti-phospho-MKK3 (serine 189 and threonine 193) (Cell Signaling Technology, 1:500 dilution), mouse monoclonal anti-MKK3 (Assay designs, 1:1000 dilution), mouse monoclonal anti-MLTK (Abnova, 1:500 dilution), rabbit polyclonal anti-MLK3 (Cell Signaling Technology, 1:500 dilution), mouse monoclonal anti-PKNα (BD Biosciences Pharmingen, 1:1000 dilution), rabbit polyclonal anti-ERK1/2 (Santa Cruz Biotechnology, 1:500 dilution), rabbit polyclonal anti-phospho-ERK1/2 (threonine 202 and tyrosine 204) (Santa Cruz Biotechnology, 1:500 dilution), rabbit polyclonal anti-JNK (Cell Signaling Technologies, 1:500 dilution), rabbit polyclonal anti-phospho-JNK (threonine 183 and tyrosine 185) (Cell Signaling Technologies, 1:500 dilution), mouse monoclonal anti-actin (Sigma, 1:1000 dilution), mouse monoclonal anti-histidine tag (Qiagen 100 μg/ml, 1:1000 dilution).
Statistical Analysis
Statistical significance was analyzed using a Kruskal-Wallis test followed by Mann-Whitney U tests with the Bonferroni corrections.
RESULTS
AKAP-Lbc Mediates α1-AR-induced p38α MAPK Activation
Evidence collected over the last decade indicates that protein kinases of the MAPK family including ERK, JNK, and p38 play an important role in mediating many of the pathophysiological responses induced by α1-ARs (1, 9–11). Although several studies indicate that GTPase RhoA plays a central role in mediating activation of MAPK pathways downstream of α1-ARs (1, 10, 14), it is currently not clear how signals are specifically transferred from activated RhoA to the activation of selected MAPKs.
To initially determine the implication of RhoA in pathways linking α1-ARs to the activation of ERK1/2, JNK, and p38, HEK-293 cells expressing the HA-tagged α1-AR were treated for 2 h in the absence or presence of 1 μg/ml of a cell permeable form of the C3 botulism toxin and subsequently incubated with or without 10−4 m epinephrine for 10 min. Activation of ERK1/2 and JNK in cell lysates was assessed by Western blot using antibodies recognizing the phosphorylated forms of ERK1/2 and JNK. On the other hand, p38α activity was determined using a kinase assay that measured the ability of immunoprecipitated endogenous p38α to induce the phosphorylation of purified GST-ATF2. Interestingly, inhibition of RhoA impaired by 58 and 69% the ability of α1b-ARs to induce p38α activation under basal conditions and following epinephrine stimulation, respectively (Fig. 1, A, panel 1, lanes 7 and 8, and B), without affecting α1-AR-mediated phosphorylation of ERK1/2 and JNK (supplemental Fig. S1, A and C, panel 1, lanes 7 and 8, and B and D). This suggests that RhoA is involved in the pathway that links α1b-ARs to the activation of p38α.
FIGURE 1.
AKAP-Lbc mediates α1-AR-induced p38α activation. A, HEK-293 cells were transfected with the empty pRK5 plasmid or the cDNA encoding the HA-tagged α1b-AR. After a 24-h serum starvation, cells were incubated for 2 h with or without 1 μg/ml of purified C3 toxin and incubated for 15 min with or without (Ctrl) 10−4 m epinephrine (EPI). Cell lysates where subjected to immunoprecipitation using anti-p38α monoclonal antibodies. Kinase reactions were performed by incubating p38α immunoprecipitates with 1 μg of purified GST-ATF2 and in the presence of ATP. Phospho-GST-ATF2 was detected by immunoblot using rabbit polyclonal antibodies recognizing phosphothreonine 71 of ATF2 (panel 1). The amounts of GST-ATF2, p38α, and HA-α1b-ARs were assessed using polyclonal antibodies against ATF2 (panel 2), p38α (panel 3), and the HA epitope (panel 4), respectively. B, quantitative analysis of phosphorylated ATF2 was obtained by densitometry. The amount of phospho-ATF2 was normalized to the total amount of ATF2 and p38α. Results are expressed as mean ± S.E. of 3 different experiments. §, p < 0.05 as compared with phospho-ATF2 levels measured in untreated control cells expressing HA-α1b-ARs. *, p < 0.05 as compared with phospho-ATF2 levels measured in epinephrine-treated control cells expressing HA-α1b-ARs. C, HEK-293 cells infected with control lentiviruses or lentiviruses encoding AKAP-Lbc shRNAs were transfected with the empty pRK5 plasmid or the cDNA encoding the HA-tagged α1b-AR. After a 24-h serum starvation, cells were incubated for 15 min with or without (Ctrl) 10−4 m epinephrine, lysed, and subjected to immunoprecipitation using monoclonal anti-p38α antibodies. Kinase reactions and detection of phospho-ATF2 (panel 1), ATF2 (panel 2), p38α (panel 3), and HA-α1b-ARs (panel 4) were performed as indicated in A. Expression of endogenous AKAP-Lbc and actin was detected using affinity purified anti-AKAP-Lbc polyclonal antibodies (panel 5) and anti-actin monoclonal antibodies (panel 6). D, quantitative analysis of phospho-ATF2 was obtained by densitometry as indicated in B. §, p < 0.05 as compared with phospho-ATF2 levels measured in untreated control cells expressing HA-α1b-ARs. *, p < 0.05 as compared with phospho-ATF2 levels measured in epinephrine-treated control cells expressing HA-α1b-ARs. E, HEK-293 cells were transfected with cDNA encoding the FLAG-tagged S1565A mutant of AKAP-Lbc. After a 24-h serum starvation, cells were incubated for 2 h with or without 1 μg/ml of purified C3 toxin. Kinase activity of immunoprecipitated p38α and detection of phospho-ATF2 (panel 1), ATF2 (panel 2), and p38α (panel 3) in cell lysates was determined as indicated in A. Expression of the FLAG-tagged AKAP-Lbc S1565A mutant was assessed using monoclonal antibodies against the FLAG tag (panel 4). F, quantitative analysis of phosphorylated ATF2 was obtained by densitometry as indicated in B. *, p < 0.05 as compared with phospho-ATF2 levels measured in untreated cells expressing FLAG-AKAP-Lbc S1565A.
Based on these results and our previous evidence that the RhoA-specific guanine nucleotide exchange factor AKAP-Lbc mediates RhoA activation downstream of α1b-ARs (2), we raised the question of whether AKAP-Lbc might organize the signaling cascade linking α1b-ARs to the activation of p38α. To address this hypothesis, we initially determined the impact of silencing AKAP-Lbc expression in HEK-293 cells on the ability of α1-ARs to induce the activation of p38α.
AKAP-Lbc silencing was achieved by infecting cells using lentiviruses encoding two distinct shRNAs directed against a sequence within the Dbl homology domain (shRNA1) and N-terminal regulatory region (shRNA2) of AKAP-Lbc, respectively. Both shRNAs could inhibit AKAP-Lbc expression by about 90% as compared with cells infected with control lentiviruses (Fig. 1C, panel 5).
Infected cells were transfected with the cDNA encoding the HA-tagged α1b-AR, serum starved for 24 h, and incubated in the absence or presence of epinephrine for 10 min. p38α was then immunoprecipitated and its activity assessed as indicated above.
Silencing of AKAP-Lbc expression significantly reduced the ability of α1b-ARs to induce p38α activation both under basal conditions and following epinephrine stimulation. Basal p38α activation was inhibited between 67 (shRNA1) and 74% (shRNA2), whereas inhibition of epinephrine-induced p38α activation was between 63 (shRNA1) and 72% (shRNA2) (Fig. 1C, panel 1, lanes 9–12, and D). Interestingly, re-expression of a silencing resistant mutant of AKAP-Lbc (2) in silenced cells rescued the ability of α1b-AR to promote the activation of p38α (supplemental Fig. S2, A, panel 1, lanes 5 and 6, and B).
These results suggest that the inhibition of p38α activation was strictly dependent on reduced AKAP-Lbc expression and not due to an off-target effect. Control experiments revealed that the ability of α1b-ARs to promote phosphorylation of endogenous ERK1/2 and JNK was not affected by AKAP-Lbc silencing (supplemental Fig. 3, A, panels 1 and 3, lanes 7 and 8). These results strongly suggest that AKAP-Lbc specifically contributes to the activation of p38α MAPK induced by α1b-ARs.
To directly determine whether AKAP-Lbc can enhance p38α activation through its ability to activate RhoA, we assessed whether RhoA inhibition could affect the p38α activating potential of the S1565A mutant of AKAP-Lbc, which displays constitutive Rho-GEF activity (19). HEK-293 cells transfected with the FLAG-tagged AKAP-Lbc S1565A mutant were serum starved for 24 h and incubated for 2 h in the absence or presence of 1 μg/ml of C3 botulinum toxin. As shown in Fig. 1E, overexpression of the FLAG-tagged AKAP-Lbc S1565A mutant induced a 2.9-fold enhancement of p38α kinase activity, which was reduced after RhoA inhibition (Fig. 1, E, panel 1, lanes 2 and 3, and F). Control experiments revealed that this constitutively active AKAP-Lbc mutant was not able to promote phosphorylation of endogenous ERK1/2 and JNK (supplemental Fig. S3, B and C, upper panel, lane 2). Altogether, these findings indicate that the AKAP-Lbc·RhoA complex specifically mediates α1-AR-induced p38α activation.
AKAP-Lbc Interacts with p38α
Our current findings reveal that AKAP-Lbc and RhoA mediate the activation of p38α but not that of ERK1/2 and JNK downstream of α1b-ARs. This suggests the existence of molecular mechanisms that allow the AKAP-Lbc signaling complex to select and activate the p38 effector pathway. One attractive hypothesis would be that AKAP-Lbc and p38α might be maintained within the same macromolecular unit. In this configuration, activating signals could be integrated by AKAP-Lbc and rapidly transmitted to p38α.
To assess whether AKAP-Lbc could form a complex with p38, we determined whether HA-tagged p38α could be co-immunoprecipitated with FLAG-tagged AKAP-Lbc from lysates of transfected HEK-293 cells. As shown in Fig. 2A, anti-FLAG antibodies could immunoprecipitate HA-p38α from cells expressing the FLAG-tagged AKAP-Lbc, but not from cells transfected with the empty pFLAG vector (Fig. 2A, upper panel, lanes 1 and 2). Using a similar approach we could show that HA-JNK1 and GFP-ERK1 do not form stable complexes with AKAP-Lbc vector (Fig. 2, B and C, upper panel, lanes 1 and 2). These results suggest that AKAP-Lbc specifically binds and activates p38α. The p38 family of MAPK is constituted by four members (p38α, p38β, p38γ, and p38δ) (22). Co-immunoprecipitation experiments performed using the different recombinant p38 kinases indicate that FLAG-AKAP-Lbc interacts mainly with p38α, to a lesser extent with p38β, but not with p38γ and p38δ (results not shown).
FIGURE 2.
AKAP-Lbc assembles a p38α activation complex. A–C, extracts from HEK-293 cells transfected with the plasmids encoding HA-tagged p38α (A), HA-tagged JNK1 (B), or GFP-tagged ERK1 (C) in combination with the empty FLAG vector (lane 1) or the vector encoding FLAG-tagged AKAP-Lbc (lane 2) were subjected to immunoprecipitation with anti-FLAG antibodies. Western blots of the immunoprecipitates and the cell extracts were revealed using anti-HA monoclonal antibodies to detect HA-p38α and HA-JNK1 (A and B, upper and middle panels), anti-GFP polyclonal antibodies to detect GFP-ERK1 (C, upper and middle panels), or anti-FLAG monoclonal antibodies to detect FLAG-AKAP-Lbc (lower panels). D, HEK-293 cells were transfected with the plasmids encoding HA-tagged MKK4, MKK3, or MKK6 in combination with the vector encoding FLAG-tagged AKAP-Lbc. Immunoprecipitations and Western blots were performed as indicated in A. E and F, HEK-293 cells were transfected with the plasmids encoding HA-tagged MLK3, MEKK1, TAK1 (E) or MLTK (F), in combination with the empty FLAG vector or the plasmid encoding FLAG-tagged AKAP-Lbc. Immunoprecipitations and Western blots were performed as indicated in A. G, extracts from HEK-293 cells transfected with the empty FLAG vector or the plasmid encoding FLAG-tagged AKAP-Lbc were subjected to immunoprecipitation with anti-FLAG antibodies. Western blots of the immunoprecipitates and the cell extracts were revealed using anti-PKNα monoclonal antibodies. Results are representative of at least three independent experiments.
AKAP-Lbc Assembles a p38α Activation Module
MAPKs are activated by protein kinase cascades in which the MAPK is phosphorylated and activated by a MAP kinase kinase (MAPKK) that is, in turn, phosphorylated and activated by a MAP kinase kinase kinase (MAPKKK). It is well established that p38α can be activated by the MAPKKs MKK3, MKK4, and MKK6 (23). In turn, these three p38-activating kinases can be phosphorylated and activated by several MAPKKKs such as TAK1, members of the mixed lineage kinase (MLK) family including MLK3, MLTK, and DLK, and several members of the MEKK family of protein kinases (23, 24). Because these kinases have been described to be organized into signaling complexes to create functional MAPK modules, we have investigated the possibility that kinases known to act upstream of p38α would also associate with AKAP-Lbc.
To address this point, we performed coimmunoprecipitation experiments from HEK-293 cells that were transiently transfected with the cDNA encoding the HA-tagged forms of the MAPKKs MKK3, MKK4, and MKK6 (Fig. 2D), as well as of the MAPKKKs MLK3, MEK1, TAK1 (Fig. 2E), and MLTK (Fig. 2F) in combination with the empty FLAG vector (pFLAG) or the FLAG-tagged AKAP-Lbc. After immunoprecipitating the anchoring protein using anti-FLAG antibodies, anti-HA antibodies were used to immunoblot the immunoprecipitated samples. Western blots revealed that MKK3 (Fig. 2D, upper panel, lane 5) as well as kinases belonging to MLK family including MLK3 and MLTK (Fig. 2, E, upper panel, lane 4, and F, upper panel, lane 2) could specifically co-immunoprecipitate with AKAP-Lbc, whereas the MAPKKs MKK4 and MKK6 as well as the MAPKKKs MEKK1 and TAK1 did not (Fig. 2, D, upper panel, lanes 4 and 6, and E, upper panel, lanes 5 and 6). These results suggest that AKAP-Lbc can interact with a signaling module composed of p38α, its upstream kinase MKK3, and MAPKKKs of the MLK family such as MLK3 or MLTK.
However, MLKs have never been reported as direct effectors of RhoA. Accordingly, control experiments performed in our laboratory using purified kinases failed to detect MLK activation by RhoA (results not shown). This raises the question of how the AKAP-Lbc·RhoA complex can transmit signals to the MLK-MKK3-p38α module. In this context, previous evidence indicates that protein kinase Nα (PKNα), a well characterized effector of RhoA, can act as an upstream activating kinase of MLTK (25). Based on these findings, we raised the hypothesis of whether PKNα could also bind to AKAP-Lbc. Interestingly, Western blots performed on FLAG-AKAP-Lbc immunoprecipitates indicate that endogenous PKNα can co-immunoprecipitate with the anchoring protein (Fig. 2G, upper panel, lane 2).
In a similar set of experiments, we could show that p38α, MKK3, MLTK, and PKNα endogenously expressed in HEK293 cells could form a complex with endogenous AKAP-Lbc. This is shown by the fact that p38α, MKK3, MLTK, and PKNα could be detected in AKAP-Lbc immunoprecipitates (Fig. 3, A, upper and middle panels, lane 3, and B, upper and middle panels, lane 3). On the other hand, no interaction between endogenous AKAP-Lbc and MLK3 could be detected (Fig. 3C, upper panel, lane 3). Therefore, whereas overexpressed AKAP-Lbc has the potential of binding both MLK3 and MLTK (Fig. 2, E and F), endogenous AKAP-Lbc seems to preferentially assemble a complex that specifically contains MLTK. Collectively these results suggest that AKAP-Lbc can assemble a large signaling complex containing PKNα, MLTK, MKK3, and p38α that can link RhoA activation to the stimulation of the p38 transduction pathway.
FIGURE 3.
AKAP-Lbc interacts with endogenous p38α, MKK3, MLTK, and PKNα. HEK-293 cell extracts were subjected to immunoprecipitation with either non-immune IgGs or affinity purified anti-AKAP-Lbc polyclonal antibodies. Western blots of the immunoprecipitates and the cell extracts were revealed using anti-p38α (A, upper panel), anti-MKK3 (A, middle panel), anti-PKNα (B, upper panel), anti-MLTK (B, middle panel), anti-MLK3 (C, upper panel), or affinity purified anti-AKAP-Lbc polyclonal antibodies (lower panels). Results are representative of at least three independent experiments.
Mapping of the Kinase Binding Sites on AKAP-Lbc
To identify the binding site for p38α as well as its upstream activating kinases on AKAP-Lbc, we initially generated a series of FLAG-tagged AKAP-Lbc fragments encompassing residues 1–503, 504–1000, 1001–1387, 1388–1922, 1923–2336, and 2337–2817 (supplemental Fig. S4A). The fragments were initially expressed in HEK-293 cells in combination with HA-MKK3 and interactions assessed by co-immunoprecipitation. The FLAG-tagged fragments were immunoprecipitated from cell lysates using anti-FLAG antibodies and the presence of associated kinases detected using anti-HA antibodies. Our results indicate that MKK3 interact exclusively with the fragment of AKAP-Lbc included between residues 1388 and 1922 (supplemental Fig. S4, upper panel, lane 5).
We have subsequently further narrowed the binding site using shorter FLAG-tagged AKAP-Lbc fragments derived from the 1388–1922 region (Fig. 4A). As shown in Fig. 4B, our results indicate that HA-MKK3 interacts with AKAP-Lbc in a minimal region encompassing residues 1585–1715 (Fig. 4B, upper panel).
FIGURE 4.
Mapping the kinase interaction sites on AKAP-Lbc. A, schematic representation of the AKAP-Lbc fragments used for the mapping experiments. The minimal binding site (residues 1585–1715) is boxed. LC3 and 14-3-3 binding sites as well as the C1 region (C1) are shown. B, HEK-293 cells were transfected with HA-tagged MKK3 in combination with either the empty FLAG vector or FLAG-tagged fragments of AKAP-Lbc indicated in A. Cell lysates were subjected to immunoprecipitation with anti-FLAG antibodies. Western blots of the immunoprecipitates and cell extracts were revealed using anti-HA polyclonal antibodies to detect the HA-tagged MKK3 (upper and middle panels), or anti-FLAG monoclonal antibodies to detect the FLAG-tagged AKAP-Lbc fragments (lower panel). C–F, extracts from HEK-293 cells transfected with plasmids encoding the empty FLAG vector, FLAG-AKAP-Lbc, or the FLAG-AKAP-Lbc Δ1585–1715 mutant in combination with the vectors encoding HA-tagged p38α (C), MKK3 (D), MLTK (E), or the empty pRK5 vector (F). Western blots of the immunoprecipitates (IP) and the cell extracts were revealed using anti-HA polyclonal antibodies to detect HA-tagged p38α, MKK3, and MLTK (C–E, upper and middle panels), anti-PKNα monoclonal antibodies to detect endogenous PKNα (F, upper and middle panels), or anti-FLAG monoclonal antibodies to detect FLAG-AKAP-Lbc (lower panels).
To validate these findings and assess whether the identified domain was also required for binding the other kinases, we determined the impact of deleting residues 1585–1715 from the AKAP-Lbc on its ability to associate with HA-p38α, HA-MKK3, HA-MLTK, and endogenous PKNα in co-immunoprecipitation experiments (Fig. 4, C–F). The deletion reduced significantly the ability of all the tested kinases to co-immunoprecipitate with the FLAG-tagged AKAP-Lbc (Fig. 4, C–F, upper panel, second and third lanes). These findings suggest that residues 1585–1715 form a binding site that recruits p38α as well as its upstream activating kinases MKK3, MLTK, and PKNα.
PKNα Directly Binds AKAP-Lbc, p38α, MKK3, and MLTK
Although our current results suggest that AKAP-Lbc interacts with PKNα, MLTK, MKK3, and p38α, they do not indicate how the complex is organized. Based on previous findings showing that PKNα can act both as an upstream activating kinase of MLTK and as a scaffolding protein (25) we hypothesized that it could recruit p38α, MKK3, and MLTK to AKAP-Lbc. Therefore, we determined whether PKNα could directly associate with AKAP-Lbc as well as with the other kinases.
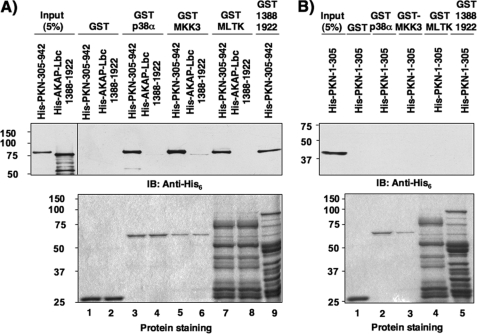
To assess whether the interaction of p38α, MKK3, and MLTK with AKAP-Lbc occurs through a direct interaction or whether it is mediated through PKNα, we monitored the ability of purified GST fusion proteins of p38α, MKK3, and MLTK, and AKAP-Lbc fragment 1388–1922 to associate with purified His6-tagged N-terminal or C-terminal fragments of PKNα (His-PKN-(1–305) and His-PKN-(305–942), respectively), or with the His6-tagged 1388–1922 fragment of AKAP-Lbc using an in vitro pulldown assay. Interestingly, our results indicate that p38α, MKK3, and MLTK, as well as AKAP-Lbc can directly bind the C-terminal but not the N-terminal fragment of PKNα (Fig. 5, A, upper panel, lanes 3, 5, 7, and 9, B, upper panel, lanes 2–6). A weak direct interaction could also be detected between AKAP-Lbc and MKK3 (Fig. 5A, upper panel, lane 6), whereas no binding was observed between AKAP-Lbc and p38α or MLTK (Fig. 5A, upper panel, lanes 4 and 8). These results indicate that AKAP-Lbc can directly bind PKNα, which, in turn can recruit p38α, MKK3, and MLTK. The association between AKAP-Lbc and MKK3 could stabilize the formation of the complex.
FIGURE 5.
PKNα directly interacts with MLTK, MKK3, p38α, and AKAP-Lbc. A, bacterially purified His6-tagged fragments (100 nm) encompassing residues 305–942 of PKNα and 1388–1922 of AKAP-Lbc were incubated with glutathione-Sepharose beads coupled to 2 μg of GST alone, or GST-tagged p38α, MKK3, MLTK, and AKAP-Lbc-(1388–1922). Associated His6-tagged fragments were detected using anti-His6 monoclonal antibodies (upper panel). A control protein staining indicating the expression level of the different GST-tagged constructs used in the pulldown assay is shown (lower panel). B, bacterially purified His6-tagged fragments encompassing residues 1–305 of PKNα (100 nm) were incubated with glutathione-Sepharose beads coupled to 2 μg of GST alone, or GST-tagged p38α, MKK3, MLTK, and AKAP-Lbc 1388–1922. Associated His6-tagged fragments were detected as indicated in A.
PKNα Is Required for Proper Assembly of the AKAP-Lbc·p38α Signaling Complex
To assess whether PKNα could contribute to assembly of the AKAP-Lbc·p38α complex inside cells, we determined the impact of silencing PKNα expression in HEK-293 cells on the interaction of AKAP-Lbc with p38α and MKK3.
PKNα silencing was achieved by infecting cells using lentiviruses encoding shRNAs directed against PKNα. Using this approach, PKNα expression could be inhibited by 80–90% as compared with cells infected with control lentiviruses (Fig. 6A, panel 6).
FIGURE 6.
Silencing of PKNα inhibits AKAP-Lbc-mediated activation of MLTK. A, extracts from HEK-293 cells infected with control lentiviruses (control) or lentiviruses encoding PKNα shRNAs (PKNα shRNA1) were subjected to immunoprecipitation (IP) with either non-immune IgGs or affinity purified anti-AKAP-Lbc polyclonal antibodies. Western blots of the immunoprecipitates and the cell extracts were revealed using antibodies against p38α (panels 1 and 2), MKK3 (panels 3 and 4), AKAP-Lbc (panel 5), PKNα (panel 6), or actin (panel 7). B, HEK-293 cells infected with control lentiviruses or lentiviruses encoding PKNα shRNAs (shRNA1 and shRNA2) were transfected with the plasmids encoding FLAG-MLTK in the presence of the vector encoding GFP or the GFP-tagged AKAP-Lbc S1565A mutant. After a 24-h serum starvation, cells were lysed and FLAG-MLTK was subjected to immunoprecipitation using anti-FLAG monoclonal antibodies. Kinase reactions were performed by incubating FLAG-MLTK immunoprecipitates with 1 μg of purified GST-MKK3 and in the presence of ATP. Phospho-GST-MKK3 was detected by immunoblot (IB) using a rabbit polyclonal antibody recognizing phosphoserine 189 and phosphothreonine 193 of MKK3 (panel 1). The amounts of immunoprecipitated FLAG-MLTK as well as the expression of AKAP-Lbc S1565A-GFP and PKNα in cell lysates were assessed using antibodies against the FLAG tag (panel 2), GFP (panel 3), and PKNα (panel 4), respectively. Results are representative of three independent experiments.
AKAP-Lbc was immunoprecipitated from infected cells using affinity purified anti-AKAP-Lbc antibodies and the presence of associated p38α and MKK3 revealed by Western blot. As shown in Fig. 6A, silencing of PKNα expression significantly reduced the amount of p38α and MKK3 interacting with endogenous AKAP-Lbc (Fig. 6A, panels 1 and 3, lane 4). This suggests that PKNα favors the association of p38α and MKK3 with AKAP-Lbc.
PKNα Mediates AKAP-Lbc-induced Activation of MLTK
Previous findings have shown that PKNα can directly phosphorylate and activate MLTK (25). Based on this evidence, we determined whether PKNα was required to transmit activating signals from AKAP-Lbc to MLTK. To address this point, we assessed whether silencing of PKNα using shRNAs targeting two distinct regions of the kinase (PKN shRNA1 and PKN shRNA2) could affect the ability of the S1565A mutant of AKAP-Lbc, which displays constitutively Rho-GEF activity, to activate MLTK.
Infected cells were transfected with the cDNAs encoding FLAG-MLTK and the GFP-tagged AKAP-Lbc S1565A mutant. After a 24-h serum starvation, FLAG-MLTK was then immunoprecipitated and its ability to phosphorylate purified GST-MKK3 determined using an in vitro kinase assay. Interestingly, silencing of PKNα expression significantly reduced the ability of AKAP-Lbc S1565A to promote MLTK activation (Fig. 6B, panel 1, lanes 5 and 6) suggesting that PKNα is required for AKAP-Lbc-mediated MLTK activity.
Disruption of AKAP-Lbc Complex Impairs α1-AR-mediated p38α Activation
Based on the mapping studies presented above, we investigated the possibility of whether a fragment of AKAP-Lbc encompassing residues 1585–1715 could be used as a competitor to disrupt the endogenous complexes formed by AKAP-Lbc and the various kinases. Such a competitor fragment could represent a valuable tool to study the role of the AKAP-Lbc signaling complex in the activation of p38α inside cells.
A GFP fusion of the competitor fragment was expressed in HEK-293 cells and its ability to inhibit the binding of HA-MKK3, HA-MLTK, as well as PKNα to the FLAG-tagged fragment of AKAP-Lbc encompassing residues 1388–1922 was assessed by co-immunoprecipitation (supplemental Fig. S5). Interestingly, overexpression of the fragment reduced the interaction between AKAP-Lbc and the various kinases by more than 80% suggesting that it can act as an efficient competitive inhibitor (supplemental Fig. S5, A–C, upper panel, lane 3). Based on these results we determined the impact of overexpressing the GFP-tagged competitor fragment on the ability of α1b-adrenergic receptors to induce p38α activation in HEK-293 cells.
Interestingly, expression of the competitor fragment reduced by 62 and 58% the ability of α1b-ARs to promote p38α activation under basal conditions and in response to epinephrine stimulation, respectively (Fig. 7, A, panel 1, lanes 7 and 8, and B), without affecting receptor-induced phosphorylation of ERK1/2 and JNK1 (supplemental Fig. S6, A and B, panel 1, lanes 3 and 4). These findings suggest that the integrity of the complex formed by AKAP-Lbc and the various kinases is required for the activation of p38α induced by α1b-ARs.
FIGURE 7.
Disruption of the AKAP-Lbc complex inhibits α1b-AR-induced p38α activation. A, HEK-293 cells were transfected with vectors encoding GFP or GFP-tagged AKAP-Lbc fragment 1585–1715 in the absence or presence of the plasmid encoding the HA-tagged α1-AR. After a 24-h serum starvation, cells were incubated for 15 min with or without 10−4 m epinephrine (EPI), lysed, and subjected to immunoprecipitation using anti-p38α monoclonal antibodies. Kinase reactions were performed by incubating p38α immunoprecipitates with 1 μg of purified GST-ATF2 and in the presence of ATP. Detection of phospho-ATF2 (panel 1), ATF2 (panel 2), p38α (panel 3), and HA-tagged α1-AR (panel 5) were performed as indicated in Fig. 1A. Expression of GFP as well as the GFP-tagged AKAP-Lbc fragment 1585–1715 (panel 4) was detected using polyclonal anti-GFP antibodies. B, quantitative analysis of phosphorylated ATF2 was obtained by densitometry. The amount of phospho-ATF2 was normalized to the total amount of ATF2. Results are expressed as mean ± S.E. of 3 different experiments.). §, p < 0.05 as compared with phospho-ATF2 levels measured in untreated cells expressing HA-α1b-ARs and GFP. *, p < 0.05 as compared with phospho-ATF2 levels measured in epinephrine-treated cells expressing HA-α1b-ARs and GFP.
Binding of 14-3-33 to AKAP-Lbc Inhibits the Recruitment of PKNα and Reduces p38α Activation
We previously demonstrated that recruitment of the regulatory protein 14-3-3 to a motif located at position 1565 within the N-terminal regulatory region of AKAP-Lbc strongly inhibits the Rho-GEF activity of the anchoring protein (19). Our current results indicate that this site is located in close proximity of the binding domain for the p38α activation complex. This raises the possibility that 14-3-3 recruitment could also interfere with the interaction of AKAP-Lbc with the p38α signaling complex and therefore inhibit AKAP-Lbc-mediated p38 activation.
To address this question, we initially determined whether overexpression of 14-3-3β in HEK-293 cells could affect the ability of AKAP-Lbc to associate with PKNα. HEK-293 cells were transfected with FLAG AKAP-Lbc together with increasing amounts of GFP-tagged 14-3-3β. After immunoprecipitating the anchoring protein using anti-FLAG antibodies, anti-PKNα antibodies were used to immunoblot the immunoprecipitated samples. Western blots revealed that overexpression of increasing amounts of 14-3-3β progressively reduced the ability of PKNα to co-immunoprecipitate with AKAP-Lbc (Fig. 8, A, panel 1, lanes 2–4, and B). In line with these results, we could show that the S1565A mutant of AKAP-Lbc, which is unable to bind 14-3-3, display a 2-fold higher ability to associate with endogenous PKNα when compared with wild type AKAP-Lbc (Fig. 8, C, panel 1, lanes 2 and 3, and D). This indicates that recruitment of 14-3-3 inhibits PKNα binding to AKAP-Lbc.
FIGURE 8.
14-3-3 inhibits the interaction between AKAP-Lbc and PKNα. A, HEK-293 cells were transfected with the empty pFLAG vector or the vector encoding FLAG-AKAP-Lbc in combination with increasing amounts (indicated above each lane) of the plasmid encoding 14-3-3β-GFP. Cell extracts were subjected to immunoprecipitation with anti-FLAG antibodies. Western blots of the immunoprecipitates and cell extracts were revealed using anti-PKNα polyclonal antibodies (panels 1 and 2), anti-14-3-3β polyclonal antibodies (panels 3 and 4), or anti-FLAG monoclonal antibodies to detect the FLAG-AKAP-Lbc (panel 5). B, densitometry of the bands corresponding to PKNα coimmunoprecipitated with AKAP-Lbc. The amount of PKNα in the immunoprecipitates was normalized to the PKNα content of the cell extracts. Results are expressed as mean ± S.E. of three independent experiments. *, p < 0.05 as compared with the levels of co-immunoprecipitated PKNα measured in cells expressing only FLAG-AKAP-Lbc. C, extracts from HEK-293 cells transfected with plasmids encoding the empty FLAG vector, FLAG-AKAP-Lbc, or FLAG-AKAP-Lbc S1565A. Cell extracts were subjected to immunoprecipitation (IP) with anti-FLAG antibodies. Western blots of the immunoprecipitates and the cell extracts were revealed as indicated in A. D, densitometry of the bands corresponding to PKNα coimmunoprecipitated with AKAP-Lbc was performed as indicated in B. Results are expressed as mean ± S.E. of three independent experiments. *, p < 0.05 as compared with the levels of co-immunoprecipitated PKNα measured in cells expressing FLAG-AKAP-Lbc. E, extracts from HEK-293 cells transfected with the vectors encoding FLAG-AKAP-Lbc-GFP or FLAG-AKAP-Lbc S1565A. After a 24-h serum starvation, cells were lysed and subjected to immunoprecipitation using monoclonal anti-p38α antibodies. Kinase reactions and detection of phospho-ATF2 (panel 1), ATF2 (panel 2), and p38α (panel 3) were performed as indicated in Fig. 1A. Expression of the AKAP-Lbc constructs was detected using anti-FLAG monoclonal antibodies (panel 4). F, quantitative analysis of phospho-ATF2 was obtained as indicated in Fig. 1B. Results are expressed as mean ± S.E. of three different experiments. *, p < 0.05 as compared with phospho-ATF2 levels measured in cells expressing FLAG-AKAP-Lbc.
To determine whether this reduction of PKNα binding induced by 14-3-3 could affect the ability of AKAP-Lbc to induce p38α activation, we compared the p38 activating potential of the wild type and 14-3-3 binding deficient forms of AKAP-Lbc. We could show that deletion of the 14-3-3 binding site increases by 2.7-fold the activation of p38α induced by the anchoring protein (Fig. 8, E, upper panel, lanes 2 and 3, and F). Collectively, these results suggest that 14-3-3 exerts an inhibitory effect on the ability of AKAP-Lbc to recruit and activate the p38α signaling complex.
DISCUSSION
MAP kinase pathways are crucial mediators of several pathophysiological responses induced by α1-adrenergic receptors (1, 9–11). Although evidence collected over the last years indicates that MAPK cascades are organized in transduction modules (13), it is currently unknown how such signaling complexes are assembled and activated in response to α1-adrenergic receptor stimulation to generate specific cellular responses. Our findings now indicate that the RhoA-selective exchange factor AKAP-Lbc recruits a signaling module containing the RhoA effector PKNα and the MLTK, MKK3, and p38α kinases, which transduce activating signals from α1b-ARs down to p38α. In particular, whereas we have previously shown that α1b-ARs mediate AKAP-Lbc activation through the α subunit of the heterotrimeric G protein G12 (2), our current model proposes that activated AKAP-Lbc promotes, through RhoA, the sequential activation PKNα, MLTK, MKK3, and p38α within the complex. On the other hand, inactivation of AKAP-Lbc-mediated p38 signaling occurs following the recruitment of 14-3-3 to AKAP-Lbc. This promotes the dissociation of the p38α activation module from AKAP-Lbc (Fig. 9) and deactivates AKAP-Lbc Rho-GEF activity (19). Therefore, AKAP-Lbc represents a molecular platform where signals that activate or deactivate p38 signaling converge.
FIGURE 9.
Model for the AKAP-Lbc·p38α activation complex. AKAP-Lbc assembles a signaling complex that includes the scaffolding protein PKNα as well as MLTK, MKK3, and p38α. The AKAP-Lbc signaling complex is activated in response to α1-AR stimulation through a Gα12-mediated signaling pathway (17). Activated AKAP-Lbc promotes the formation of RhoA-GTP, which, in turn, induces the activation of a signaling cascade that includes PKNα, MLTK, MKK3, and p38α. The recruitment of 14-3-3 inhibits AKAP-Lbc Rho-GEF activity (19), impairs the interaction between PKNα and AKAP-Lbc, and reduces p38 activation.
p38 kinases were originally described to mediate cellular responses to various types of stresses (23, 26). During the last decade, however, it has become increasingly clear that members of this kinase family can participate in signaling pathways activated by of a variety of other membrane receptors, including cytokine and G protein-coupled receptors, to promote cellular functions such as proliferation, growth, inflammation, and contraction (23, 26). In particular, it was shown that activation of p38α by α1-ARs can regulate arterial smooth muscle cell contractility (27) and promote cardiomyocyte sarcomere remodeling during cardiac hypertrophy (10).
Because of the implication of p38 kinases in such a variety of crucial responses, the molecular mechanisms involved in their activation has been the subject of intensive investigation. In this context, it is well established that GTPases of the Rho family, including RhoA, Rac1, and cdc42, are key mediators of p38 activation induced by membrane receptors (10, 14, 24, 28). However, how activation of Rho GTPases by upstream stimuli is translated into the activation of a specific p38 pathway is far from being entirely elucidated.
Two studies provided initial evidence that the GTPase Rac-1 and its upstream activator Tiam can recruit signaling complexes containing p38 and its upstream activating kinases. In a first study, Rac-1 was shown to directly bind to Osm, a scaffold protein that recruits MEKK3 and MKK3 (29). This complex has been implicated in hyperosmotic shock-induced p38 activation. In a second study, the Rac-1 activator Tiam was shown to recruit a p38 signaling complex formed by the scaffold protein JIP2 and the kinases MLK3 and MKK3 (30). This study, however, did not determine which extracellular stimuli activate this signaling module or whether Tiam and JIP2 can form a complex at the endogenous level.
More recently, elegant studies showed that the pro-myogenic cell surface protein Cdo can recruit two proteins named Bnip-2 and JLP, which act as scaffolds for the Cdc42 and p38, respectively (31, 32). The assembly of this signaling complex promotes Cdc42-dependent p38 activation.
In this context, our current findings that AKAP-Lbc can mediate α1-AR-induced p38 activation through the assembly of a signaling module composed of PKNα, MLTK, MKK3, and p38α, provide new mechanistic insights on how specific signals can be vehiculated from membrane receptors to p38α. They also highlight the key function of PKNα as a scaffold protein that facilitates RhoA-dependent p38 activation. This view is supported by our results showing that PKNα can directly interact with AKAP-Lbc, MLTK, MKK3, and p38α (Fig. 5), and that silencing of endogenous PKNα in HEK-293 cells can reduce the interaction of endogenous p38α and MKK3 with AKAP-Lbc as well as AKAP-Lbc-mediated MLTK activation (Fig. 6).
Interestingly, previous studies have shown that PKNα can also promote the activation of p38γ (28) potentially through its ability to bind and activate MLTK and MKK6 (25). This suggests that PKNα can recruit a different combination of signaling enzymes to modulate the activation of different p38 isoforms. The molecular mechanisms regulating the interaction between PKNα and p38 activating kinases are currently unknown and will deserve further investigation. Based on our co-immunoprecipitation experiments, which failed to detect an interaction between p38γ and AKAP-Lbc (results not shown) one could raise the hypothesis that AKAP-Lbc might selectively stabilize the interaction between PKNα and p38α.
In addition to PKNα, three other scaffold proteins including JIP2, JIP4, and Osm have been shown to promote p38 activation (13, 29, 33). Interestingly, recent studies identified an interaction between recombinant JIP4 and a splice variant of AKAP-Lbc, called Brx, that contains only the last 1429 residues the anchoring protein (34). However, whereas these studies mapped the interaction determinants for JIP4 to the last 400 amino acids of Brx, they did not determine whether the two proteins can interact at the endogenous level.
In control experiments, we could not detect the expression of JIP4 in HEK-293 cell lysates (results not shown), suggesting that JIP4 is unlikely to be involved in the recruitment of p38α and its upstream kinases to AKAP-Lbc in this cell line. In line with this conclusion, our results indicate that deletion of the PKNα binding site (residues 1585–1715) from AKAP-Lbc abolishes the ability of the anchoring protein to recruit the p38α and its upstream kinases MKK3 and MLTK (Fig. 4). This suggests that the interaction between AKAP-Lbc and p38α is mediated by the PKNα binding domain and does not involve additional interaction sites. It is possible, however, that the ability of AKAP-Lbc and Brx to recruit PKNα or JIP4 might be influenced by the relative abundance of these scaffolding proteins in different cell types.
Our current findings support the view that AKAP-Lbc specifically mediates α1-AR-induced p38α activation without affecting activation of ERK1/2 and JNK (Figs. 1 and 7, and supplemental Figs. S1 and S6). At the molecular level, this can be explained by the fact that AKAP-Lbc forms a complex with p38α and not with ERK and JNK (Figs. 2 and 3). Therefore, the ability of AKAP-Lbc to transmit activating signals to specific MAPK pathways is dictated by its ability to recruit specific combinations of kinases. These results suggest therefore that different signaling complexes might mediate α1-AR-induced activation of ERK1/2 and JNK. In this respect, recent previous findings indicate that the Rho-GEF p115 can recruit a scaffolding protein named CNK1, which binds the kinases MLK2 and MKK7 to coordinate the activation of JNK1 (35). Further investigations will be required to determine whether p115 or a different signaling complex is involved in the organization of the JNK pathway downstream of α1b-ARs.
Members of the 14-3-3 family regulate a variety of transduction pathways either by affecting the catalytic activity, the subcellular localization, or by regulating protein-protein interaction properties of signaling molecules (36). It is well established that 14-3-3 has an antagonistic effect on the p38 signaling pathway (37, 38). This could be in part mediated by the inhibitory action of 14-3-3 on Ask1, a MAPKKK that acts upstream of p38 (39). However, our current findings now suggest that 14-3-3 can inhibit AKAP-Lbc-mediated p38α activation by inducing the dissociation of the PKNα·MLTK·MKK3·p38α complex from AKAP-Lbc (Fig. 8) as well as by inhibiting the Rho-GEF activity of the anchoring protein (19). Therefore, our results provide a new molecular explanation on how 14-3-3 proteins can impair p38 signaling inside cells.
Given the proximity of the binding sites for 14-3-3 and PKNα it is tempting to speculate that 14-3-3 recruitment might inhibit the interaction between AKAP-Lbc and PKNα by directly masking the PKNα interaction site on the anchoring protein. This is reminiscent of the mechanism through which 14-3-3 has been shown to inhibit the interaction between the pro-apoptotic protein Bad and pro-survival Bcl-2 family members (40).
In conclusion, the implications of our findings are 2-fold. First, they identify key molecular mechanisms controlling signaling specificity downstream of α1b-ARs. By assembling a macromolecular signaling complex containing RhoA, PKNα, MLTK, MKK3, and p38α, AKAP-Lbc controls the specific transduction of signals from α1b-ARs to p38α. Second, they provide a novel hypothesis explaining the inhibitory action of 14-3-3 on the p38 pathway, suggesting that AKAP-Lbc might represent a molecular platform integrating 14-3-3 and p38 signaling.
Supplementary Material
Acknowledgments
We acknowledge Monique Nenniger-Tosato for excellent technical assistance, Prof. Susanna Cotecchia for helpful discussions and suggestions, Dr. C. Widmann for providing HA-tagged JNK1, MKK3, MKK6, MEK1, and MEKK1, as well as FLAG-tagged JNK1 constructs, Dr. S. Gutkind for providing the vector encoding Myc-PKNα, Dr. L. B. Holzman for providing the plasmid encoding HA-MLK3, and Dr. J. Ninomiya-Tsuji for providing the HA-TAK1 vector.
This work was supported by Grant 3100A0-122020 of the Fonds National Suisse de la Recherche Scientifique (to D. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
- α1-AR
- α1-adrenergic receptor
- GEF
- guanine nucleotide exchange factor
- AKAP
- A-kinase anchoring protein
- MLK
- mixed lineage kinase.
REFERENCES
- 1. Maruyama Y., Nishida M., Sugimoto Y., Tanabe S., Turner J. H., Kozasa T., Wada T., Nagao T., Kurose H. (2002) Circ. Res. 91, 961–969 [DOI] [PubMed] [Google Scholar]
- 2. Appert-Collin A., Cotecchia S., Nenniger-Tosato M., Pedrazzini T., Diviani D. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 10140–10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Philipp M., Hein L. (2004) Pharmacol. Ther. 101, 65–74 [DOI] [PubMed] [Google Scholar]
- 4. Zhang H., Faber J. E. (2001) Circ. Res. 89, 815–822 [DOI] [PubMed] [Google Scholar]
- 5. Erami C., Zhang H., Tanoue A., Tsujimoto G., Thomas S. A., Faber J. E. (2005) Am. J. Physiol. Heart Circ. Physiol. 289, H744–753 [DOI] [PubMed] [Google Scholar]
- 6. Milano C. A., Dolber P. C., Rockman H. A., Bond R. A., Venable M. E., Allen L. F., Lefkowitz R. J. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 10109–10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Knowlton K. U., Rockman H. A., Itani M., Vovan A., Seidman C. E., Chien K. R. (1995) J. Clin. Invest. 96, 1311–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Connell T. D., Ishizaka S., Nakamura A., Swigart P. M., Rodrigo M. C., Simpson G. L., Cotecchia S., Rokosh D. G., Grossman W., Foster E., Simpson P. C. (2003) J. Clin. Invest. 111, 1783–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clerk A., Sugden P. H. (1999) Am. J. Cardiol. 83, 64H–69H [DOI] [PubMed] [Google Scholar]
- 10. Charron F., Tsimiklis G., Arcand M., Robitaille L., Liang Q., Molkentin J. D., Meloche S., Nemer M. (2001) Genes Dev. 15, 2702–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang H., Chalothorn D., Jackson L. F., Lee D. C., Faber J. E. (2004) Circ. Res. 95, 989–997 [DOI] [PubMed] [Google Scholar]
- 12. Widmann C., Gibson S., Jarpe M. B., Johnson G. L. (1999) Physiol. Rev. 79, 143–180 [DOI] [PubMed] [Google Scholar]
- 13. Morrison D. K., Davis R. J. (2003) Annu. Rev. Cell Dev. Biol. 19, 91–118 [DOI] [PubMed] [Google Scholar]
- 14. Brown J. H., Del Re D. P., Sussman M. A. (2006) Circ. Res. 98, 730–742 [DOI] [PubMed] [Google Scholar]
- 15. Cerione R. A., Zheng Y. (1996) Curr. Opin. Cell Biol. 8, 216–222 [DOI] [PubMed] [Google Scholar]
- 16. Zheng Y. (2001) Trends Biochem. Sci. 26, 724–732 [DOI] [PubMed] [Google Scholar]
- 17. Diviani D., Soderling J., Scott J. D. (2001) J. Biol. Chem. 276, 44247–44257 [DOI] [PubMed] [Google Scholar]
- 18. Welch E. J., Jones B. W., Scott J. D. (2010) Mol. Interv. 10, 86–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diviani D., Abuin L., Cotecchia S., Pansier L. (2004) EMBO J. 23, 2811–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F. H., Verma I. M., Trono D. (1996) Science 272, 263–267 [DOI] [PubMed] [Google Scholar]
- 21. Bridge A. J., Pebernard S., Ducraux A., Nicoulaz A. L., Iggo R. (2003) Nat. Genet. 34, 263–264 [DOI] [PubMed] [Google Scholar]
- 22. Raman M., Chen W., Cobb M. H. (2007) Oncogene 26, 3100–3112 [DOI] [PubMed] [Google Scholar]
- 23. Coulthard L. R., White D. E., Jones D. L., McDermott M. F., Burchill S. A. (2009) Trends Mol. Med. 15, 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gallo K. A., Johnson G. L. (2002) Nat. Rev. Mol. Cell Biol. 3, 663–672 [DOI] [PubMed] [Google Scholar]
- 25. Takahashi M., Gotoh Y., Isagawa T., Nishimura T., Goyama E., Kim H. S., Mukai H., Ono Y. (2003) J. Biochem. 133, 181–187 [DOI] [PubMed] [Google Scholar]
- 26. Nebreda A. R., Porras A. (2000) Trends Biochem. Sci. 25, 257–260 [DOI] [PubMed] [Google Scholar]
- 27. Srinivasan R., Forman S., Quinlan R. A., Ohanian J., Ohanian V. (2008) Am. J. Physiol. Heart Circ. Physiol. 294, H961–969 [DOI] [PubMed] [Google Scholar]
- 28. Marinissen M. J., Chiariello M., Gutkind J. S. (2001) Genes Dev. 15, 535–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uhlik M. T., Abell A. N., Johnson N. L., Sun W., Cuevas B. D., Lobel-Rice K. E., Horne E. A., Dell'Acqua M. L., Johnson G. L. (2003) Nat. Cell Biol. 5, 1104–1110 [DOI] [PubMed] [Google Scholar]
- 30. Buchsbaum R. J., Connolly B. A., Feig L. A. (2002) Mol. Cell. Biol. 22, 4073–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kang J. S., Bae G. U., Yi M. J., Yang Y. J., Oh J. E., Takaesu G., Zhou Y. T., Low B. C., Krauss R. S. (2008) J. Cell Biol. 182, 497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu M., Krauss R. S. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 4212–4217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kelkar N., Standen C. L., Davis R. J. (2005) Mol. Cell. Biol. 25, 2733–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kino T., Takatori H., Manoli I., Wang Y., Tiulpakov A., Blackman M. R., Su Y. A., Chrousos G. P., DeCherney A. H., Segars J. H. (2009) Sci. Signal. 2, ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jaffe A. B., Hall A., Schmidt A. (2005) Curr. Biol. 15, 405–412 [DOI] [PubMed] [Google Scholar]
- 36. Yaffe M. B. (2002) FEBS Lett. 513, 53–57 [DOI] [PubMed] [Google Scholar]
- 37. Xing H., Zhang S., Weinheimer C., Kovacs A., Muslin A. J. (2000) EMBO J. 19, 349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang S., Ren J., Zhang C. E., Treskov I., Wang Y., Muslin A. J. (2003) Circ. Res. 93, 1026–1028 [DOI] [PubMed] [Google Scholar]
- 39. Zhang L., Chen J., Fu H. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 8511–8515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zha J., Harada H., Yang E., Jockel J., Korsmeyer S. J. (1996) Cell 87, 619–628 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.