Abstract
The multifunctional Ca2+/calmodulin-dependent protein kinase II (CaMKII) promotes vascular smooth muscle (VSMC) proliferation. However, the signaling pathways mediating CAMKII-dependent proliferative effects in vivo are poorly understood. This study tested the hypothesis that CaMKIIδ mediates neointimal proliferation after carotid artery ligation by regulating expression and activity of cell cycle regulators, particularly at the G1/S checkpoint. Data herein indicate that 14 days after carotid ligation, C57Bl/6 mice developed a marked neointima with robust CaMKII protein expression. In particular, only the CaMKII isoform δ was increased as demonstrated by quantitative RT-PCR. Genetic deletion of CaMKII δ prevented injury-induced neointimal hyperplasia and cell proliferation in the intima and media. In ligated carotids of control mice, the proliferative cell cycle markers cdk2, cyclin E, and cyclin D1 were activated. In contrast, in CaMKIIδ−/− mice, we detected a reduction in proliferative cell cycle regulators as well as an increase in the cell cycle inhibitor p21. This expression profile was confirmed in cultured CaMKIIδ−/− VSMC, in which cdk2 and cdk4 activity was decreased. Toward understanding how CAMKIIδ affects p53, a transcriptional regulator of p21, we examined p53 pathway components. Our data indicate that p53 is elevated in CAMKIIδ−/− VSMC, whereas phosphorylation of the p53-specific E3 ligase, Mdm2, was decreased. In conclusion, CaMKII stimulates neointima proliferation after vascular injury by regulating cell proliferation through inhibition of p21 and induction of Mdm-2-mediated degradation of p53.
Keywords: Atherosclerosis, Calcium Calmodulin-dependent Protein Kinase (CaMK), Cell Cycle, Signal Transduction, Smooth Muscle, Neointima, Vascular Injury
Introduction
Smooth muscle proliferation contributes to vascular remodeling and obstructive vasculopathies such as atherosclerosis and restenosis following percutaneous coronary interventions (1). Therefore, understanding potential mechanisms to reduce or inhibit neointimal hyperplasia is an important goal in cardiovascular medicine. Several reports have emphasized the role of cell cycle regulation in proliferation after vascular injury. In mammalian cells, cell cycle progression is tightly controlled by cyclin/cyclin-dependent kinases (cdk)2 complexes and their inhibitors, p21 and p27. Inhibiting positive cell cycle regulators decreases neointimal proliferation as demonstrated by data in which antisense cdk2 kinase oligonucleotides inhibit intimal formation following vascular injury (2, 3). Additionally, p21 induction inhibits VSMC proliferation and limits neointimal formation in vivo after balloon catheter injury (4). However, the precise signaling pathways that initiate the changes in cell cycle regulatory proteins remain unclear.
The multifunctional Ca2+/calmodulin-dependent protein kinase II (CaMKII) is strongly expressed in vascular smooth muscle cells (VSMC) (5), where it is required for angiotensin-II-mediated vascular smooth muscle hypertrophy (5) and force maintenance in vascular smooth muscle cell contraction (6). Accumulating evidence suggests that CaMKII is critical for in vitro VSMC proliferation (7) and migration (8). For example, depletion of CaMKII δ2 with a specific shRNA inhibited neointimal formation and adventitial thickening after balloon injury of rat carotid arteries (9). However, the molecular mechanisms by which CaMKII regulates VSMC proliferation remain are largely unknown.
Recent cancer biology studies have identified CaMKII and CaMKII-dependent signaling pathways as regulators of the cell cycle machinery. Cell cycle arrest in response to CaMKII inhibition has been reported in both G1 (10, 11) and in G2/M phase (7). Various effects of CAMKII inhibition on cell cycle progression have been reported in different models: decreased activity of cdk2 and 4 in NIH 3T3 cells (11), decreased phosphorylation and subsequent proteasomal degradation of the cell cycle inhibitor p27 in colon cancer cell lines (12) and up-regulation of the cell cycle inhibitor p21 with concomitant down-regulation of the positive regulators cyclin D1, cyclin E, and cdk2 in human ovarian cell lines (13).
In light of these studies that document a role for CAMKII in both neointimal formation as well as control of cell cycle regulators, we hypothesized that CaMKII mediates VSMC proliferation and neointimal formation via regulation of G1 cell cycle progression. Our data demonstrate that CaMKIIδ deletion significantly reduced neointimal formation after carotid artery ligation in vivo and abrogated cell proliferation in the intima and media after injury. The decreased proliferation was associated with increased expression of the cell cycle inhibitor p21. In cultured aortic VSMC isolated from CaMKIIδ−/− mice, a similar anti-proliferative expression pattern was present with increased expression of p21. p21 strongly associated with cdk2/cyclin E and cdk4/cyclin D complexes in CaMKIIδ−/− cells and accordingly, the cdk kinase activities were decreased. Previous studies report that CaMKII activates Akt in VSMC (14), and inhibition of Akt decreases neointimal formation after vascular injury through increased p21 expression (15). In our experiments, CaMKIIδ deletion led to decreased Akt activation and increased p21 expression. p53, the transcriptional regulator of p21, was up-regulated in CaMKIIδ−/− cells. In addition, the phosphorylation of the Mdm2, the E3 ligase that initiates ubiquitination of p53, was decreased with CaMKIIδ deletion. Overall, the results demonstrate for the first time that CaMKIIδ regulates VSMC proliferation through the Akt pathway via transcriptional regulation of p21.
EXPERIMENTAL PROCEDURES
Reagents
The following antibodies were used in this study: anti-α-smooth muscle actin, anti-cyclin D, anti-cyclin E, anti-p21, anti-PCNA, anti-Mdm2, anti-GAPDH (Santa Cruz Biotechnology), anti-BrdU (Invitrogen), anti-cdk2, anti-cdk4, anti-cyclin D1, anti-p21, anti-p-Akt, anti-Akt, anti-p-Mdm2 (Cell Signaling Technology), anti-p27 (Milipore), anti-annexin V (Abcam), anti-total CaMKII (5).
Mice
Male and female CaMKII δ−/− (16), a kind gift by Dr. Eric Olsen, University of Texas Southwestern Medical Center, and C57Bl/6 wild-type littermate mice were used according to the University of Iowa Institutional Animal Care and Use Committee guidelines. All procedures were in compliance with the standards for the care and use of laboratory animals of the Institute of Laboratory Animal Resource, National Academy of Science.
Carotid Injury Model
10–12-week-old wild-type C57Bl/6 mice and CaMKIIδ−/− mice were anesthetized with ketamine and xylazine (2 mg and 0.3 mg, respectively, intraperitoneal (IP)). The left common carotid artery was ligated through a midline neck incision (17). 7, 14, or 28 days after injury, all animals were anesthetized and perfused with PBS, followed by 4% paraformaldehyde for 3 min. The carotid arteries were excised and embedded in paraffin. Five micron cross sections were taken starting at the carotid ligation site for Verhoeff-van Gieson elastin and HE staining. Total vessel, luminal, intimal, and medial areas were measured using NIH Image J. Intimal and medial VSM cells were counted after nuclear staining with DAPI. 1, 2, or 4 weeks after injury, some animals received two subcutaneous injections of the thymidine analog BrdU (2 mg per injection). These injections were given 12 h and 1 h before the mice were sacrificed.
Immunohistochemistry
Mouse aorta and carotid arteries were fixed in 4% paraformaldehyde and cryoprotected in 30% sucrose/OCT and embedded in OCT. 10-μm frozen sections were collected on Superfrost Plus slides. Frozen sections were washed in PBS and then preincubated in 5% goat serum for 30 min followed by incubation in primary antibodies for 1 h at room temperature. After washes in PBS for 30 min at room temperature, the primary antibody was detected with Alexa 568-conjugated secondary antibodies (Invitrogen). Sections were counterstained with Vectashield containing DAPI (Vector Labs) to visualize nuclei. Images were captured with Zeiss LSM 710m Laser scanning microscope. Densitometry for different antigens was performed using NIH Image J.
Quantitative PCR
Total RNA was isolated per manufacturer's recommendation, including digestion with proteinase K and DNase I to eliminate genomic DNA contamination. cDNA was transcribed from 1 μg RNA using Superscript III enzyme (Invitrogen) and random nanomer primers. Message expression was quantified using an iQ Lightcycler (Bio-Rad) with SYBR green dye and normalized to acidic ribosomal phosphoprotein (ARP) mRNA. Specificity and replication efficiency were tested for each primer pair.
Cell Culture
Mouse aortic smooth muscle cells were isolated by enzymatic dispersion (18). Briefly, mouse VSMCs were used at passages 4–10. Cells were cultured in DMEM supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 8 mm HEPES, and 2 mm l-glutamine at 37 °C in a humidified 95% air and 5% CO2 incubator. The purity of the mouse VSMCs preparation in culture was confirmed by immunocytochemistry for α-smooth muscle actin.
Cell Proliferation and DNA Synthesis Assay
For proliferation measurements, VSMCs were seeded in 6-well plates at 105 cells/ml, and cultured in DMEM containing 10% FBS until reaching 70% confluence. Cells were counted in triplicates for three consecutive days using a Beckman Coulter automated cell counter. For DNA synthesis measurements, VSMCs were plated in 12-well plates at 5 × 104cells/ml. At about 70% confluence, the cells were growth-arrested in serum-free media for 24 h. Cells were then grown in media with 10% FBS in the presence of 0.5 μCi [3H]thymidine/well for 24 h. The labeling reaction was terminated by aspirating the medium and subjecting cultures to washes with PBS containing 10% trichloroacetic acid and ethanol/ether. Acid soluble [3H]thymidine was extracted with 0.5 m NaOH, which was mixed with liquid mixture and counted in scintillation counter as previously described (19).
Immunoblot and Immunoprecipitation
Whole tissue extracts from mouse aorta, carotid arteries or mouse VSM cells were prepared in ice-cold RIPA lysis buffer and used for immunoblotting (5). For immunoprecipitation, 200–300 μg of protein was precipitated with 2 μg of antibody in the presence of dynabeads (Invitrogen) for 2 h at room temperature. Cell lysates or tissue homogenates were centrifuged at 4 °C for 15 min at maximal speed, and the supernatant was stored at −80 °C for further analysis. Samples and prestained molecular weight markers were separated in SDS-polyacrylamide gels (4–20%) and transferred to a polyvinylidene difluoride membrane. After blocking with 5% BSA, the membranes were incubated with different primary antibodies for 1 h or overnight. The blots were then incubated with a horseradish peroxidase-conjugated secondary antibody and developed by a chemiluminescence detection system described by the manufacturer (Santa Cruz Biotechnology).
Cdk2 and Cdk4 Kinase Activity Assay
C57Bl/6 and CaMKIIδ−/− VSMC were lysed at about 70% confluence. 200 μg of protein was immunoprecipitated with anti-cdk2, anti-cdk4 antibody or rabbit serum in the presence of dynabeads. The precipitates were incubated with [32P]ATP and histone H1 or retinoblastoma protein (Rb) for 30 min at 30 °C and separated on 12% polyacrylamide gels as described (20).
CaMKII Kinase Activity Assay
CaMKII activity of fresh cell lysates from C57Bl/6 and CaMKIIδ−/− VSMC was measured with syntide 2 as substrate as described (21).
Adenoviral Transduction
VSMC were incubated with adenoviruses containing genes for Myc-tagged CaMKIIδ2 or the HA-tagged CaMKII peptide inhibitor CaMKIIN at MOI of 500 overnight. Subsequent experiments were conducted 72 h after adenoviral transduction (5).
Statistical Analysis
Data are shown as mean ± S.E. unless noted otherwise. The SigmaPlot statistical package was used for the quantitative analyses of parameters such as intima-medial lesion area and intimal-medial SMC number (ANOVA and Student's t test). A probability value <0.05 was considered significant.
RESULTS
CaMKII Expression in the Neointima after Carotid Ligation
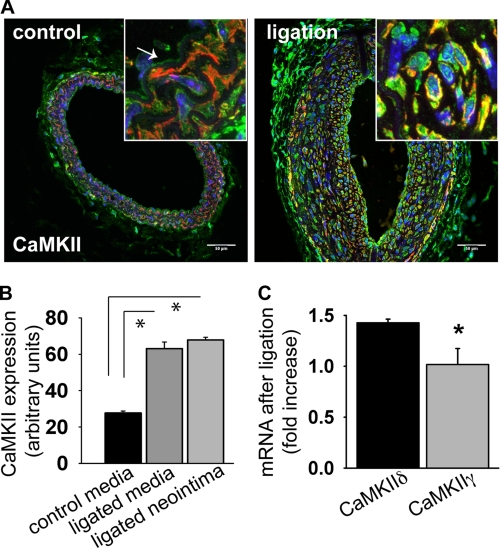
We first sought to characterize the expression pattern of CAMKII following carotid ligation. As demonstrated in Fig. 1A, CaMKII is expressed in the endothelium and media of unligated carotid arteries from C57Bl/6 mice (left panel). We next performed carotid ligation and found, 14 days after ligation (17), intimal hyperplasia up to 2 mm proximal to the ligature. Strikingly, a significant increase in CaMKII expression was detected in the neointima and media of the ligated carotid arteries as compared with unligated arteries (Fig. 1, A, right panel and B). CaMKII isoforms γ and δ are reported to be expressed in arteries (22, 23), therefore we performed qRT-PCR to identify which isoforms are up-regulated after carotid ligation. 14 days after surgery, CaMKIIδ mRNA was increased by 42.6%+3.6% (p < 0.05) whereas CaMKIIγ mRNA was unchanged in response to ligation (Fig. 1C).
FIGURE 1.
CaMKII expression after carotid ligation. A, cross section of an untreated control carotid artery of a C57Bl/6 mouse (left panel) shows CaMKII immunoreactivity in the endothelium and media. 14 days of carotid ligation, strong CaMKII immunoreactivity (green) is seen in the neointima (smooth-muscle actin red, DAPI blue). Inset: detail of the carotid media (control) and neointima (ligated) at ×63 magnification. Representative example is ∼2.0 mm proximal to the ligation. Scale bar, 50 μm. B, densitometric quantitation of CaMKII expression in the media of the untreated control and neointima and media of a ligated C57Bl/6 mouse carotid artery 14 days after ligation. The measurements are given in arbitrary units adjusted for area and background. C, CaMKII isoform mRNA expression in carotid arteries of C57Bl/6 mouse 14 days after ligation by qrtPCR. Five carotids were pooled per measurement; data are representative of three independent experiments (*, p < 0.05).
CaMKIIδ−/− Mice Display Normal Vascular Morphology and Blood Pressure Response
For further studies, we used CaMKIIδ−/− mice that lack functional CaMKII due to deletion of the ATP binding site in exons 1 and 2 (16). First, we quantified the CaMKIIγ and δ mRNA expression to evaluate whether CaMKIIγ is up-regulated to compensate for the absence of the δ isoform. Whereas CaMKIIδ mRNA was not detectable, CaMKIIγ was increased 1.7-fold in aortic RNA samples of CaMKIIδ−/− mice (supplemental Fig. S1). We did not detect any difference in the morphology of carotid arteries of CaMKIIδ−/− mice or a difference in CaMKII expression by immunohistochemistry (Fig. 2A), likely due the compensatory up-regulation of CaMKIIγ expression. As CaMKII has been postulated to mediate smooth muscle contraction and blood pressure regulation, we determined basic hemodynamic parameters of CaMKIIδ−/− mice. No difference in systolic blood pressure was detected at baseline by tail cuff measurement (114 ± 2.1 mmHg in CaMKIIδ−/− mice versus119 ± 1.1 mmHg in wild-type littermates, ns). Under Ang-II infusion at pressor dose, the increase in systolic blood pressure was comparable in controls and CaMKIIδ−/− mice (16 versus 17 mm Hg ns, supplemental Fig. S1B). These findings suggest CaMKIIδ deletion does not result in obvious vascular pathology.
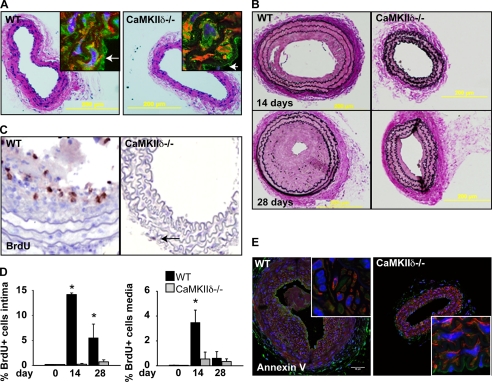
FIGURE 2.
CaMKIIδ deletion decreases neointima formation and cell proliferation following carotid artery ligation. A, H&E staining of representative sections of C57Bl/6 littermate control and CaMKIIδ−/− mouse carotid arteries. Inset: CaMKII immunofluorescence (green) in the carotid media (smooth-muscle actin-red, DAPI-blue, 63×). B, Verhoeff-van Gieson elastin staining of representative sections of C57Bl/6 littermate control and a CaMKIIδ−/− mouse carotid arteries 14 and 28 days after total ligation. C, BrdU labeling in situ 14 days after ligation. D, quantitation of BrdU-labeled cells in the intima (left) and media (right). E, representative annexin V staining of C57Bl/6 littermate control and CaMKIIδ−/− mouse carotid arteries 14 days after ligation Inset: no significant annexinV labeling is detected (63×). *, p < 0.05.
CaMKIIδ−/− Mice Are Protected against Neointimal Hyperplasia after Carotid Ligation
Interruption of carotid flow caused significant neointimal formation in a majority of wild-type mice after 14 days (Fig. 2B, upper panels), whereas only 2 out of 14 CaMKIIδ−/− mice (81% versus 14%, p < 0.01) developed a neointima. The neointimal area in control mice was significantly greater than the CaMKIIδ−/− mice 14 and 28 days following carotid ligation (Table 1). Next, we compared cell numbers in control and CaMKIIδ−/− ligated arteries. As shown in Table 1, CaMKIIδ−/− mice had reduced cell number in both the intima and media compared with littermate controls at both time points examined. Studies of cell proliferation demonstrate that, in control mice, 14.2% of cells in the neointima and 3.1% of cells in the media stained positive for BrdU 14 days post-ligation, while 5.5% of neointimal and 0.6% of medial cells from CaMKIIδ−/− mice were BrdU-positive (Fig. 2, C and D). This trend was maintained in the neointima at 28 days following ligation. These data strongly suggest that CaMKIIδ mediates neointimal formation by stimulating cell proliferation after vascular injury by carotid ligation.
TABLE 1.
Morphometrical measurements in WT and CaMKIIδ−/− mice
| Measurement | Group | 0 Days | 14 Days | 28 Days | |||
|---|---|---|---|---|---|---|---|
| Intimal area (mm2) | WT | 0.0003 | (0.00001)a | 0.0216 | (0.0037) | 0.0588 | (0.0184) |
| CaMKIIδ−/− | 0.0003 | (0.00001) | 0.0039b | (0.003) | 0.0220b | (0.0139) | |
| Total cell # Intima | WT | n/a | 82 | (14) | 373 | (104) | |
| CaMKIIδ−/− | n/a | 18b | (14) | 104b | (63) | ||
| Medial area (mm2) | WT | 0.0249 | (0.003) | 0.0377 | (0.002) | 0.0333 | (0.0040) |
| CaMKIIδ−/− | 0.0224 | (0.0002) | 0.0253b | (0.0019) | 0.0248b | (0.0015) | |
| Total cell # Media | WT | 97 | (1) | 132 | (15) | 127 | (20) |
| CaMKIIδ−/− | 92 | (2) | 73b | (4) | 85b | (11) | |
| Area of stenosis (%) | WT | n/a | 42.1 | (6.8) | 91.7 | (4.1) | |
| CaMKIIδ−/− | n/a | 8.0b | (6.5) | 58.5 | (14.8) | ||
a Numbers in parentheses represent the S.E.
b p < 0.05, WT vs. CaMKIIδ−/−.
Because it has been shown that CaMKII mediates apoptosis in cardiac myocytes (24), and that apoptosis may contribute to neointima formation after carotid ligation (17), we investigated the role of CaMKIIδ in apoptosis following carotid ligation. Using annexin V staining 14 days after carotid ligation, we did not detect evidence for increased apoptosis in the neointima in control or CaMKIIδ−/− mice (Fig. 2E). Taken together, these findings show that CaMKIIδ deletion is associated primarily with reduced VSMC proliferation in the intima and media without significantly affecting apoptosis.
CaMKIIδ Determines the Expression of Cell Cycle Regulatory Proteins
Data in Figs. 1 and 2 identified CaMKII as a regulator of VSMC proliferation in response to carotid ligation injury. We next sought to understand how CaMKII mechanistically promotes neointima growth. CaMKII controls various cell cycle regulators of the G1 phase (11, 13). Therefore, we hypothesized that CaMKIIδ induces VSMC proliferation after carotid ligation through regulation of key cell cycle proteins.
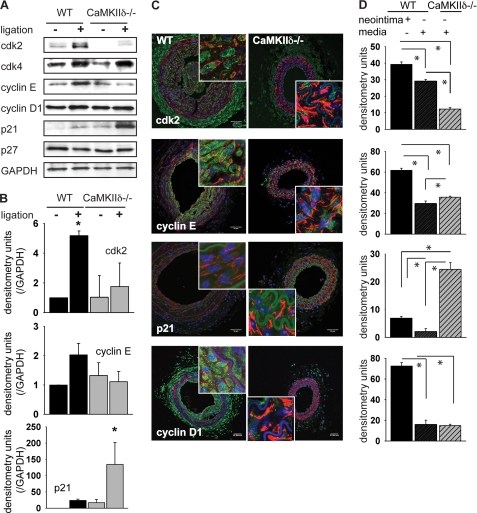
To assess how CaMKII affects expression of these cell cycle regulatory proteins, carotid arteries were harvested from wild-type or CaMKIIδ−/− mice 2 weeks after ligation. As expected, in wild-type mice, positive regulators of cellular proliferation, cyclin E and cdk2, were markedly up-regulated after injury compared with uninjured arteries (Fig. 3, A and B). Cyclin D expression was slightly elevated after ligation injury, and the cell cycle inhibitor p21 was expressed at low levels in the wild-type mice. In contrast, CaMKIIδ−/− mice showed little to no increase in cdk2 and cyclin E, whereas the cell cycle inhibitor p21 was significantly elevated after ligation. The p27 and cyclin D expression levels remained unchanged after ligation and were comparable to wild-type mice. These results indicate that CaMKIIδ coherently regulates expression of cell cycle-related proteins in vascular injury. After deletion of CaMKIIδ, the cell cycle enhancing regulators cdk2 and cyclin E are decreased whereas the inhibitor p21 is strongly expressed. Overall, this expression pattern favors cell cycle arrest.
FIGURE 3.
CaMKIIδ deletion affects the expression of cell cycle regulators after carotid artery ligation. A, ligated and contralateral unligated carotid arteries were explanted 14 days after ligation. 10 μg of lysate was resolved by SDS-PAGE and blotted with antibodies as indicated. (n = 6–8 mice per group). B, quantitation of three independent experiments by densitometric analysis (*, p < 0.05). C, cdk2, cyclinD1, and E, p21 immunofluorescence (green) in C57Bl/6 littermate control and CaMKIIδ−/− mouse carotid artery 14 days after ligation (smooth muscle-actin-red, DAPI-blue, scale bar 25 μm). Inset: detail of the neointima and media of a ligated control and of the media of a ligated CaMKIIδ−/− carotid artery. D, densitometric quantitation of cdk2, cyclinD1, and E, p21 in the neointima and media of the ligated control and in the media of the ligated CaMKIIδ−/− carotid artery, adjusted for area and background. Because no measurable neointima developed after ligation in CaMKIIδ−/− carotid arteries, no densitometry of the CaMKIIδ−/− neointima is provided.
Immunoblots of carotid lysates give an integrated read-out of the carotids without making distinctions between cell types. Hence, we examined localization of cell cycle proteins by immunofluorescence. The highest expression of cdk2, cyclin E, and cyclin D1 was detected in smooth-muscle actin-positive cells within the neointima of injured carotid arteries (Fig. 3, C and D). This region is where the greatest degree of cell proliferation occurred (Fig. 2D). However, significantly reduced expression of the positive cell cycle regulatory proteins was detected in the media of CaMKIIδ−/− mice following carotid injury compared with control mice (Fig. 3, C and D). The cell cycle inhibitor p21 was virtually absent in control mice but detectable in the media of CaMKIIδ−/− mice. These data suggest that CaMKII promotes expression of positive cell cycle regulators and negatively regulates p21 expression in the media following vascular injury.
CaMKIIδ Deletion Decreased VSMC Proliferation but Not Overall CaMKII Activity
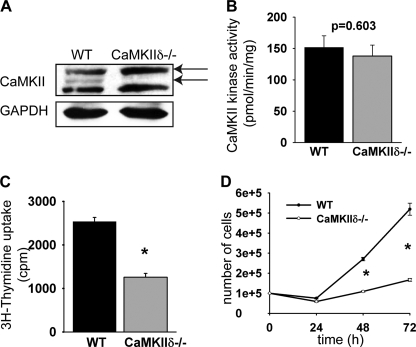
To further characterize the signaling pathways by which CaMKII regulates cell cycle regulator expression, we isolated aortic smooth muscle cells from CaMKIIδ−/− mice and littermates controls. Similar to our findings in uninjured arteries, CaMKIIγ mRNA was increased by 47 + 11% compared with wild-type mice while CaMKIIδ mRNA was undetectable in CaMKIIδ−/− VSMC (supplemental Fig. S2). In immunoblots with a pan-CaMKII antibody, we detected two specific bands in lysates from wild type VSMC, one at 54kD that corresponds to CaMKIIγ and one at 52 kDa representing CaMKIIδ (9). In CaMKIIδ−/− VSMC, the lower band representing CaMKIIδ was missing as expected (Fig. 4A). However, the band at 54 kDa was more prominent than in the wild-type cells, which may account for the increased CaMKIIγ mRNA copy numbers in CaMKIIδ−/− VSMC. A lower band at 50 kDa was also seen. The identity of this band is not entirely clear, but it is likely unspecific. Next, we investigated if genetic deletion of CaMKIIδ affected total CaMKII activity. We did not detect a significant difference in total Ca2+/CaM-dependent CaMKII activity in the two cell types (Fig. 4B, 85% in CaMKIIδ−/− cells versus 100% in control cells, ns).
FIGURE 4.
Characterization of VSMC isolated from CaMKIIδ−/− aortas. A, immunoblots for total CaMKII. The lower band (∼52 kDa) represents CaMKIIδ and is absent in CaMKIIδ−/− cells. The lowest band is most likely unspecific. B, CaMKII activity was measured as a function of [32P]ATP incorporation into a synthetic substrate (syntide-2). C, [3H]thymidine uptake assays. [3H]thymidine incorporation is displayed as cpm. D, cell counts of CaMKIIδ−/− and wild-type VSMCs that were plated at low density and grown in media with 10% FBS. Three samples were counted in duplicate. (*, p < 0.05).
Data in Fig. 2 and Table 1 demonstrate that CaMKII expression controls proliferation of the neointimal cell population. We confirmed that CaMKIIδ−/− VSMC are have impaired cell growth by [3H]thymidine incorporation. Specifically, CaMKIIδ deletion reduced DNA synthesis under growth conditions by 51 + 3%, (Fig. 4C, p < 0.05), and the rate proliferation of CaMKIIδ−/− VSMC was significantly lower compared with wild-type cells (Fig. 4D). These data confirm that CaMKIIδ deletion decreases VSMC proliferation, and this phenotype is not compensated by increased expression of CaMKIIγ.
Expression of Cell Cycle Regulators in Proliferating CaMKIIδ−/− VSMC
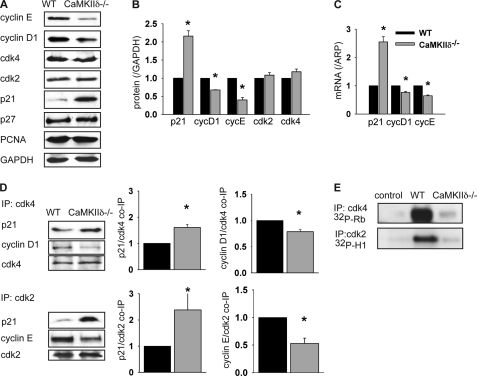
Consistent with data in arterial sections, p21 protein expression was significantly increased in CaMKIIδ−/− VSMC compared with wild-type control cells (2.1-fold, p < 0.05) without any significant changes in p27 protein levels. Cyclin E and to a lesser degree cyclin D1 protein expression were significantly diminished with deficiency of CaMKIIδ (0.32- and 0.67-fold, p < 0.05), whereas cdk2 and -4 and PCNA expression was not affected by CaMKIIδ deletion (Fig. 5, A and B). Comparison of mRNA in CaMKIIδ−/− and control VSMC revealed a 2.55-fold increase in p21 mRNA levels and decreased mRNA levels of cyclin D1 and E in cells lacking CaMKIIδ (0.83- and 0.64-fold, p < 0.05; Fig. 5C). The mRNA expression of p27 and PCNA was not affected by CaMKIIδ deletion (data not shown). It has been shown that p21 is targeted for degradation by the ubiquitin-proteasome pathway (25), but after treatment with the cycloheximide, we did not detect a difference in protein stability in CaMKIIδ−/− cells or controls (data not shown). In summary, these data indicate that CaMKIIδ regulates the expression of cell cycle markers at the transcriptional level.
FIGURE 5.
CaMKIIδ regulates the expression of cell cycle regulators in VSMCs. A, CaMKIIδ−/− and wild type VSMC were lysed at 70% confluence. 20 μg of protein was separated and blotted with indicated antibodies. A representative blot of three independent experiments is shown. B, densitometry of three independent experiments. C, quantitative real time PCR for cell cycle regulators in CaMKIIδ−/− and wild-type aortic VSMC. D, cell lysates of CaMKIIδ−/− and wild-type VSMC were incubated with anti-cdk2 or cdk4 antibodies, and the immunoprecipitates analyzed by immunoblotting with p21, cyclin D1, and cdk4 or cyclin E and cdk 2 antibodies. Middle and right panels: quantitation of cyclin D1, E, and p21 co-immunoprecipitation from three independent experiments. E, cdk2 and -4 kinase activity assays were performed with histone H-1 or Rb as substrate as described. Samples were separated by SDS-PAGE and analyzed by autoradiography. A representative blot of three experiments is shown (*, p < 0.05).
CaMKIIδ Deletion Increased Association of p21 with cdk2 and -4
Because of the elevated expression of p21 in CaMKIIδ−/− VSMC, we hypothesized that the association of p21 with cdk2/cyclin E and cdk4/cyclin D1 complexes is also increased, which would lead to decreased kinase activity. As shown in Fig. 5D, p21 co-immunoprecipitated with both cdk2 and cdk4 in lysates from wild-type VSMC. In CaMKIIδ−/− cells, the association of p21 with cdk2 and 4 was increased 1.6- and 2.3-fold, respectively, compared with control samples (p < 0.05). In addition, the association of cyclin D1 with cdk4 (0.78-fold) and of cyclin E with cdk2 (0.53-fold) was significantly decreased with CaMKIIδ deletion (Fig. 5D), suggesting that p21 sequesters the cyclin-dependent kinases away from their cognate cyclins. The increased association of the inhibitor p21 with cdk2 and 4 should therefore result in decreased kinase activity in CaMKIIδ−/− cells. Indeed, we detected decreased cdk2 and 4 kinase activity in CaMKIIδ−/− cells compared with controls (Fig. 5E). These data strongly suggest that CaMKIIδ deletion leads to decreased cell proliferation by reducing the cdk2 and -4 activity in proliferating VSMC.
Akt Phosphorylation Is Decreased with CaMKIIδ Deletion
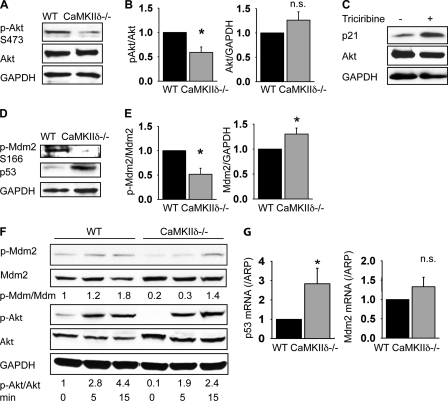
p21 expression is regulated by p53, and it has been shown that p53 degradation leads to decreased p21 expression and occurs through a signaling cascade that involves activation of Akt. Of note, Akt activation has been reported to be CaMKII-dependent in vascular smooth muscle cells (14). Because the expression of the cell cycle inhibitor p21 is strongly increased in CaMKIIδ−/− cells (Fig. 5, A and B), we systematically examined expression and phosphorylation of the pathway components that might lead to CaMKII-dependent p21 regulation. The phosphorylation of Akt at Ser-473 was significantly decreased in CaMKIIδ−/− cells (Fig. 6, A and B). Incubation of VSMC with the Akt inhibitor triciribine increased p21 expression (Fig. 6C).
FIGURE 6.
Akt activation is decreased in CaMKIIδ deletion. A, immunoblots for phosphorylated Akt (Ser-473), total Akt, and GAPDH in CaMKIIδ−/− and wild-type VSMC. B, densitometric quantitation of phosphorylated Akt and Akt expression from three independent experiments. C, wild-type VSMC were growth-arrested for 24 h in serum-free media before incubation in media with 10% FBS in the absence or presence of the Akt inhibitor triciribine (10 mm) for 24 h. A representative blot of three experiments is shown. D, immunoblots for phosphorylated Mdm2 (Ser-166), p53, and GAPDH. A representative blot of three independent experiments is shown. E, quantitation of phosphorylated Mdm2 and total Mdm2 expression. Three independent experiments are shown. F, time course of Akt and Mdm2 phosphorylation in response to DMEM with 10% FBS after serum starvation. G, p53 and Mdm2 mRNA expression in proliferating CaMKIIδ−/− and wild-type VSMC. Cells were lysed at 70% confluence (p < 0.05).
Mdm2, the E3 ligase that targets p53 for ubiquitination and subsequent proteosomal degradation, is activated by Akt phosphorylation at Ser-166 (26). Our data demonstrate that Mdm2 phosphorylation at Ser-166 was significantly decreased in CaMKIIδ−/− VSMC, while Mdm2 protein was up-regulated (Fig. 6D). An example of the phosphorylation time course for Akt and Mdm2 is given in Fig. 6F. Furthermore, loss of CaMKIIδ blocked Mdm-2-mediated p53 degradation as evidenced by the high expression of p53 in CaMKIIδ−/− VSMC compared with controls (Fig. 6D). Our data demonstrate that loss of CaMKIIδ directly correlates with decreased Akt and Mdm-2 phosphorylation and increased p53 levels. To assess whether the increase in p53 in CaMKIIδ−/− VSMC is caused by mechanisms other than post-transcriptional regulation, we also examined p53 and Mdm2 mRNA expression by qRT-PCR. We detected significantly increased p53 mRNA expression in VSMC lacking CaMKIIδ−/− (2.88-fold, p < 0.05), whereas Mdm2 mRNA levels were not significantly increased (Fig. 6G). Collectively these results implicate CaMKII-mediated activation of the Akt/Mdm2/p53 signaling nexus as the mechanism by which CaMKII promotes proliferation in VSMC.
CaMKIIδ Overexpression Decreases p21 and p53 Expression in CaMKIIδ−/− VSMC
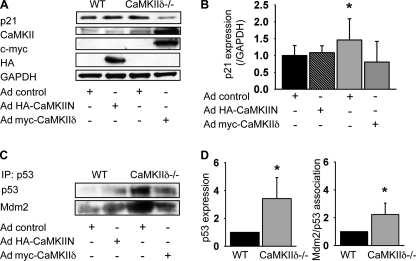
We hypothesized that the effects we describe were due to lack of CaMKIIδ, not the augmented expression of CaMKIIγ. Therefore we overexpressed CaMKIIδ2 by adenoviral transduction in CaMKIIδ−/− VSMC and found that overexpression of CaMKIIδ2 strongly decreased the expression of p21 (Fig. 7, A and B). The CaMKII peptide inhibitor CaMKIIN can also be used to block the activation of all CaMKII isoforms. The overexpression of CaMKIIN in control VSMC did not significantly increase p21 protein levels (Fig. 7, A and B). The overexpression of CaMKIIδ2 in CaMKIIδ−/− VSMC decreased p53 expression, whereas CaMKII inhibition with CaMKIIN in wild-type cells resulted in elevated p53 expression (Fig. 7C). The association of Mdm2 with p53 paralleled the expression of p53 (Fig. 7, C and D).
FIGURE 7.
p21 and p53 expression are regulated by CaMKIIδ. A, adenoviral overexpression of the CaMKII inhibitory peptide CaMKIIN in control cells and of CaMKIIδ2 in CaMKIIδ−/− VSMC. Immunoblots for p21, CaMKII, c-Myc, HA, and GAPDH. Representative blot of three independent experiments. B, quantitation of p21 expression from three independent experiments. C, immunoprecipitation of p53 in 200 μg of lysate of control VSMC transduced with adenovirus expressing CaMKIIN or CaMKIIδ−/− VSMC transduced with adenovirus expressing CaMKIIδ2. The blots were stripped and reprobed for Mdm2. D, quantitation of p53 expression and Mdm2 co-precipitation in control and CaMKIIδ−/− VSMC from three independent experiments as described in C (p < 0.05). Equal amount of protein were used for p53 immunoprecipitation.
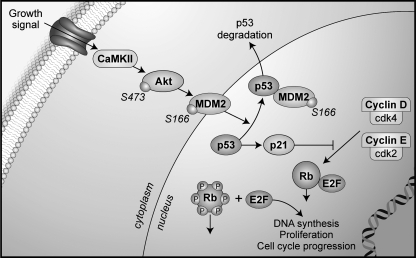
In summary, CaMKII regulates p21 protein levels through transcriptional and post-transcriptional mechanisms. With CaMKIIδ deletion, Akt phosphorylation is decreased, which in turn results in decreased activation of p53 degradation. In addition, p53 mRNA levels are increased in CaMKIIδ−/− VSMC. Akt inhibition ultimately results in increased p21 mRNA transcription (Fig. 8), suggesting that Akt is a mediator of the growth response to CaMKII in VSMC by regulation of p21 expression.
FIGURE 8.
Model of mechanism for CaMKIIδ function in neointima formation. CaMKII activates Akt, which then phosphorylates Mdm2. Phosphorylated Mdm2 associates with p53, the master regulator of p21 mRNA transcription, which results in p53 degradation. Under CaMKIIδ deletion, Akt phosphorylation is decreased and p21 mRNA transcription is increased, which results in stronger association of p21 with cdk complexes and thereby decreased cdk kinase activity and lack of proliferation.
DISCUSSION
In this study, we show that CaMKIIδ deletion significantly reduced in vivo neointimal formation after carotid artery ligation and abrogated cell proliferation in intima and media after injury. After vascular injury, CaMKIIδ was strongly expressed in neointima and media. In CaMKIIδ−/− mice, the expression of the cell cycle inhibitor p21 in VSMC in the media was strongly increased. In contrast, in wild-type mice, the cell cycle promoters cdk2, cyclin E and D were strongly increased. In proliferating cultured VSMC from CaMKIIδ−/− mice, mRNA and protein levels of the cell cycle inhibitor p21 were significantly elevated. The association of p21 with cyclin E/cdk2 and cyclin D/cdk4 complexes was increased in CaMKIIδ−/− cells and resulted in decreased cdk2 and 4 kinase activity. CaMKIIδ deletion diminished activation of Akt and Mdm2, the ubiquitin ligase for p53, a master regulator of p21 transcription. Taken together, our data define the pathway by which CaMKII promotes proliferation of VSMC following vascular injury.
Decreased cell proliferation after CaMKII inhibition has been reported in VSMC (7) and a variety of cell lines (11–13, 27). In this study, we identified p21 as important target of CaMKII regulation in VSMC. p21 is up-regulated in human osteosarcoma and ovarian adenocarcinoma cells in response to CaMKII inhibition (13, 27). Our data demonstrate increased p21 mRNA and protein levels in proliferating CaMKIIδ−/− VSMC and in the neointima of CaMKIIδ−/− mice. Akt activation by Ser-437 phosphorylation is mediated by CaMKII and has been proposed as upstream signaling event in VSMC proliferation (14). p53 expression and corresponding activity is intricately regulated at both the transcriptional and post-translational levels (28). At the post-translational level, active, phosphorylated Akt regulates the degradation of p53, a strong transcriptional activator of p21, through phosphorylation of the intermediate p53-regulatory protein Mdm2 (13). Mdm2 targets p53 for degradation by acting as an E3 ubiquitin ligase (29). Mdm2 phosphorylation on serines 166 and 168 by Akt is a prerequisite for its E3-ligase activity (26, 30). Decreased Mdm2 phosphorylation with CaMKIIδ deletion would result in decreased p53 degradation, increased p21 transcription, and cell cycle arrest. Interestingly, we observed significantly higher p53 mRNA levels in CaMKIIδ−/− cells which points toward a second regulatory function for CaMKII, but this mechanism remains unclear and may be through modulating p53 transcription or mRNA stability.
In CaMKIIδ−/− cells, cyclin E and D mRNA and protein levels were moderately decreased (Fig. 5, A–C). Whereas the underlying pathways remain to be elucidated, several studies provide evidence for potential mechanisms of CaMKII regulation. Cyclin D is transcriptionally induced by NFκB (31) and AP-1, which is comprised by c-Jun and c-Fos (32). CaMKII has been reported to activate the NFκB signaling pathway at several steps (33, 34). Furthermore, CaMKII induces c-Jun expression in proliferating VSMC through HDAC4 phosphorylation and MEF2 derepression (35). It is notable that our immunoblots in CaMKIIδ−/− VSMC showed decreased cyclin D levels. In contrast, in CaMKIIδ−/− carotid arteries, the decrease was more apparent by immunofluorescence than by immunoblots, which may be due to differences in sample preparation. This finding may warrant further investigation. Cyclin E expression is strongly controlled by E2F, and CaMKII inhibition is associated with decreased phosphorylation of retinoblastoma protein and E2F transactivation (27). Further studies are warranted to understand the precise mechanism by which CaMKII controls expression of these positive cell cycle regulatory proteins.
Decreased apoptosis has been reported as contributor to neointimal formation after carotid ligation (17, 36). In this study, however, we detected low apoptotic activity without a difference in CaMKIIδ−/− mice 14 days after ligation compared with wild-type littermates. CaMKII mediates apoptosis in cardiac myocytes (24), however, whether CaMKII regulates VSMC apoptosis remains to be investigated.
CaMKII is expressed in VSMC (5) and promotes migration (8), proliferation (7), hypertrophy (5), and contraction (6) in vitro. Unfortunately, our understanding of its function in the vasculature in vivo has been hampered by a lack of relevant models. Most data to date rely on administration of the CaMKII inhibitor KN-93 (5, 37) that has clear CaMKII-independent effects (38). To our knowledge, this is the first study to take advantage of the recently developed CaMKIIδ−/− mice to study CaMKII isoform-specific effects in the vasculature (16, 39, 40).
Our results raise the question whether the decrease in neointimal formation, cell proliferation, and change in cell cycle regulation are CaMKIIδ-specific or due to decreased total CaMKII activity. In both aorta and VSMC from CaMKIIδ−/− mice, we detected a robust anti-proliferative phenotype despite up-regulation of CaMKIIγ expression (supplemental Figs. S1 and S2). In addition, the total Ca2+/calmodulin-dependent CaMKII activity was not significantly lower in CaMKIIδ−/− VSMC compared with controls (Fig. 4B). Our data favor the theory that CaMKII isoforms in smooth muscle cells have specific functional targets. We believe that the antiproliferative effects we detected are explained by absence of the CaMKIIδ isoform rather than increases in CaMKIIγ expression in CaMKIIδ−/− mice. This is based on three observations in this study: 1) we detected increased transcription of CaMKIIδ, but not γ, after carotid ligation of wild type arteries that display strong total CaMKII expression in the neointima (Fig. 1C), 2) the adenoviral overexpression of CaMKIIδ in CaMKIIδ−/− VSMC reverses the changes in p21 expression (Fig. 7A), 3) the decrease in Akt phosphorylation (Fig. 6A) negates the possibility that the effects we found are due to increased compensatory CaMKIIγ kinase activity.
In summary, this study provides in vivo and in vitro evidence that CaMKIIδ controls VSMC proliferation through modulation of cell cycle regulators. CaMKIIδ deletion caused decreased VSMC proliferation, neointimal formation, and programmatic changes in cell cycle control, including increased p21 protein expression and decreased cdk activity. These data provide novel insights into the mechanisms involved in VSMC growth and raise questions regarding whether CaMKII inhibition could decrease VSMC proliferation in clinical settings, such as neointimal formation after angioplasty, arteriosclerosis, and transplant-associated arteriopathy.
Supplementary Material
Acknowledgments
We thank Litao Xie and Dr. Chantal Allamargot (Central Microscopy Research Facilities, University of Iowa) for expert technical support and Dr. Kristina W. Thiel for assistance in the preparation of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants HL 079031, and R01 HL 70250 (to M. E. A.); National Institutes of Health Grants R01 HL 084583 and R01 HL 083422, and Pew Scholars Trust (to P. J. M.). This work was also supported in part by funding from the following grants: an American Heart Association Scientist Development Grant, a VA Merit grant by the Office of Research and Development, Dept. of Veterans Affairs and a Carver Trust Medical Research Initiative Grant (to I. M. G.), and the University of Iowa Cardiovascular Center Interdisciplinary Research Fellowship (to W. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- cdk
- cyclin-dependent kinase
- CaMKIIδ
- calcium/calmodulin-dependent protein kinase II isoform δ
- Mdm2
- mouse double minute protein 2
- VSMC
- vascular smooth muscle cells
- PI3K
- phosphoinositide-3-kinase
- BrdU
- bromodeoxyuridine
- ARP
- acidic ribosomal phosphoprotein.
REFERENCES
- 1. Babapulle M. N., Joseph L., Bélisle P., Brophy J. M., Eisenberg M. J. (2004) Lancet 364, 583–591 [DOI] [PubMed] [Google Scholar]
- 2. Morishita R., Gibbons G. H., Ellison K. E., Nakajima M., von der Leyen H., Zhang L., Kaneda Y., Ogihara T., Dzau V. J. (1994) J. Clin. Invest. 93, 1458–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen D., Krasinski K., Sylvester A., Chen J., Nisen P. D., Andrés V. (1997) J. Clin. Invest. 99, 2334–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tanner F. C., Boehm M., Akyürek L. M., San H., Yang Z. Y., Tashiro J., Nabel G. J., Nabel E. G. (2000) Circulation 101, 2022–2025 [DOI] [PubMed] [Google Scholar]
- 5. Li H., Li W., Gupta A. K., Mohler P. J., Anderson M. E., Grumbach I. M. (2010) Am. J. Physiol. Heart Circ. Physiol. 298, H688–H698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim I., Je H. D., Gallant C., Zhan Q., Riper D. V., Badwey J. A., Singer H. A., Morgan K. G. (2000) J. Physiol. 526, 367–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. House S. J., Ginnan R. G., Armstrong S. E., Singer H. A. (2007) Am. J. Physiol. Cell. Physiol. 292, C2276—C2287 [DOI] [PubMed] [Google Scholar]
- 8. Mercure M. Z., Ginnan R., Singer H. A. (2008) Am. J. Physiol. Cell. Physiol. 294, C1465–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. House S. J., Singer H. A. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 441–447 [DOI] [PubMed] [Google Scholar]
- 10. Tombes R. M., Grant S., Westin E. H., Krystal G. (1995) Cell Growth & Differ. 6, 1063–1070 [PubMed] [Google Scholar]
- 11. Morris T. A., DeLorenzo R. J., Tombes R. M. (1998) Exp. Cell Res. 240, 218–227 [DOI] [PubMed] [Google Scholar]
- 12. Li N., Wang C., Wu Y., Liu X., Cao X. (2009) J. Biol. Chem. 284, 3021–3027 [DOI] [PubMed] [Google Scholar]
- 13. Ma S., Yang Y., Wang C., Hui N., Gu L., Zhong H., Cai Z., Wang Q., Zhang Q., Li N., Cao X. (2009) J. Biol. Chem. 284, 24773–24782 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Li F., Malik K. U. (2005) Am. J. Physiol. Heart Circ. Physiol. 288, H2306–H2316 [DOI] [PubMed] [Google Scholar]
- 15. Stabile E., Zhou Y. F., Saji M., Castagna M., Shou M., Kinnaird T. D., Baffour R., Ringel M. D., Epstein S. E., Fuchs S. (2003) Circ. Res. 93, 1059–1065 [DOI] [PubMed] [Google Scholar]
- 16. Backs J., Backs T., Neef S., Kreusser M. M., Lehmann L. H., Patrick D. M., Grueter C. E., Qi X., Richardson J. A., Hill J. A., Katus H. A., Bassel-Duby R., Maier L. S., Olson E. N. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 2342–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar A., Lindner V. (1997) Arterioscler. Thromb. Vasc. Biol. 17, 2238–2244 [DOI] [PubMed] [Google Scholar]
- 18. Ray J. L., Leach R., Herbert J. M., Benson M. (2001) Methods Cell Sci. 23, 185–188 [DOI] [PubMed] [Google Scholar]
- 19. Ushio-Fukai M., Alexander R. W., Akers M., Griendling K. K. (1998) J. Biol. Chem. 273, 15022–15029 [DOI] [PubMed] [Google Scholar]
- 20. Matsushime H., Quelle D. E., Shurtleff S. A., Shibuya M., Sherr C. J., Kato J. Y. (1994) Mol. Cell. Biol. 14, 2066–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu Y., Temple J., Zhang R., Dzhura I., Zhang W., Trimble R., Roden D. M., Passier R., Olson E. N., Colbran R. J., Anderson M. E. (2002) Circulation 106, 1288–1293 [DOI] [PubMed] [Google Scholar]
- 22. Schworer C. M., Rothblum L. I., Thekkumkara T. J., Singer H. A. (1993) J. Biol. Chem. 268, 14443–14449 [PubMed] [Google Scholar]
- 23. Zhou Z. L., Ikebe M. (1994) Biochem. J. 299, 489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Erickson J. R., Joiner M. L., Guan X., Kutschke W., Yang J., Oddis C. V., Bartlett R. K., Lowe J. S., O'Donnell S. E., Aykin-Burns N., Zimmerman M. C., Zimmerman K., Ham A. J., Weiss R. M., Spitz D. R., Shea M. A., Colbran R. J., Mohler P. J., Anderson M. E. (2008) Cell 133, 462–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rousseau D., Cannella D., Boulaire J., Fitzgerald P., Fotedar A., Fotedar R. (1999) Oncogene 18, 4313–4325 [DOI] [PubMed] [Google Scholar]
- 26. Mayo L. D., Donner D. B. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 11598–11603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yuan K., Chung L. W., Siegal G. P., Zayzafoon M. (2007) Lab. Invest. 87, 938–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lu X. (2010) Cold Spring Harb Perspect. Biol. 2, a000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haupt Y., Maya R., Kazaz A., Oren M. (1997) Nature 387, 296–299 [DOI] [PubMed] [Google Scholar]
- 30. Zhou B. P., Liao Y., Xia W., Zou Y., Spohn B., Hung M. C. (2001) Nat. Cell Biol. 3, 973–982 [DOI] [PubMed] [Google Scholar]
- 31. Guttridge D. C., Albanese C., Reuther J. Y., Pestell R. G., Baldwin A. S., Jr. (1999) Mol. Cell. Biol. 19, 5785–5799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bakiri L., Lallemand D., Bossy-Wetzel E., Yaniv M. (2000) EMBO J. 19, 2056–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ishiguro K., Green T., Rapley J., Wachtel H., Giallourakis C., Landry A., Cao Z., Lu N., Takafumi A., Goto H., Daly M. J., Xavier R. J. (2006) Mol. Cell. Biol. 26, 5497–5508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh M. V., Kapoun A., Higgins L., Kutschke W., Thurman J. M., Zhang R., Singh M., Yang J., Guan X., Lowe J. S., Weiss R. M., Zimmermann K., Yull F. E., Blackwell T. S., Mohler P. J., Anderson M. E. (2009) J. Clin. Invest. 119, 986–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gordon J. W., Pagiatakis C., Salma J., Du M., Andreucci J. J., Zhao J., Hou G., Perry R. L., Dan Q., Courtman D., Bendeck M. P., McDermott J. C. (2009) J. Biol. Chem. 284, 19027–19042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Y., Takeshita K., Liu P. Y., Satoh M., Oyama N., Mukai Y., Chin M. T., Krebs L., Kotlikoff M. I., Radtke F., Gridley T., Liao J. K. (2009) Circulation 119, 2686–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muthalif M. M., Karzoun N. A., Benter I. F., Gaber L., Ljuca F., Uddin M. R., Khandekar Z., Estes A., Malik K. U. (2002) Hypertension 39, 704–709 [DOI] [PubMed] [Google Scholar]
- 38. Rezazadeh S., Claydon T. W., Fedida D. (2006) J. Pharmacol. Exp. Ther. 317, 292–299 [DOI] [PubMed] [Google Scholar]
- 39. Backs J., Stein P., Backs T., Duncan F. E., Grueter C. E., McAnally J., Qi X., Schultz R. M., Olson E. N. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ling H., Zhang T., Pereira L., Means C. K., Cheng H., Gu Y., Dalton N. D., Peterson K. L., Chen J., Bers D., Heller Brown J. (2009) J. Clin. Invest. 119, 1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.