Abstract
During early pregnancy, the endometrium undergoes pronounced hormone-dependent functional changes in preparation for embryo implantation. Local autocrine-paracrine signaling at the fetal-maternal interface is crucial for establishment of pregnancy. We previously reported that the transcription factor C/EBPβ, which is expressed at the implantation sites in pregnant mice, acts as a key mediator of steroid hormone responsiveness in the endometrium. Mice lacking C/EBPβ fail to support implantation due to defects in epithelial proliferation and stromal cell differentiation. In the present study, C/EBPβ expression was dramatically stimulated in the endometrium of baboons (Papio anubis) during the window of uterine receptivity in response to a local infusion of chorionic gonadotropin, an embryonic signal. A robust induction of C/EBPβ expression was also seen at the implantation site in the baboon and the human. Collectively, our results indicate that C/EBPβ is a biomarker of endometrial receptivity and plays a conserved functional role during implantation in the primate.
Keywords: Endometrium, Implantation, Uterine Receptivity
INTRODUCTION
Implantation involves a complex series of signaling events between the developing embryo and the endometrium1–4. In many species, including the rodents, it is well established that the endometrial tissue undergoes extensive proliferation, differentiation and remodeling in response to the steroid hormones estrogen and progesterone5. The uterine luminal epithelium attains a functional receptive state, which makes it competent for blastocyst attachment. The underlying stromal cells undergo a hormonally induced transformation, known as decidualization, which is essential for supporting the growth and survival of the implanted embryo. In primates, additional paracrine signals from the developing embryo produce further morphological and biochemical changes in the steroid-primed uterus to promote uterine receptivity and implantation6. Although the identities of the molecular pathways that control uterine receptivity and decidualization are not fully understood, it is clear that perturbations in these pathways cause uterine dysfunctions leading to early pregnancy loss and female infertility. Therefore, there is a critical need to identify molecules that would serve as useful biomarkers of the receptive state of human endometrium.
Recent gene targeting studies using transgenic mouse models revealed that a variety of factors, including transcription regulators, cytokines, morphogens, and growth factors, function downstream of steroid hormone signaling to control early phases of fetal-maternal interactions during implantation7–9. We have previously identified C/EBPβ, a CCAAT box-binding transcription factor, as a key steroid-regulated molecule involved in proliferation and differentiation of uterine epithelial and stromal cells in the mouse10. In pregnant mice, uterine expression of C/EBPβ is observed in the uterine luminal epithelium during the receptive phase. Thereafter the C/EBPβ expression shifts to the decidualizing stromal cells surrounding the implanted embryo. The uteri of C/EBPβ-null mice are non-receptive to embryo implantation and exhibit a complete lack of response to a deciduogenic stimulus, indicating a critical role of this transcription factor in regulating uterine receptivity and stromal differentiation program 10,11.
A rational next step in our research is to evaluate C/EBPβ as a potential marker of uterine receptivity in the primate. Despite many similarities in the processes of implantation and placentation in rodents and primates, differences also exist between these species. For example, in humans and non-human primates, a limited differentiation of endometrial stroma occurs spontaneously during the secretory phase of the menstrual cycle. This stromal transformation, which is initiated in the absence of an embryo, is critical for uterine receptivity and becomes more wide spread with progressive phases of implantation during the first trimester of pregnancy. In contrast, in rodents, decidualization occurs in response to implanting embryos12. In humans, for ethical reasons, distinct stages of implantation and pregnancy are difficult to study due to the lack of available tissue. Thus, a nonhuman primate, such as the baboon (Papio anubis), is an important research model for basic and preclinical studies relevant to humans. The present study assesses C/EBPβ as a marker of receptivity of baboon endometrium during normal and simulated pregnancy conditions. Our findings suggest that C/EBPβ expression is intimately associated with attainment of uterine receptivity in the primates.
MATERIALS and METHODS
Materials
Recombinant human chorionic gonadotropin (hCG) was obtained from Dr. A. F. Parlow (National Hormone and Pituitary Program, Harbor-UCLA Medical center, Torrance, CA) and from EMD Serono (Rockland, MA). Rabbit polyclonal antibody against C/EBPβ was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Recombinant human IL-1β and IL-1 receptor antagonist (IL-1Ra) were obtained from R&D systems (Minneapolis, MN).
Tissue Collection
Endometrial tissues (n= three/stage) were obtained from normal cycling, and pregnant female baboons (Papio anubis) as described previously15. All experimental procedures were approved by the Animal Care Committee of the University of Illinois (Chicago, IL). Uteri from pregnant baboons (days 15–60 of pregnancy) were obtained by endometriectomy or hysterectomy. The stage of pregnancy was confirmed by ultrasound and by circulating levels of baboon CG, estrogen, and progesterone, as described previously 13, 14. After visualization of the site of attachment, the uterine tissue directly under or immediately adjacent to the implantation site was obtained.
For simulated pregnancy studies, normally cycling baboons were treated with hCG. Uterine tissues were obtained as described previously15 from untreated or hCG-treated animals (n=3 for each group). Ovulation was detected in cycling female baboons by measuring peripheral serum levels of estradiol, beginning 7 days after the first day of menses. The day of the estradiol surge was designated day -1; the day of the ovulatory LH surge as day 0, and the day of ovulation as day 1. On day 5 post-ovulation (PO), an oviductal cannula was attached to an Alzet osmotic minipump (ALZA Corp., Mountain View, CA), and recombinant hCG was infused at a rate of 1.25 IU/h for 5 days, as previously described. Endometrial tissues of hCG-treated animals were obtained on days 10–15 PO. In some experiments, IL-1β or IL-1Ra (12 ng/d) was infused together with hCG. The IL-1β or IL-1Ra infusion was begun on day 10 PO together with hCG and was continued for an additional 5 days. The experiment was terminated on day 15 PO (which corresponds to day 5 after implantation in pregnant baboons).
Human implantation sites were obtained at 8 and 9 weeks of gestation from curettage specimens at the time of elective terminations. The use of these archival tissues for research was approved by the Institutional Review Boards of the University of Illinois and John H. Stroger Jr. Hospital of Cook County, Chicago, Illinois. Tissues were formalin fixed and paraffin embedded.
Immunohistochemistry
Uterine tissues were fixed in Bouin’s solution or 10% buffered formalin for 24 h at room temperature, dehydrated in graded ethanol, cleared in xylene, and embedded in paraffin. 4μm paraffin-embedded uterine sections were stained for C/EBPβ as described previously10,11. Intensity of immunostaining was measured using semiquantitative histologic scoring (HSCORE) system. At least seven non-overlapping fields of 40X magnification for each time point were used for the quantitation. The equation HSCORE=ΣPi(I+1), where I represents the staining intensity (values of 1, 2, or 3 signify weak, moderate, or strong staining, respectively) was used to calculate HSCORES.
RESULTS
Induction of C/EBPβ in the receptive baboon endometrium
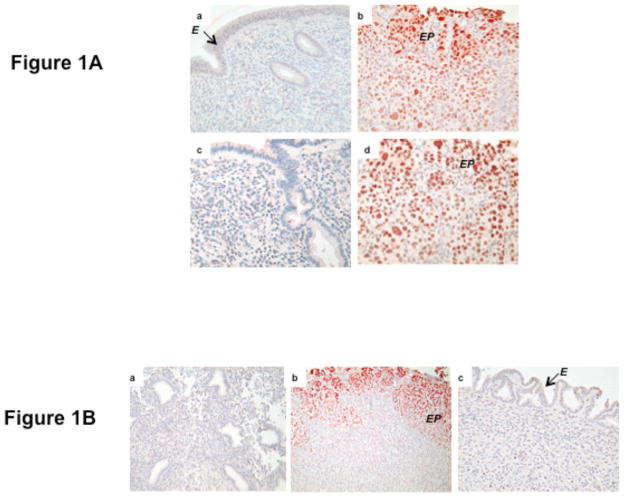
Previous studies have shown that in normally cycling baboon endometrial receptivity is induced in the absence of an embryo following infusion of human chorionic gonadotropin (hCG) into the uterine cavity of the female16. Administration of hCG, which mimics an embryonic signal, to steroid hormone-primed baboon results in epithelial plaque formation due to hypertrophy of the surface epithelium, stromal cell differentiation and an increase in glandular secretory activity, which are early indicators of maternal response to pregnancy. We used this simulated pregnancy model (described in Materials and Methods) to examine the expression of C/EBPβ in the baboon endometrium. As shown in Fig. 1A, a low level of C/EBPβ expression was observed in the endometrial tissue of untreated animals on days 11 PO or 14 PO (panels a and c). In contrast, a dramatic increase in C/EBPβ expression was seen on day 11 PO or day 14 PO upon infusion of hCG into the uterine cavity of the cycling baboon starting day 5 PO (panelsb and d). As shown in Fig. 1A (panelsb and d), greatly enhanced nuclear staining of C/EBPβ was observed in the epithelial plaque (indicated as EP) cells and the underlying stromal fibroblasts, indicating that the expression of this factor coincides with the receptive state of baboon endometrium.
Fig. 1. Immunohistochemical localization of C/EBPβ in the baboon endometrium.
A. Paraffin-embedded uterine tissues were processed and analyzed by immunohistochemical staining for C/EBPβ, as described in Materials and Methods. Panels a, endometrium on day 11 PO (10X); b, endometrium on day 11 PO infused with hCG (20X); c, endometrium on day 14 PO (10X); and d, endometrium on day 14 PO infused with hCG (20X). Arrow indicates epithelium (E) and EP denotes epithelial plaque.
B. Immunohistochemical localization of C/EBPβ in baboon endometrium on day 15 PO. Panels a, untreated; b, treated with hCG and IL-1β; and c, treated with hCG and IL-1Ra. Red deposits indicate sites of specific immunostaining. Arrow indicates epithelium (E) and EP denotes epithelial plaque.
Previous studies reported that IL-1β, a proinflammatory cytokine, plays a critical role in hCG induced changes that are associated with the establishment of uterine receptivity 15, 17. Treatment with IL-1Ra, an IL-1β receptor antagonist, prevented hCG-induced formation of epithelial plaque, resulting in a non-receptive uterus. We, therefore, examined the effects of the blockade of IL-1β signaling on hCG-induced C/EBPβ expression in the simulated pregnancy model. As shown in Fig. 1B, a low level of C/EBPβ expression was detected on day 15 PO. However, marked enhancement in C/EBPβ expression was observed on day 15 PO in the endometrial sections treated with a combination of hCG and IL-1β (Fig. 1B, panel b). The staining of C/EBPβ was localized in the epithelial cell clusters and the sub epithelial stromal cells. Strikingly, C/EBPβ expression was abolished when IL-1Ra was administered along with hCG into the uteri of cycling baboons (panel c). These results indicated that IL-1β signaling pathway plays a critical role in hCG-induced expression of C/EBPβ in the receptive baboon endometrium. Our studies also revealed that agents that prevent the creation of a receptive uterus also blocked C/EBPβ expression. The appearance of this transcription factor in the hormone-primed endometrium is thus potentially functionally linked with uterine receptivity.
Expression of C/EBPβ in baboon decidua at the implantation site
We next monitored the expression of C/EBPβ in the baboon endometrium during normal pregnancy (Fig. 2). Attachment of the embryo to the uterine wall, leading to the establishment of pregnancy, constitutes the final phase of uterine receptivity. The prominent morphological features of this phase are glandular hypertrophy and differentiation of stromal cells into decidual cells. The expression of C/EBPβ was observed in the pre-decidual stromal cells as early as days 10, 14 and 15 of pregnancy (Fig. 2A, panels a,b, and c, respectively). Quantification of C/EBPβ immuno-positive cells in the stroma showed a gradual increase from day 10 to day 15 of pregnancy (Fig. 2B). With the progress of pregnancy, intense expression of C/EBPβ was observed in the stromal cells surrounding the implantation site (IS) on day 21 (Fig. 2C, panels a and b), day 40 (Fig. 2C, panel c), and day 57 (Fig. 2C, panel d). Upon closer examination, it was evident that the C/EBPβ immunostaining in the maternal decidua on days 40, 57, and 60 is localized in the nucleus and it most likely represented the active form of this transcription factor (Fig. 2D). Collectively, these results indicated that C/EBPβ is expressed in the pre-decidual as well as decidual stromal cells at the baboon implantation site during early pregnancy, and its expression continues in these cells during mid pregnancy. These results are consistent with our previous description of a marked nuclear localization of C/EBPβ protein in both proliferating and differentiating stromal cells surrounding the implanted embryos as the decidualization process progressed in pregnant mouse uterus 10.
Fig. 2. Immunohistochemical localization of C/EBPβ in pregnant baboon endometrium.
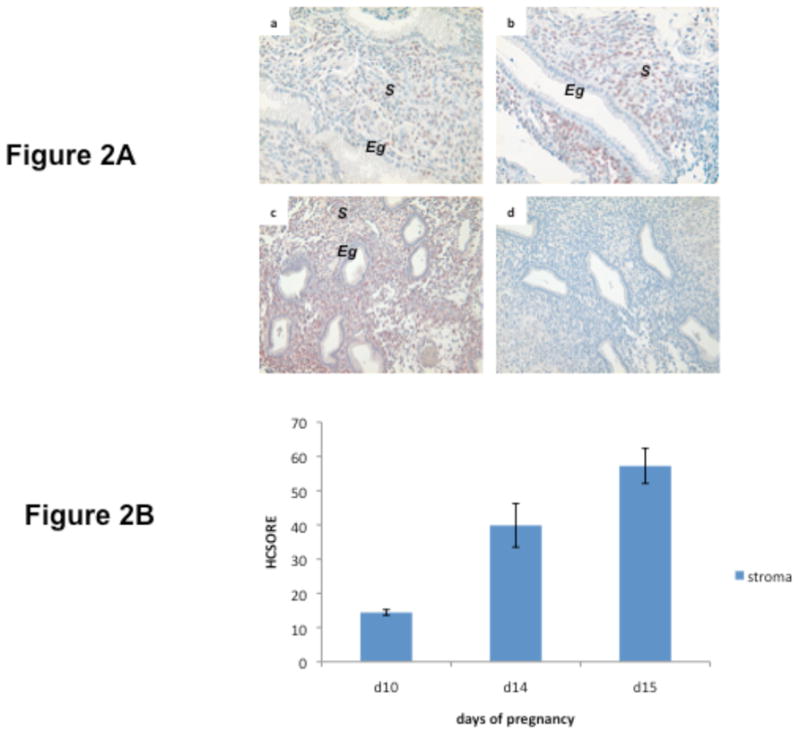

A. Localization of C/EBPβ immunostaining on day 10 (panel a), day 14 (panel b), and day 15 (panel c) of pregnancy. Panel d, represents control day15 endometrial section incubated with no primary antibody. The images shown in the panels are at 10X magnification. Eg: endometrial gland, S: stroma
B. Quantitation of nuclear C/EBPβ immunostaining in baboon endometrium. HSCORES of C/EBPβ positive cells in the baboon endometrial stroma revealed a significant increase from day 10 to day 15 of pregnancy (p<0.001).
C. Expression of C/EBPβ at the baboon implantation site. Panel a, day 21: Panel b is a magnified (10X) image of panel a; panel c, day 40; and panel d, day 57. Panel e represents a control (day 21) endometrial section incubated with no primary antibody. Panels a, c, d and e are at 4X magnification. CV: chorionic villi, D: decidua, IS: implantation site.
D. Localization of C/EBPβ immunostaining in decidua on day 40 (panel a), day 57 (panels b and c, 20X), and day 60 (panel d) of gestation. Panels a, b, and d are at 10X magnification. BV: blood vessels, D: decidua, DEC: decidual cells (indicated by arrow).
Expression of C/EBPβ in baboon placenta
During pregnancy in the baboon, in addition to C/EBPβ expression in the decidua, a high level of this protein was observed in the placenta through gestation day 60 (Fig. 3). C/EBPβ staining was prominent in the syncytiotrophoblast (ST, indicated by arrow in panels b, c and d) of chorionic villi of the baboon placenta on days 40, 57 and 60 of pregnancy (panels a, b, and c, respectively). Remarkably, the villous cytotrophoblast (CT, indicated by arrow in panel d) of the baboon placenta was negative for C/EBPβ immunostaining at all stages of gestation (Fig. 3). The strong placental expression of C/EBPβ pointed to a possible role of this transcription factor during trophoblast differentiation and function.
Fig. 3. Immunohistochemical localization of C/EBPβ in the baboon placenta.

Localization of C/EBPβ immunostaining on day 40 (panel a, 10X), day 57 (panel b, 10X), and day 60 (panel c 10X, panel d, 20X) of gestation. CV: chorionic villi, DEC: decidua, ST: syncytiotrophoblast (indicated by arrow), CT: cytotrophoblast (indicated by arrow).
Expression of C/EBPβ in decidua and placenta at the implantation site in the human
We have recently reported the expression C/EBPβ in human endometrium during the menstrual cycle18. A marked increase in nuclear C/EBPβ immunostaining was observed in the glandular and stromal cells beginning on about cycle day 20, when the human endometrium attains the embryo-receptive status, and it continued for the remainder of the cycle27. To further examine the biological significance of C/EBPβ function during pregnancy, we examined its expression at implantation sites in archival human endometrial tissues. As shown in Fig. 4A, a moderate level of C/EBPβ expression was detected in the endometrial surface epithelium (SE) during first trimester of pregnancy (indicated by arrow, panels a and b, 8 weeks, panel c, 9 weeks). However, intense nuclear expression of C/EBPβ was observed in decidual stromal cells at the implantation site during this period (indicated as “S” in Fig. 4A, panels b,- and c;. As shown in Fig. 4B, there was no significant difference in stromal C/EBPβ immunostaining between weeks 8 and 9 of pregnancy. No C/EBPβ expression was observed in the endometrial glands in any of the samples studied. The expression of C/EBPβ was also localized in the decidua (indicated as D in Fig. 4C, panels b, c, and d) and villous syncytiotrophoblast in the human first trimester placenta (Fig. 4C, indicated as ST in panels a and d). In contrast, the cytotrophoblast remained negative for C/EBPβ immunostaining. The pattern of C/EBPβ expression in the decidua (panels b, c, and d) and placenta (PL, panel d) at the human implantation site is remarkably similar to that observed in the pregnant baboon endometrium.
Fig. 4. Immunohistochemical localization of C/EBPβ at human implantation site.


A. Localization of C/EBPβ immunostaining in sections of first trimester human implantation sites at 8 weeks (panels a and b, 20X), and 9 weeks (panel c). Panel d represents a control 8 weeks endometrial section incubated with no primary antibody. Se: surface epithelium (indicated by arrow), S: s stromal cells, and Eg: endometrial gland.
B. Quantitation of nuclear C/EBPβ immunostaining in human endometrium. HSCORES of stromal C/EBPβ positive cells in the human endometrium at the implantation site at 8 and 9 weeks of pregnancy. There is no significant difference in C/EBPβ immunostaining between weeks 8 and 9 (p=0.758).
C. Localization of C/EBPβ in the syncytiotroblast of human placenta (panel a, 4X indicated by arrow), and decidual cells of the placental-decidual interface (panel b, 4X: panel c, 10X: and panel d, 10X) at implantation site during first trimester of human pregnancy. CV: chorionic villi, D: decidua, and PL: placenta. The arrows point to DEC: decidual cells, ET: extravillous trophoblast, and ST: syncytiotrophoblast. These are typical results from archival tissues obtained from four individuals.
DISCUSSION
C/EBPβ is a transcription factor that belongs to the C/EBP family of basic leucine-zipper proteins, which also includes C/EBPα and C/EBPγ 19. Our previous studies revealed a critical role of this factor in the regulation of murine uterine function during implantation 10, 11. In the mouse, lack of C/EBPβ in the uterus leads to multiple functional abnormalities, including impaired epithelial cell proliferation in response to estrogen and a loss of decidual response to an artificial stimulus10. Embryo transfer experiments conducted in ovariectomized C/EBPβ-null mice supplemented with estrogen and progesterone revealed that the mutant uterus is refractory to embryo attachment 10. Because C/EBPβ is a critical mediator of steroid hormone responsiveness in pregnant mouse uterus, it is important to understand its pattern of expression in human and non-human primates during pregnancy. The present study reports pronounced nuclear expression of C/EBPβ in the decidua and placenta at the implantation sites of pregnant human and baboon. The documentation of expression of a key steroid-regulated factor, with established functional role in the mouse uterus, at the implantation sites of human and nonhuman primates represents an important first step in advancing translational research in uterine biology.
We have uncovered an important functional link between C/EBPβ expression and the receptive status of baboon endometrium. Endometrial receptivity for embryo implantation in the baboon occurs in distinct phases20–23. Phase I, regulated by the steroid hormones estrogen and progesterone, spans days 8 to 10 post-ovulation (PO) of the normal menstrual cycle. This phase is characterized by the presence of columnar epithelium and an increase in stromal cell proliferation. We noted that a low level of C/EBPβ was expressed in the stromal cells at this phase of the cycle, presumably in response to estrogen and progesterone. Phase II of uterine receptivity is induced by signals from the blastocyst superimposed on the hormone-primed receptive endometrium. Morphologically phase II is remarkably distinct from phase I. During phase II, hypertrophic surface epithelial cells form epithelial plaque, which is an early indicator of maternal response to pregnancy. Previous studies have shown that stage II of the receptive endometrium can be induced in the absence of an embryo by infusion of hCG into the uterine cavity of the cycling baboon16. Administration of hCG, which mimics an embryonic signal, to steroid hormone-primed baboon endometrium creates simulated pregnancy conditions and results in epithelial plaque formation. Remarkably, we observed that hCG treatment leads to a robust stimulation of C/EBPβ expression in the epithelial plaque and in the subluminal stromal cells during simulated pregnancy. These results suggested an intimate connection between the expression of this transcription factor in endometrial cells and creation of the receptive state of the uterus. Most importantly, this functional link is conserved among species as varied as the rodents and primates.
Previous studies have shown that hCG-mediated morphological and functional transformation of the baboon endometrium, such as the formation of epithelial plaque, is critically dependent on signaling by cytokines such as IL-1β 23. Uterine infusion of the IL-1 receptor antagonist IL-1Ra, which prevents binding and activation of IL-1β signaling pathway, suppressed hCG-induced uterine receptivity in baboons. Interestingly, the IL-1Ra-mediated inhibition of uterine receptivity was associated with a concomitant loss of C/EBPβ expression in the epithelial plaque and in sub-luminal stromal cells. It is conceivable that steroid hormones, along with hCG, IL-1β, and presumably other embryonic factors, are critical for maximal expression and function of C/EBPβ in the receptive baboon endometrium.
The final stage of uterine receptivity (Phase III) is initiated following embryo attachment and implantation20–22. This phase is marked by a significant increase in permeability of the subepithelial capillaries surrounding the blastocyst, along with glandular hypertrophy and initiation of stromal decidualization. Our study shows that the pre-decidual cells in the early pregnant baboon endometrium as well as the decidual cells near the spiral arteries in the mid-pregnancy maternal decidua express a high level of C/EBPβ protein. This high expression level in decidual cells is maintained until day 60 of gestation. Based on our previous studies in the murine model, where C/EBPβ continues to be expressed in the stromal cells throughout the decidualization period, it is likely that this transcription factor is involved in the induction as well as maintenance of stromal differentiation program in the baboon decidua10.
Interestingly, a previous study employing primary cultures of human endometrial stromal cells suggested that C/EBPβ is a potential mediator of the cAMP-dependent endometrial stromal decidualization in the human24. In this study, the authors showed that the protein kinase A (PKA)-dependent activation of the decidual prolactin promoter, a major differentiation marker in human endometrial stromal cells, involves cooperative interaction of forkhead/winged-helix transcription factor with C/EBPβ. We recently analyzed in vivo expression of C/EBPβ in the human endometrium during the menstrual cycle18. Although the epithelial expression of C/EBPβ did not change significantly during the cycle, an increased expression of C/EBPβ was observed in the stromal cells during the mid secretory phase of the cycle, which overlaps with the putative window of implantation. The study, however, did not address the C/EBPβ expression profile in the human endometrium during pregnancy.
Our present study reveals prominent expression of C/EBPβ at the implantation site during the first trimester of pregnancy. While the endometrial expression of this factor is mostly localized in the stromal cells, moderate expression is also observed in surface epithelium at 8 weeks of gestation. The pattern of C/EBPβ expression in the human implantation site resembles the widespread and intense expression of this transcription factor in decidual stromal cells surrounding the implanted embryo in pregnant mouse uterus. While it is clear that induction of C/EBPβ in the mouse arises from the interplay of estrogen and progesterone within the uterine compartments, regulation of this factor in the human endometrium is not fully understood. Preliminary studies in our laboratory indicate that estrogen, progesterone, and cAMP are necessary for maximal expression of C/EBPβ in human endometrial stromal cells (W. Wang, R. N. Taylor, I. C. Bagchi and M. K. Bagchi, unpublished data).
Our study also shows C/EBPβ expression in the baboon and human placenta. Baboon placenta at days 40 to 60 of gestation and first trimester human placenta showed elevated C/EBPβ expression in the syncytiotrophoblast cells of both free and anchoring villi. In contrast, cytotrophoblast cells were devoid of C/EBPβ expression at all stages in both species. Although the precise role of C/EBPβ in placental function is unclear, previous studies reported that it regulates the expression of critical placenta-specific genes during pregnancy. For example, the homeodomain gene, Distal-less 3, which plays an essential role in normal placental development, is regulated by C/EBPβ in human placental cells25. Furthermore, the gene encoding human aromatase cytochrome P450, a critical enzyme involved in the conversion of androgens to estrogens, is also transcriptionally regulated by C/EBPβ in human placental cells26.
In summary, our studies document, for the first time, the expression of C/EBPβ, critical regulator of implantation in the mouse, at implantation sites of human and nonhuman primates. The results presented in this paper are consistent with a conserved role of this key transcription factor in the regulation of uterine receptivity, stromal decidualization, and placental function during early pregnancy. These findings also support the hypothesis that C/EBPβ expression in the endometrium and placenta is a prerequisite for successful embryo implantation in primates.
Acknowledgments
Acknowledgement of grant support: This work was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreements U54 HD 055787 (MKB) and HD 040093 (ATF) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR16515-01 from the National Center for Research Resources, National Institutes of Health (ICB). We thank Dr. Karen Ferrer for providing the archival human implantation sites used in this study.
Contributor Information
Athilakshmi Kannan, Email: kalki32@uiuc.edu.
Asgerally T Fazleabas, Email: asgi@uic.edu.
Indrani C Bagchi, Email: ibagchi@illinois.edu.
References
- 1.Parr MBPEL. The Implantation Reaction. In: Wynn RM, Jollie WP, editors. Biology of the Uterus. New York: Plenum; 1989. pp. 233–277. [Google Scholar]
- 2.Psychoyos A. In: Handbook Of Physiology. Greep RO, Astwood EG, Geiger SR, editors. Washington, DC: Am. Physiol. Soc; 1973. pp. 187–215. [Google Scholar]
- 3.Weitlauf HM. In: The Physiology of Reproduction. Knobil E, Neill JD, editors. New York: Raven; 1994. pp. 391–440. [Google Scholar]
- 4.Yoshinga K. uterine receptivity for blastocyst implantation. Ann N Y Acad Sci. 1988;541:424–431. doi: 10.1111/j.1749-6632.1988.tb22279.x. [DOI] [PubMed] [Google Scholar]
- 5.Carson DD, Bagchi I, Dey SK, et al. Embryo implantation. Dev Biol. 2000 Jul 15;223(2):217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- 6.Enders AC, Lantz KC, Peterson PE, et al. From blastocyst to placenta: the morphology of implantation in the baboon. Hum Reprod Update. 1997 Nov–Dec;3(6):561–573. doi: 10.1093/humupd/3.6.561. [DOI] [PubMed] [Google Scholar]
- 7.Lee K, Jeong JW, Kwak I, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nature Genetics. 2006;38:1204–1209. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- 8.Lim H, Ma L, Ma W, et al. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol. 1999;13:1005–1017. doi: 10.1210/mend.13.6.0284. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Cheon YP, Kannan A, et al. A novel pathway involving progesterone receptor, 12/15-lipoxygenase-derived eicosanoids, and peroxisome proliferator-activated receptor gamma regulates implantation in mice. J Biol Chem. 2004 Mar 19;279(12):11570–11581. doi: 10.1074/jbc.M311773200. [DOI] [PubMed] [Google Scholar]
- 10.Mantena SR, Kannan A, Cheon YP, et al. C/EBPbeta is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc Natl Acad Sci U S A. 2006 Feb 7;103(6):1870–1875. doi: 10.1073/pnas.0507261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagchi MK, Mantena SR, Kannan A, et al. Control of uterine cell proliferation and differentiation by C/EBPbeta: functional implications for establishment of early pregnancy. Cell Cycle. 2006 May;5(9):922–925. doi: 10.4161/cc.5.9.2712. [DOI] [PubMed] [Google Scholar]
- 12.Paria BC, Huet-Hudson YM, Dey SK. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortman JD, Herring JM, Miller JB, et al. Chorionic gonadotropin, estradiol, and progesterone levels in baboons (Papio anubis) during early pregnancy and spontaneous abortion. Biol Reprod. 1993 Oct;49(4):737–742. doi: 10.1095/biolreprod49.4.737. [DOI] [PubMed] [Google Scholar]
- 14.Herring JM, Fortman JD, Anderson RJ, et al. Ultrasonic determination of fetal parameters in baboons (Papio anubis) Lab Anim Sci. 1991 Dec;41(6):602–605. [PubMed] [Google Scholar]
- 15.Strakova Z, Mavrogianis P, Meng X, et al. In vivo infusion of interleukin-1beta and chorionic gonadotropin induces endometrial changes that mimic early pregnancy events in the baboon. Endocrinology. 2005 Sep;146(9):4097–4104. doi: 10.1210/en.2005-0380. [DOI] [PubMed] [Google Scholar]
- 16.Fazleabas AT, Donnelly KM, Srinivasan S, et al. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2543–2548. doi: 10.1073/pnas.96.5.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strakova Z, Srisuparp S, Fazleabas AT. IL-1beta during in vitro decidualization in primate. J Reprod Immunol. 2002 May–Jun;55(1–2):35–47. doi: 10.1016/s0165-0378(01)00141-3. [DOI] [PubMed] [Google Scholar]
- 18.Plante BJ, Kannan A, Bagchi MK, et al. Cyclic regulation of transcription factor C/EBP beta in human endometrium. Reprod Biol Endocrinol. 2009 Feb 17;7(1):15. doi: 10.1186/1477-7827-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wedel A, Ziegler-Heitbrock HW. The C/EBP family of transcription factors. Immunobiology. 1995 Jul;193(2–4):171–185. doi: 10.1016/s0171-2985(11)80541-3. [DOI] [PubMed] [Google Scholar]
- 20.Fazleabas AT, Kim JJ, Srinivasan S, et al. Implantation in the baboon: endometrial responses. Semin Reprod Endocrinol. 1999;17(3):257–265. doi: 10.1055/s-2007-1016233. [DOI] [PubMed] [Google Scholar]
- 21.Fazleabas AT, Kim JJ, Strakova Z. Implantation: embryonic signals and the modulation of the uterine environment--a review. Placenta. 2004 Apr;25( Suppl A):S26–31. doi: 10.1016/j.placenta.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Cameo P, Srisuparp S, Strakova Z, et al. Chorionic gonadotropin and uterine dialogue in the primate. Reprod Biol Endocrinol. 2004 Jul 5;2:50. doi: 10.1186/1477-7827-2-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon C, Valbuena D, Krussel J, et al. Interleukin-1 receptor antagonist prevents embryonic implantation by a direct effect on the endometrial epithelium. Fertil Steril. 1998 Nov;70(5):896–906. doi: 10.1016/s0015-0282(98)00275-1. [DOI] [PubMed] [Google Scholar]
- 24.Christian M, Zhang X, Schneider-Merck T, et al. Cyclic AMP-induced Forkhead Transcription Factor, FKHR, Cooperates with CCAAT/enhancer-binding protein b in differentiating human endometrial stromal cells. J Biol Chem. 2002 June 7;277(23):20825–20832. doi: 10.1074/jbc.M201018200. [DOI] [PubMed] [Google Scholar]
- 25.Holland MP, Bliss SP, Berghorn KA, et al. A role for CCAAT/enhancer-binding protein beta in the basal regulation of the distal-less 3 gene promoter in placental cells. Endocrinology. 2004 Mar;145(3):1096–1105. doi: 10.1210/en.2003-0777. [DOI] [PubMed] [Google Scholar]
- 26.Toda K, Yang LX, Shizuta Y. Transcriptional regulation of the human aromatase cytochrome P450 gene expression in human placental cells. J Steroid Biochem Mol Biol. 1995 Jun;53(1–6):181–190. doi: 10.1016/0960-0760(95)00032-u. [DOI] [PubMed] [Google Scholar]