Abstract
Intermittent hypoxia (IH) associated with recurrent apneas often leads to cardiovascular abnormalities. Previously, we showed that IH treatment elevates blood pressure and increases plasma catecholamines (CAs) in rats via reactive oxygen species (ROS)-dependent enhanced synthesis and secretion from the adrenal medulla (AM). Neuropeptide Y (NPY), a sympathetic neurotransmitter that colocalizes with CA in the AM, has been implicated in blood pressure regulation during persistent stress. Here, we investigated whether IH facilitates NPY synthesis in the rat AM and assessed the role of ROS signaling. IH increased NPY-like immunoreactivity in many dopamine-β-hydroxylase–expressing chromaffin cells with a parallel increase in preproNPY mRNA and protein. IH increased the activities of proNPY-processing enzymes, which were due, in part, to elevated protein expression and increased proteolytic processing. IH increased ROS generation, and antioxidants reversed IH-induced increases in ROS, preproNPY, and its processing to bioactive NPY in the AM. IH treatment increased blood pressure and antioxidants and inhibition of NPY amidation prevented this response. These findings suggest that IH-induced elevation in NPY expression in the rat AM is mediated by ROS-dependent augmentation of preproNPY mRNA expression and proNPY-processing enzyme activities and contributes to IH-induced elevation of blood pressure. Antioxid. Redox Signal. 14, 1179–1190.
Introduction
Obstructive sleep apnea (OSA) is emerging as an independent risk factor for hypertension and peripheral vascular diseases (11). The Sleep Heart Health Study reported that ∼24% of men and ∼9% of women in the middle-aged group are affected by OSA (48, 73). In OSA patients, recurrent upper-airway constriction causes periodic oxygen desaturation, resulting in intermittent hypoxia (IH). Daytime sympathetic nerve activity is greater in humans with severe sleep apnea (10, 13, 16, 37, 77), and rodents treated with IH exhibit enhanced sympathetic nerve activity, as evidenced by increased levels of circulating catecholamines (4, 34). Studies in rodents also showed that adrenal medullectomy prevented not only the IH-induced increase in plasma catecholamines but also the elevation in blood pressure (4). These findings suggest that adrenal medulla–derived vasoactive substances may contribute to IH-induced cardiovascular alterations.
Neuropeptide Y (NPY), a potent vascoconstrictor peptide, is expressed in specific cell bodies and processes of the central and peripheral nervous system (69). The adrenal medulla, in addition to catecholamines, expresses multiple neuropeptides, including NPY (31, 40, 62, 63). NPY occurs as a precursor preproNPY (ppNPY) protein that undergoes proteolytic processing inside the secretory vesicles involving cathepsin L (CtsL), prohormone convertase (PC), carboxypeptidase E (CPE), and peptidylglycine α-amidating monooxygenase (PAM) to form the biologically active NPY-amide (7, 14, 19, 36, 50). Several findings in the literature support a regulatory role for NPY of the sympathetic nervous system in the control of blood pressure, especially during intense, chronic stress (23, 31, 44, 71, 72, 79). For instance, in response to persistent cold or psychogenic stress, NPY is released from the sympathetic nerve terminals along with norepinephrine (NE) (12, 20, 44), causing vasoconstriction via enhancing the action(s) of NE (27, 29). Of relevance to IH are recent clinical studies that reported higher levels of plasma NPY in OSA patients (6, 39) compared with nonapneic patients; this was reversed after treatment with continuous positive airway pressure (CPAP) (6). In other studies, CPAP effectively normalized elevated blood pressures and diminished the daytime sympathetic activation seen in OSA patients (8, 22, 24, 42, 77). Taken together, these studies suggest an association between OSA-mediated alterations in plasma NPY and hypertension. However, it remains to be determined whether IH, one of the major determinants of OSA, directly affects the synthesis, release, and receptor interactions of NPY.
The objective of the present study was, therefore, to determine whether IH alters NPY synthesis in the sympathetic nervous system. We chose adrenal medulla for this investigation due to its dual role as a constituent and effector organ of the sympathetic nervous system. Moreover, adrenal medulla from diverse species has been shown to synthesize and store NPY along with NE in the large-dense core vesicles (19, 40, 62, 63). Our results demonstrate that IH treatment markedly elevates bioactive NPY levels in the adult rat adrenal medulla via mechanisms involving reactive oxygen species (ROS)-dependent upregulation of NPY transcription and proNPY processing.
Materials and Methods
Materials
Neuropeptide Y, Tween-20, malondialdehyde (MDA), N-acetyl cysteine, 4-phenyl-3-butenoic acid (PBA), and N-tris (hydroxymethyl) methyl-2-aminoethane sulfonic acid (TES) were obtained from Sigma (St. Louis, MO). N-Dansyl-Tyr-Val-Gly (N-Dan-Y-V-G) was from American Peptide Company (Sunnyvale, CA). The pyroGlu-Arg-Thr-Lys-Arg-MCA and Dansyl-Phe-Ala-Arg were synthesized commercially (Peptide 2.0 Inc., Chantilly, VA). Manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride (MnTMPyP) was purchased from ALEXIS Biochemicals (San Diego, CA). Complete protease inhibitor cocktail (EDTA-free) was obtained from Roche (Indianapolis, IN). Guanidinoethylmercaptosuccinic acid (GEMSA) was obtained from Calbiochem.
Methods
The Institutional Animal Care and Use Committee of the University of Chicago approved animal handling and experimental protocols. The studies were performed with adult male Sprague–Dawley rats weighing 200 to 250 g. Animals were housed two per cage in a temperature-controlled room on a 12-h light/dark cycle (6 AM/6 PM) and were given food and water ad libitum.
Exposure to intermittent hypoxia
Rats were exposed to alternating cycles of hypoxia (5% O2 for 15 s) and normoxia (21% O2 for 5 min) for 8 h/day between 9:00 AM and 5:00 PM for 10 days, as previously reported (51). Rats exposed to normoxia for 10 days served as controls. To determine the role of ROS, rats were given either manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride (MnTMPyP) or N-acetyl-l-cysteine (NAC), as described previously (65). To examine the role of NPY, rats were administered daily 4-phenyl-3-butenoic acid (PBA; Sigma; 300 mg/kg, IP), before IH or normoxic exposure for 10 days. PBA was solubilized in physiologic saline by adjusting the pH to 7.4 with 0.1N NaOH.
All experiments were performed within ∼16 h after the final IH exposure. Rats were anesthetized with urethane (1.2 g/kg, IP), and the surgical plane of anesthesia was determined based on the absence of limb withdrawal in response to noxious pinching of toes.
Isolation of adrenal medulla and superior cervical ganglion
Whole adrenal gland and superior cervical ganglion were removed from anesthetized rats, frozen in liquid nitrogen, and stored at −80°C until further analysis. In experiments involving analysis of various adrenal regions, medulla and cortex were dissected under ice-cold conditions.
Extraction, purification, and enzyme immunoassay of NPY
Thawed tissues were washed with physiologic saline to remove blood and homogenized in 500 μl of 0.1N acetic acid containing protease inhibitor cocktail. The resulting acid extract was boiled for 20 min, cooled, and centrifuged at 15,000 g for 20 min. The supernatant was saved, and the pellet was re-extracted in 250 μl of 0.1N acetic acid and centrifuged as described earlier. The supernatants from the two extraction steps containing peptides were pooled and further purified by using a SepPak C18 column, as described previously (65). The efficiency of NPY recovery was 75 ± 6%. NPY-like immunoreactivity (NPY-Li) in purified peptide extracts of adrenal medulla was determined by using an enzyme immunoassay (EIA) kit specific for NPY by following the manufacturer's instructions (Bachem, Chicago, IL). The sensitivity of the EIA for NPY was 50 pg/ml.
Immunofluorescence analysis
The fixation and immunostaining procedures used in this study were essentially the same as described previously (33). In brief, after anesthesia (urethane, 1.2 g/kg; IP), rats were perfused transcardially with 50 ml of 0.9% NaCl followed by 50 ml of 4% paraformaldehyde in PBS. The adrenal glands were removed, postfixed in 4% paraformaldehyde solution for 1 h, and transferred to a solution containing 20% sucrose in PBS. Sections of adrenal glands (5 μm thickness) were incubated with either polyclonal rabbit anti-NPY antibody (at 1:8,000 dilution; Sigma) or monoclonal mouse anti-DBH antibody (at 1:400 dilution; Millipore), an established marker of chromaffin cells, or polyclonal rabbit anti-ppNPY antibody (at 1:100 dilution; Novus, St. Charles, MO) or polyclonal rabbit anti-PHM antibody (JH1761; a gift from Dr. Eipper, at 1:1,000 dilution) at 4°C for 16 h. Subsequently, the sections were immunolabeled with either Texas Red conjugated goat anti-rabbit IgG or FITC-conjugated goat anti-mouse IgG (1:250; Molecular Probes, Eugene, OR) in PBS with 1% normal goat serum and 0.2% Triton X-100. After washing with PBS, sections were mounted in DAPI-containing media and visualized by using a fluorescence microscope (Eclipse E600, Nikon). Sections incubated without primary antibody were used as negative controls. Further to assess the specificity of NPY-like immunoreactivity (NPY-Li), immunohistochemical staining was performed after antibody preabsorption with excess NPY peptide (2 μg/ml; Sigma) at 4°C for 16 h.
Real-time PCR
Real-time PCR was performed by using a MiniOpticon system (Bio-Rad) with the SYBR GreenER two-step quantitative RT-PCR kit (Invitrogen), as described previously (53). In brief, RNA was extracted from adrenal medullae by using Trizol and was reverse transcribed by using superscript III reverse transcriptase. Primer sequences for real-time PCR amplification were as follows: 18S forward (fw), GTAACCCGTTGAACCCCATT; 18S reverse (rev), CCATCCAATCGGTAGTAGCG (151 base pair) and ppNPY: (fw) 5'-TATCCCTGCTCGTGTGTTTG-3' and (rev) 5'-AACGACAACAAGGGAAATGG-3' (386 base pairs) (75). Relative mRNA level was calculated by using the comparative threshold (CT) method with the formula 2−CT where CT is the difference between the threshold cycle of the given target cDNA between control (normoxia) and IH. The CT value was taken as a fractional cycle number at which the emitted fluorescence of the sample passes a fixed threshold above the baseline.
Values were compared with an internal standard gene 18S. Purity and specificity of all products were confirmed by omitting the template and by performing a standard melting-curve analysis.
Assays of PC1/3, CtsL, CPE, and PAM activities
PC1/3 assay
PC1/3 activity was assayed by using the procedure described previously (36). In brief, the adrenal medulla was homogenized in 2 ml of 0.1 M sodium acetate, pH 5.5, containing 1 mM phenylmethyl sulfonyl fluoride. A suitable aliquot of the homogenate was incubated with 200 μM pERTKR-methylcoumarin amide (MCA), a fluorogenic substrate, and 100 mM sodium acetate, pH 5.5, containing 5 mM CaCl2, 0.1% Brij 35, and EDTA-free protease-inhibitor cocktail. The formation of free MCA was measured fluorometrically by using excitation and emission wavelengths of 380 and 460 nm, respectively. The amount of MCA formed was calculated (1 fluorescence unit = 4.8 pmol MCA), and PC1/3 activity was expressed as picomoles of MCA formed per hour per milligram protein.
CtsL assay
Cathepsin L activity was determined by using a commercially available InnoZyme Cathepsin L Activity Kit (Calbiochem), as per manufacturer's instructions. The release of 7-amido-4-methylcoumarin (AMC) from the substrate, Z-Phe-Arg-AMC, was monitored fluorometrically by using excitation and emission wavelengths of 360 and 460 nm, respectively. CtsL activity was expressed as nanomoles of AMC release, inhibitable in the presence of CA 074 and a CL-specific inhibitor [Z-FY(t-Bu)-DMK] per hour per milligram protein.
CPE assay
CPE activity was assayed by using the procedure described previously (18). In brief, a suitable aliquot of the tissue homogenate was incubated with 50 mM sodium acetate buffer, pH 5.0, containing 0.01% Triton X100 and 200 μM dansyl-Phe-Ala-Arg substrate in a final volume of 250 μl at 37°C for 60 min. In parallel experiments, this reaction was performed in the presence of either an activator (1 mM CoCl2) or an inhibitor (1 μM guanidinoethylmercaptosuccinic acid; GEMSA) of CPE. At the end of the reaction, 100 μl of 0.5 M HCl and 2 ml of chloroform were added to the reaction vials, mixed, and centrifuged at 500 g for 2 min. The amount of the product formed during the reaction was determined by measuring the fluorescence in the organic layer by using excitation and emission wavelengths of 350 nm and 500 nm, respectively. CPE activity is defined as the difference between the activity stimulated by Co2+ and that inhibited by GEMSA and is expressed as picomoles per hour per milligram protein.
PAM assay
PAM activity was assayed by monitoring the rate of conversion of a synthetic peptide substrate, N-dansyl-Tyr-Val-Gly-COOH to N-dansyl-Tyr-Val-NH2, as described previously (28, 46, 65). PAM activity was expressed as picomoles of N-dansyl-Tyr-Val-NH2 formed per hour per milligram protein.
Protein assay
The protein concentration was determined by using a protein-assay kit from Bio-Rad with bovine serum albumin as the standard.
Western blot analysis
Immunoblot assays were performed as described previously (65) by using the following polyclonal rabbit antibodies: anti-PHM (JM629, gift from Dr. Eipper; at 1:1,000 dilution), anti-PC1/3 (Millipore, at 1:1,000 dilution), anti-CPE (Millipore, at 1:2,500 dilution), anti-CtsL (Santa Cruz Biotech, at 1:200 dilution), anti-DBH (Millipore; at 1:250 dilution); anti-ppNPY (Novus Biologicals, Denver, CO; at 1:100 dilution), anti-TH (Pel-Freez, Phoenix, AR; at 1:1,000 dilution), anti-β-actin (Sigma; at 1:10,000 dilution), and anti-DNP (Millipore; at 1: 150 dilution). Immunoblot analysis showed that preadsorption with synthetic NPY amide did not affect anti-ppNPY immunoreactivity, indicating specificity for ppNPY protein. After the washing steps, the blots were incubated with horseradish peroxidase–conjugated goat anti-rabbit or goat anti-mouse IgG (Santa Cruz Biotech; each at 1:10,000) for 1 h at 4°C. The immunoreactive proteins were identified by using the ECL detection kit from Amersham Biosciences. As a loading control, the expression level of β-actin (42 kDa) was monitored.
Assay of malondialdehyde
The level of malondialdehyde (MDA), an index of lipid oxidation, was determined by using the procedure described previously (52, 58, 65) with few modifications. In brief, 100 μl of either tissue homogenate or a suitable concentration of MDA standard was incubated with 50 μl of SDS (8.1%), 375 μl of acetic acid (20%), and 375 μl of thio-barbituric acid (0.8%) at 90°C for 60 min, followed by incubation at 4°C for 10 min. The reaction mixture was centrifuged at 800 g for 15 min, and the relative fluorescence intensity of the resulting supernatant was monitored by using excitation and emission wavelengths of 530 and 550 nm, respectively. The level of lipid oxidation was expressed as nanomoles of MDA formed per milligram of protein.
Estimation of protein carbonylation
Protein carbonyl level in the adrenal medulla was measured by using OxyBlot Protein Oxidation Detection Kit (Oxyblot; Chemicon International, Temecula, CA) in accordance with the manufacturer's instructions. In brief, 10 μg of adrenal medullary proteins was treated with SDS and 2,4-dinitrophenylhydrazine for 15 min at room temperature. After neutralization, the proteins in the reaction mixture were reduced with 2-mercaptoethanol and separated on a 12% gel with SDS-PAGE. Proteins were electrophoretically transferred to nitrocellulose membranes, and the nonspecific binding sites were blocked with 5% nonfat milk and immunolabeled with a primary polyclonal antibody specific for the 2,4-dinitrophenol group (DNP moiety of the protein; Millipore; at 1:150 dilution) for 16 h at 4°C. The immunolabeled proteins were identified by using a peroxidase-labeled secondary antibody (Millipore; at 1:300 dilution). The blots were developed by using an enhanced chemiluminescence reagent kit (ECL Western Blotting Detection Reagents, GE Healthcare). To compare the level of carbonylation between the control, IH, and IH+MnTMPyP groups, experiments were performed in triplicate.
Measurement of blood pressure
Arterial blood pressure (BP) was measured in rats exposed to either normoxia or 10 days of IH in the absence and presence of antioxidants (MnTMPyP and NAC) or PBA or vehicle (saline). In conscious, restrained rats, BP was measured by the tail-cuff method (9) with equipment made by IITC Inc., as described previously (54). In brief, rats were placed in the restrainer provided by the manufacturer and were allowed to acclimate for at least 1 h before measurements. In each rat, multiple BP measurements were made until four consistent values were recorded. Data from two consecutive days were used for BP analysis with the software, BpWonWin (IITC Life Science).
Data analysis
All enzyme-activity measurements were made in triplicate, whereas immunoblot analyses were made in duplicate. Data derived from seven independent experiments (seven rats in each group) were expressed as mean ± SEM. Statistical significance was evaluated with the unpaired t test or one-way ANOVA for repeated measures. The p values <0.05 were considered significant.
Results
IH increases NPY level in the adrenal medulla
The distribution of NPY in the rat adrenal gland was examined by using EIA specific for NPY. NPY-Li was found both in the adrenal medulla (AM) and cortex; however, the relative abundance of NPY-Li in the AM was nearly sevenfold higher than that in the cortex (Fig. 1A). IH increased adrenal medullary NPY-Li in a time-dependent manner (Fig. 1B) with an approximate 4.2-fold increase after 10 days of IH treatment. NPY-Li expression in the AM of rats exposed to either 14 or 30 days of IH was similar to the expression level seen after 10 days of IH (data not shown). Ten days of IH exposure also augmented NPY-Li in the superior cervical ganglion, a sympathetic nervous system component, albeit to a lesser degree than that in the AM (Fig. 1C). Conversely, a similar duration of IH treatment had no significant effect on NPY-Li in the adrenal cortex (Fig. 1C). To verify that the measured NPY-Li represents a biologically active amidated form of NPY, we analyzed the peptides extracted from the AM of normoxia and IH-exposed rats with high-performance liquid chromatography. We found that NPY-Li of the AM co-elutes with authentic NPY-amide. Because IH treatment for 10 days maximally increased the level of biologically active NPY, in the following localization and mechanistic studies, we used AM obtained from rats treated with 10 days of IH.
FIG. 1.
Effect of intermittent hypoxia (IH) on neuropeptide Y expression in the adrenal gland and superior cervical ganglion. (A) Distribution of NPY-like immunoreactivity (NPY-Li) in adrenal medulla (AM) and adrenal cortex (AC). (B) Changes in NPY-Li in AM as a function of duration of IH treatment. (C) Exposure to 10 days of IH increases NPY-Li in the superior cervical ganglion (SCG) but not in the AC. NPY levels were determined in acid extracts of tissues harvested from rats exposed to either normoxia (CON) or IH by using an enzyme immunoassay (EIA) kit specific for NPY. Data from seven independent experiments (seven rats in each group) are presented as mean ± SEM. **p < 0.01; ***p < 0.001.
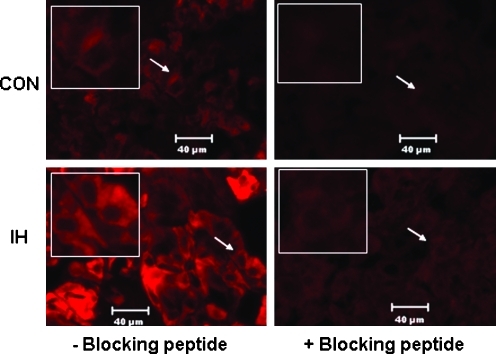
To determine the cellular source of IH-induced increase in NPY-Li in the AM, immunofluorescence analyses were performed. In rats exposed to normoxia, low-level expression of NPY-Li was detected in many AM cells (Fig. 2, top left panel). In the AM of IH-treated rats, the level of NPY-Li was markedly upregulated (Fig. 2, bottom left panel). NPY-Li was abolished after blocking the antibody with an excess amount of synthetic NPY-amide (Fig. 2, right panels). Double-labeling studies with dopamine β-hydroxylase (DBH), a marker of NE producing chromaffin cells, revealed that the IH-evoked increase in NPY-Li is confined to a subset of DBH-positive chromaffin cells (Fig. 3). Interestingly, DBH-like immunoreactivity was higher in IH-treated than in normoxic control chromaffin cells (Fig. 3, middle panels). Further, Western blot analyses showed that IH induced an approximate 2.6-fold increase in DBH protein expression without any significant alteration in TH protein expression (Fig. 4A).
FIG. 2.
Cellular localization of NPY expression in the adrenal medulla. Immunofluorescence analyses of NPY expression in the adrenal medullary sections of rats exposed to either normoxia (CON; top panels) or 10 days of IH (bottom panels). To assess the specificity of NPY-Li, sections were stained with anti-NPY antibody preadsorbed with synthetic NPY-amide peptide (+blocking peptide; right panels). Representative examples selected from seven independent experiments are presented. Other experimental details are as described in Methods. Insets: Higher magnification of areas indicated by arrows. The bar indicates 40 μm.
FIG. 3.
Colocalization of NPY with dopamine-β-hydroxylase (DBH) in a subset of chromaffin cells. Adrenal medullary sections from rats exposed to either normoxia (CON; top panels) or IH for 10 days (bottom panels) were double immunostained with anti-NPY polyclonal (left panels, red) and anti-DBH monoclonal (middle panels, green) antibodies. DBH serves as a marker of norepinephrine producing chromaffin cells. The right panels show colocalization of NPY and DBH staining, together with nuclear DAPI staining. Representative examples were selected from seven independent experiments. The bar indicates 20 μm.
FIG. 4.
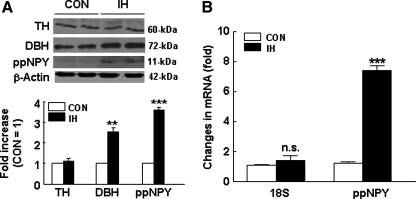
IH upregulates adrenal medullary preproNPY protein and mRNA expression. (A) Immunoblot analyses of tyrosine hydroxylase (TH), DBH, and ppNPY expression in AM of rats exposed to normoxia (CON) and 10 days of IH. The expression level of β-actin was used as loading control. Representative examples of immunoblots (top panel; from two independent experiments) and quantitative analysis (bottom panel; from seven independent experiments) of protein expression are shown. (B) Real-time PCR analysis of preproNPY (ppNPY) and 18S mRNA expression in AM of normoxic (CON) and 10-day IH-treated rats. Data are expressed as fold change. Data from seven independent experiments (seven rats in each group) are presented as mean ± SEM. **p < 0.01; ***p < 0.001. n.s., not significant.
IH elevates ppNPY mRNA and protein
Bioactive NPY is synthesized from ppNPY, a protein precursor of NPY, through sequential proteolytic processing involving CtsL, PC1/3, CPE, and PAM (7, 14, 18, 19, 36, 50). Therefore, we examined whether the IH-evoked increase in NPY-Li in the AM is due to possible alterations in either ppNPY transcription, translation, or posttranslational processing.
PreproNPY protein and mRNA expression in the control and IH-treated adrenal medullae were determined with Western blot and real-time PCR, respectively. IH increased ppNPY protein expression by about 3.6-fold (Fig. 4A), which was due in part to a marked increase in ppNPY mRNA level (about sevenfold; Fig. 4B).
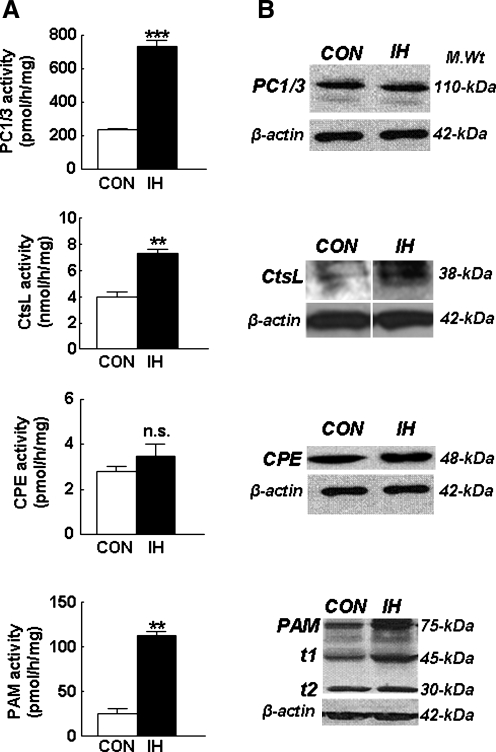
To assess whether IH alters ppNPY precursor protein processing in the AM, enzyme activities of CtsL, PC1/3, CPE, and PAM were determined by using synthetic substrates and inhibitors specific to each of these enzymes. Among these precursor protein-processing enzymes, IH selectively increased the enzymatic activities of PC1/3, CtsL and PAM by 3.1-, 1.9-, and 4.4-fold, respectively, compared with normoxic controls, without significantly altering CPE activity (Fig. 5A). To determine whether changes in protein expression contribute to IH-induced increases in enzyme activities of PC1/3, CtsL, and PAM, Western blot analyses were performed, and the results are shown in Fig. 5B. IH had no significant effect on the expression level of PC1/3 protein, whereas it increased CtsL protein expression by nearly twofold. Conversely, analysis of PAM revealed the presence of several proteolytically processed, truncated forms in the normoxic adrenal medulla, which is consistent with previous reports demonstrating tissue-specific processing of PAM (15). IH not only increased the expression of total PAM-related proteins, but also increased the level of the 45-kDa truncated form of PAM, which is enzymatically more active than the intact form of PAM (15).
FIG. 5.
IH differentially alters the activity and protein expression of proteolytic enzymes associated with proNPY processing. (A) Effects of IH on the activity (left panels) and (B) protein expression (right panels) of prohormone convertase (PC), cathepsin L (CtsL), carboxypeptidase E (CPE), and peptidyl glycine-α-amidating monooxygenase (PAM) are shown. Representative examples of immunoblot analysis of PC, CtsL, CPE, and PAM expression are shown in B. β-Actin served as loading control. CON, normoxia. Data from seven independent experiments (seven rats in each group) are expressed as mean ± SEM. **p < 0.01; ***p < 0.001; n.s., not significant.
IH increases ROS generation in the rat adrenal medulla
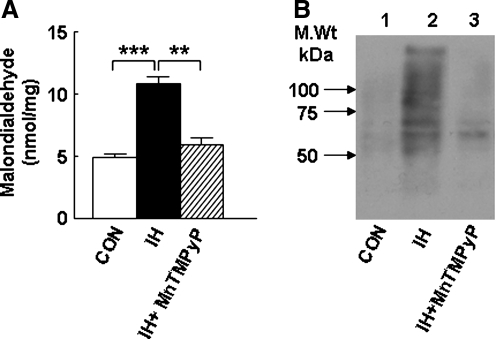
Previously, by monitoring the inhibition of aconitase enzyme activity as an index of ROS generation in vivo, we showed that IH increased ROS generation in the rat carotid body (51) and brainstem regions (57, 65). To determine whether IH induces ROS generation in the AM, we measured the level of MDA and protein carbonyl as indices of lipid and protein oxidation, respectively. In the control adrenal medulla, the MDA level was 4.9 ± 0.2 nmol/mg protein, and IH increased the MDA level by about 120% (Fig. 6A). Likewise, the level of oxidized proteins (as measured by the relative abundance of protein carbonyl) was relatively low in the normoxic control, whereas it was markedly elevated in IH-treated AM samples (Fig. 6B). Treatment of rats with MnTMPyP, a membrane-permeable antioxidant, before IH exposure, significantly attenuated IH-evoked increases in MDA and protein carbonyl levels. These results suggest that IH caused an increase in the generation of ROS in the AM.
FIG. 6.
IH increases indices of oxidative stress in the adrenal medulla. (A) Effects of IH on malondialdehyde and (B) protein carbonyl levels are shown. MnTMPyP (5 mg/kg/day; IP), a membrane-permeable scavenger of superoxide anion, reverses IH-induced changes in malondialdehyde and protein carbonyl to normoxic (CON) levels. Other experimental details are as described in Methods. Data from seven independent experiments (seven rats in each group) are shown as mean ± SEM. **p < 0.01; ***p < 0.001.
ROS signaling mediates IH-induced upregulation of NPY-Li, ppNPY, and proNPY processing
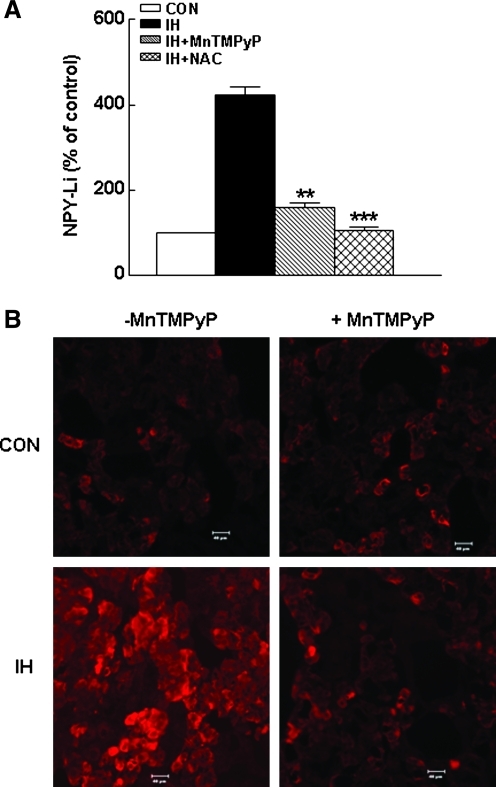
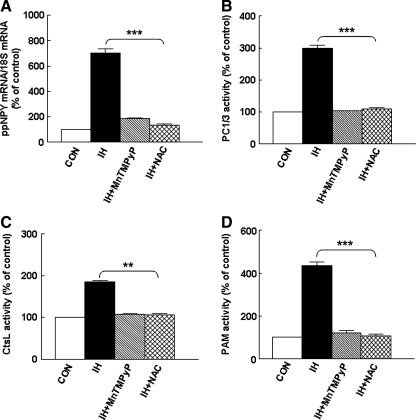
To examine whether ROS signaling contributes to IH-induced upregulation of NPY in the adrenal medulla, rats were administered MnTMPyP, a superoxide dismutase (SOD) mimetic, daily before IH exposure. Treatment with the SOD mimetic attenuated the IH-induced elevation of the NPY-Li level in the adrenal medulla (IH + SOD mimetic, 160 ± 10% vs. IH, 423 ± 20%; p < 0.01; n = 7 rats in each group; Fig. 7A) and NPY immunostaining in the chromaffin cells (Fig. 7B). Furthermore, MnTMPyP treatment either attenuated or abolished IH-mediated increases in ppNPY mRNA (Fig. 8A), and enzymatic activities of PC1/3, CtsL, and PAM (Fig. 8B–D). Likewise, treatment with N-acetylcysteine, a ROS scavenger, also reversed the increases in tissue levels of NPY-Li, ppNPY mRNA, and activities of proteolytic enzymes associated with proNPY processing elicited by IH to normoxic control levels (Figs. 7A and 8).
FIG. 7.
Antioxidants reverse IH-induced upregulation of NPY-Li. (A) Effects of MnTMPyP and N-acetylcysteine (NAC) treatments on tissue concentration of NPY-Li and (B) expression of NPY-Li in adrenal medullary cells are shown. Representative example of the effect of MnTMPyP on NPY-Li is shown in B. Data from seven independent experiments (seven rats in each group) are expressed as mean ± SEM. **p < 0.01; ***p < 0.001. The bar indicates 40 μm.
FIG. 8.
Antioxidants prevent IH-induced upregulation of preproNPY mRNA expression and activity of proteolytic enzymes associated with proNPY processing. Effects of MnTMPyP and NAC on preproNPY (ppNPY) mRNA expression (A) and on enzymatic activities of (B) prohormone convertase (PC1/3), (C) cathepsin L (CtsL), and (D) PAM in the adrenal medulla of normoxic (CON) and IH-treated rats. Data from seven independent experiments (seven rats in each group) are presented as mean ± SEM. **p < 0.01; ***p < 0.001.
ROS-dependent NPY signaling is critical for IH-induced elevation in blood pressure
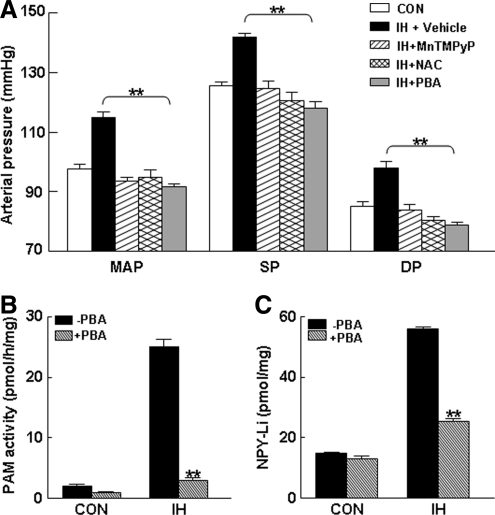
NPY signaling has been suggested to play a prominent role in blood pressure regulation during persistent and intense stress (23, 31, 72, 79). Because IH treatment not only increases blood pressure in rodents (4, 34) but also markedly upregulates the NPY level in the AM through ROS signaling, as shown earlier, we hypothesized that ROS-dependent NPY signaling may play a role in the IH-induced increase in blood pressure. To test this possibility, we determined the effects of ROS scavengers and PBA, an irreversible mechanism-based inhibitor of the amidation reaction (45, 49, 59) affecting NPY synthesis in the IH-evoked increase in blood pressure. In IH-treated rats, mean arterial blood pressure increased by 16.4 ± 1.3 mm Hg and was associated with increases in both systolic and diastolic pressure (Fig. 9A). Daily treatment of rats with either antioxidants or PBA effectively prevented IH-induced elevations in mean arterial, systolic, and diastolic pressures (Fig. 9A). Administration of vehicle alone had no significant effect on the blood pressure in the control (normoxia) rats. Likewise, vehicle had no effect on the blood pressure elevation seen in IH-treated rats. In IH-treated rats, PBA treatment concurrently reduced both PAM activity (Fig. 9B) and NPY-Li (Fig. 9C) to near control levels.
FIG. 9.
Reversal of IH-induced hypertension by antioxidants and PAM inhibitor. (A) Effects of MnTMPyP and NAC (antioxidants), 4-phenyl-3-butenoic acid (PBA, an inhibitor of PAM), and vehicle (saline) on IH-induced elevation in blood pressure. Effects of systemic administration of PBA on PAM activity (B) and NPY-Li (C) in AM of rats exposed to normoxia (CON) and 10 days of IH. Data from seven independent experiments (seven rats in each group) are presented as mean ± SEM. **p < 0.01. MAP, mean arterial pressure; SP, systolic pressure; DP, diastolic pressure.
Discussion
The overall goals of the present study were to determine whether IH affects the synthesis of NPY, a potent vasoconstrictor, in the rat AM and to elucidate the role of ROS signaling in IH-evoked responses. Our results demonstrate that NPY expression is markedly augmented by IH through ROS-mediated increases in ppNPY mRNA and protein expression and proNPY processing. Furthermore, our data show that pretreatment with either antioxidants or inhibitor in the final step of ppNPY processing was effective in preventing IH-induced increases in blood pressure.
A novel finding of the present study is that IH-evoked upregulation of NPY-Li is restricted to a subset of chromaffin cells that express DBH, which catalyzes the synthesis of NE from dopamine. Based on the type of catecholamine released, chromaffin cells are divided into two subpopulations, noradrenergic and adrenergic (3). Given that NPY often colocalizes with NE in sympathetic neurons (40, 41, 63), it is conceivable that the IH-evoked increase in NPY-Li occurs in NE-expressing chromaffin cells. However, additional studies using specific markers of noradrenergic and adrenergic chromaffin cells are needed to confirm this possibility. Interestingly, immunoblot analyses also reveal an upregulation of DBH in conjunction with preproNPY in the AM from IH-treated rats. It is likely that the enhanced DBH expression may causally be linked to a previously reported increase in NE level of the adrenal medulla by IH (34).
IH and preproNPY mRNA expression
We found that IH increases both mRNA and protein expression of preproNPY suggesting that IH may alter molecular events underlying the transcription and translation of NPY. Previous studies showed that NPY gene expression is sensitive to both pharmacologic and physiological stimuli (20, 26, 63, 72). IH-induced increase in preproNPY mRNA in the rat AM is reminiscent of a similar augmentation of preproNPY mRNA elicited by immobilization stress and pharmacologic interventions in rat sympathetic neurons (21, 47) and the sympatho-adrenal system (26, 38, 63). Stress, in addition to stimulating neuronal input to the AM, increases circulating glucocorticoids. The immobilization stress–induced elevation in adrenal medullary preproNPY mRNA expression results, in part, from the stimulation of splanchnic sympathetic nerve activity and the ensuing cholinergic receptor activation (26, 38, 63). Further, glucocorticoids have been shown to stimulate preproNPY mRNA expression in bovine cell cultures (25). Previously, we showed that IH markedly attenuates nicotine-evoked catecholamine efflux from the adult (34) and neonatal AM (67). Therefore, it is likely that neither increased splanchnic nerve activity nor nicotinic cholinergic receptor activation plays a role in the IH-evoked increase in preproNPY transcription. Such a possibility is further supported by our recent results showing that IH attenuates the expression of nicotinic acetylcholine receptor subtypes in neonatal AM (67). A recent study reported that IH increases plasma corticosterone level in adult rats (78). It is likely that the elevated corticosterone, through activation of transcriptional machinery, contributes to IH-induced preproNPY mRNA expression in the AM. The promoter region of NPY contains several functional motifs homologous to known cis-acting regulatory elements, including Sp1, cAMP response element binding (CREB), and AP1 motifs (2, 35, 43). It remains to be determined which transcription factors activated by IH could mediate the transcriptional activation of the NPY gene.
IH and precursor propeptide processing
The generation of bioactive NPY-amide in vivo is also regulated at the level of precursor propeptide processing by the enzymes CtsL, PC1/3, CPE, and PAM (7, 14, 19, 36, 50). A recent study identified CtsL as the major prohormone convertase catalyzing the formation of NPY (1-38) from proNPY (1-128), whereas PC1/3 plays only a minor role in proNPY processing (19). The biologic effects mediated by NPY require PAM-mediated C-terminal amidation, which is a prerequisite for receptor activation. The finding that CtsL and PAM activities are increased in the AM of IH-treated rats suggests that IH induces a compensatory upregulation of the precursor propeptide processing to cope with the augmented generation of proNPY. Furthermore, PAM inhibition by PBA effectively prevented IH-induced increase in NPY-amide level in the AM. Taken together, these findings suggest that the increase in the level of bioactive NPY in IH-treated adrenal medullae is, in part, the result of an increased conversion of proNPY to bioactive NPY-amide.
Previously, we reported that IH increases the activity of TH, the rate-limiting enzyme in catecholamine biosynthesis, without altering TH protein expression and that the IH-induced increase in TH activity is primarily due to enzyme activation involving posttranslational serine phosphorylation (57). The activity of PAM is increased after proteolytic processing, presumably through the removal of the inhibitory domain, resulting in a more-active form of the enzyme (15). Available evidence suggests that PC1 activity could be enhanced through increased posttranslational proteolytic modification in the dense core vesicles (56, 64). Therefore, it is possible that IH, in addition to increasing protein expression, may also activate propeptide processing enzymes in the AM through posttranslational proteolytic processing.
ROS signaling in IH-induced facilitation of preproNPY mRNA and proNPY processing
How does IH alter both preproNPY mRNA and proNPY processing? Studies in rodents (30, 53, 55, 57, 58, 70, 76), cell cultures (74), and humans (5, 68) have provided evidence for ROS signaling in IH-induced cellular, vascular, and neuronal responses. Several lines of evidence suggest that IH-induced increase in NPY in the rat AM is mediated by activation of a ROS signaling pathway. First, IH increased not only the adrenal medullary MDA level, but also the level of protein carbonylation (indices of oxidative stress). Second, daily administration of either MnTMPyP or NAC before IH exposure effectively blocked IH-induced increases in MDA, protein carbonylation, and NPY amide levels and attenuated increases in CtsL, PC1/3, and PAM enzymatic activities in IH-treated rats. Intracellular ROS could be derived from multiple sources, including cytosolic and membrane-bound oxidases, oxygenases, and the mitochondrial electron-transport chain. Although in the present study, we did not examine the chemical identity of ROS induced by IH in the AM, based on our previous studies in rodents and cell cultures treated with IH (53, 74), it is likely that either NADPH oxidase, the mitochondrial electron-transport chain, or both, may contribute to IH-evoked ROS generation and functional alterations in the AM.
It is interesting to note that IH augments NPY expression progressively in a duration-dependent manner. Such cumulative increases in NPY across exposure days may likely be due to IH-induced changes in ROS levels and the ensuing ROS signaling affecting either transcription or translation or processing of preproNPY. Although we have not monitored ROS levels at various durations of IH, our recent study in PC12 cells demonstrated that IH, in the initial periods, activates NADPH oxidase to increase ROS transiently, which facilitates the inhibition of the mitochondrial electron-transport chain at complex I for sustained production of ROS (32). Whether IH sequentially induces the activation of NADPH oxidase and the subsequent inhibition of complex I of the electron-transport chain and whether this ROS-induced ROS generation contributes to IH-induced NPY expression in the rat AM, however, remains to be determined.
Functional implications
The present study demonstrated that IH, a form of repetitive stress, markedly increases adrenal medullary NPY-Li levels through mechanism(s) involving alterations at the level of transcription, translation, and precursor propeptide processing, all of which are mediated through ROS signaling (Fig. 10). What might be the functional consequence of IH-evoked NPY elevation in the adrenal medulla? Neuroendocrine chromaffin cells of the AM have the same embryonic origin as postganglionic sympathetic neurons, and they function to secrete norepinephrine and epinephrine into the circulation, contributing to physiologic homeostasis and stress response. Available evidence suggests that NPY plays important modulatory roles in catecholamine biosynthesis by affecting the activity of tyrosine hydroxylase and in stimulus-evoked release of neurotransmitters, including catecholamines in the human and mouse AM (60, 61). Previously, we showed that IH not only increases NE and epinephrine levels in the adult (34) and neonatal (66) AM but also induces their efflux in response to acute hypoxia. It is noteworthy that adrenal medullectomy (4), as well as blockade of IH-induced NPY production in the adrenal medulla via treatment with either antioxidants or inhibitor of the amidation reaction, prevented the IH-induced increase in blood pressure. Although endothelin-1 and angiotensin II are implicated in IH-induced blood-pressure regulation (1, 17) their bioactivities do not require amidation. Therefore, it is likely that the IH-induced increase in adrenal medullary NPY plays an important role in blood-pressure regulation during recurrent apneas, possibly by regulating catecholamine synthesis or secretion or both. However, whether other AM-derived neuropeptides, in addition to NPY, also contribute to alterations in blood pressure by IH remains to be determined.
FIG. 10.
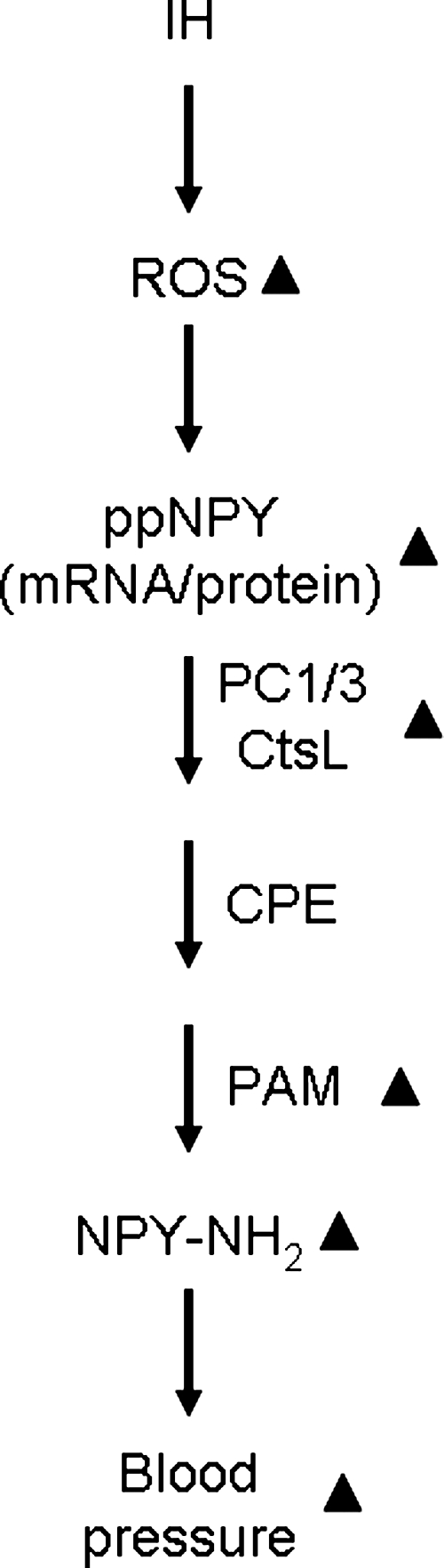
Schematic representation of IH-induced increase in adrenal medullary NPY by activation of reactive oxygen species (ROS) signaling pathway. ▴, upregulation; ppNPY, preproNPY; PC1/3, prohormone convertase 1/3; CtsL, cathepsin L; CPE, carboxypeptidase E; PAM, peptidylglycine-α-amidating monooxygenase.
In summary, results from this study suggest that IH-induced increase in NPY in the AM constitutes one of the major homeostatic mechanisms that are activated in response to IH. This may likely contribute to NE/epinephrine secretion–synthesis coupling and the consequent blood-pressure elevation seen in OSA patients and IH-treated rodents.
Abbreviations Used
- AM
adrenal medulla
- CPE
carboxypeptidase E
- CtsL
cathepsin L
- DBH
dopamine β hydroxylase
- IH
intermittent hypoxia
- MDA
malondialdehyde
- MnTMPyP
manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride
- NAC
N-acetylcysteine
- NE
norepinephrine
- NPY
neuropeptide Y
- PAM
peptidylglycine-α-amidating monooxygenase
- PBA
4-phenyl-3-butenoic acid
- PC1/3
prohormone convertase 1/3
- ROS
reactive oxygen species
Acknowledgments
We thank Brian Kinsman for critical reading of the manuscript. This study was supported by National Heart, Lung, and Blood Institute grants RO1-HL-89616 and PO1-HL-90554.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Allahdadi KJ. Cherng TW. Pai H. Silva AQ. Walker BR. Nelin LD. Kanagy NL. Endothelin type A receptor antagonist normalizes blood pressure in rats exposed to eucapnic intermittent hypoxia. Am J Physiol Heart Circ Physiol. 2008;295:H434–H440. doi: 10.1152/ajpheart.91477.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson G. Påhlman S. Parrow V. Johansson I. Hammerling U. Activation of the human NPY gene during neuroblastoma cell differentiation: induced transcriptional activities of AP-1 and AP-2. Cell Growth Differ. 1994;5:27–36. [PubMed] [Google Scholar]
- 3.Aunis D. Langley K. Physiological aspects of exocytosis in chromaffin cells of the adrenal medulla. Acta Physiol Scand. 1999;167:89–97. doi: 10.1046/j.1365-201x.1999.00580.x. [DOI] [PubMed] [Google Scholar]
- 4.Bao G. Metreveli N. Li R. Taylor A. Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol. 1997;83:95–101. doi: 10.1152/jappl.1997.83.1.95. [DOI] [PubMed] [Google Scholar]
- 5.Barceló A. Miralles C. Barbé F. Vila M. Pons S. Agustí AG. Abnormal lipid peroxidation in patients with sleep apnoea. Eur Respir J. 2000;16:644–647. doi: 10.1034/j.1399-3003.2000.16d13.x. [DOI] [PubMed] [Google Scholar]
- 6.Barceló A. Barbé F. Llompart E. de la Peña M. Durán-Cantolla J. Ladaria A. Bosch M. Guerra L. Agustí AG. Neuropeptide Y and leptin in patients with obstructive sleep apnea syndrome: role of obesity. Am J Respir Crit Care Med. 2005;171:183–187. doi: 10.1164/rccm.200405-579OC. [DOI] [PubMed] [Google Scholar]
- 7.Brakch N. Rist B. Beck-Sickinger AG. Goenaga J. Wittek R. Bürger E. Brunner HR. Grouzmann E. Role of prohormone convertases in pro-neuropeptide Y processing: coexpression and in vitro kinetic investigations. Biochemistry. 1997;36:16309–16320. doi: 10.1021/bi9714767. [DOI] [PubMed] [Google Scholar]
- 8.Bratel T. Wennlund A. Carlstrom K. Pituitary reactivity, androgens and catecholamines in obstructive sleep apnoea: effects of continuous positive airway pressure treatment (CPAP) Respir Med. 1999;93:1–7. doi: 10.1016/s0954-6111(99)90068-9. [DOI] [PubMed] [Google Scholar]
- 9.Bunag RD. Butterfield J. Tail-cuff blood pressure measurement without external preheating in awake rats. Hypertension. 1982;4:898–903. doi: 10.1161/01.hyp.4.6.898. [DOI] [PubMed] [Google Scholar]
- 10.Carlson JT. Hedner J. Elam M. Ejnell H. Sellgren J. Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–1768. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- 11.Cistulli PA. Sullivan CE. In: Pathophysiology of sleep apnea. In Sleep and Breathing. Saunders NA, editor; Sullivan CE, editor. New York: Dekker; 1994. p. 405. [Google Scholar]
- 12.Corder R. Castagné V. Rivet JM. Mormède P. Gaillard RC. Central and peripheral effects of repeated stress and high NaCl diet on neuropeptide Y. Physiol Behav. 1992;52:205–210. doi: 10.1016/0031-9384(92)90259-5. [DOI] [PubMed] [Google Scholar]
- 13.Dimsdale JE. Coy T. Ziegler MG. Ancoli-Israel S. Clausen J. The effect of sleep apnea on plasma and urinary catecholamines. Sleep. 1995;18:377–381. [PubMed] [Google Scholar]
- 14.Eipper BA. Mains RE. Peptide alpha-amidation. Annu Rev Physiol. 1988;50:333–344. doi: 10.1146/annurev.ph.50.030188.002001. [DOI] [PubMed] [Google Scholar]
- 15.Eipper BA. Green CB. Campbell TA. Stoffers DA. Keutmann HT. Mains RE. Ouafik L. Alternative splicing and endoproteolytic processing generate tissue-specific forms of pituitary peptidylglycine alpha-amidating monooxygenase (PAM) J Biol Chem. 1992;267:4008–4015. [PubMed] [Google Scholar]
- 16.Fletcher EC. Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep. 2003;26:15–19. doi: 10.1093/sleep/26.1.15. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher EC. Bao G. Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension. 1991;34:309–314. doi: 10.1161/01.hyp.34.2.309. [DOI] [PubMed] [Google Scholar]
- 18.Fricker LD. Berman YL. Leiter EH. Devi LA. Carboxypeptidase E activity is deficient in mice with the fat mutation: effect on peptide processing. J Biol Chem. 1996;271:30619–30624. doi: 10.1074/jbc.271.48.30619. [DOI] [PubMed] [Google Scholar]
- 19.Funkelstein L. Toneff T. Hwang SR. Reinheckel T. Peters C. Hook V. Cathepsin L participates in the production of neuropeptide Y in secretory vesicles, demonstrated by protease gene knockout and expression. J Neurochem. 2008;106:384–391. doi: 10.1111/j.1471-4159.2008.05408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han S. Chen X. Yang CL. Vickery L. Wu Y. Naes L. Macarthur H. Westfall TC. Influence of cold stress on neuropeptide Y and sympathetic neurotransmission. Peptides. 2005;26:2603–2609. doi: 10.1016/j.peptides.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Hänze J. Kummer W. Haass M. Lang RE. Effect of catecholamine depletion and denervation on neuropeptide Y (NPY) and tyrosine-hydroxylase (TH) mRNA levels in rat sympathetic ganglia. Exp Clin Endocrinol. 1994;102:54–59. doi: 10.1055/s-0029-1211266. [DOI] [PubMed] [Google Scholar]
- 22.Hedner J. Darpo B. Ejnell H. Carlson J. Caidahl K. Reduction in sympathetic activity after long-term CPAP treatment in sleep apnoea: cardiovascular implications. Eur Respir J. 1995;8:222–229. doi: 10.1183/09031936.95.08020222. [DOI] [PubMed] [Google Scholar]
- 23.Heilig H. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Heitmann J. Ehlenz K. Penzel T. Becker HF. Grote L. Voigt KH. Hermann Peter J. Vogelmeier C. Sympathetic activity is reduced by nCPAP in hypertensive obstructive sleep apnoea patients. Eur Respir J. 2004;23:255–262. doi: 10.1183/09031936.04.00015604. [DOI] [PubMed] [Google Scholar]
- 25.Higuchi H. Yang HY. Sabol SL. Rat neuropeptide Y precursor gene expression: mRNA structure, tissue distribution, and regulation by glucocorticoids, cyclic AMP, and phorbol ester. J Biol Chem. 1988;263:6288–6295. [PubMed] [Google Scholar]
- 26.Hiremagalur B. Kvetñanký R. Nankova B. Fleischer J. Geertman R. Fukuhara K. Viskupic E. Sabban EL. Stress elicits trans-synaptic activation of adrenal neuropeptide Y gene expression. Mol Brain Res. 1994;27:138–144. doi: 10.1016/0169-328x(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 27.Itoi K. Mouri T. Takahashi K. Sasaki S. Imai Y. Yoshinaga K. Synergistic pressor action of neuropeptide Y and norepinephrine in conscious rats. J Hypertens Suppl. 1986;4:S247–S250. [PubMed] [Google Scholar]
- 28.Jones BN. Tamburini PP. Consalvo AP. Young SD. Lovato SI. Gilligan JP. Jeng AY. Wennogle LP. A fluorometric assay for peptidyl alpha-amidation activity using high-performance liquid chromatography. Anal Biochem. 1988;168:272–279. doi: 10.1016/0003-2697(88)90318-1. [DOI] [PubMed] [Google Scholar]
- 29.Jørgensen JC. Interaction between norepinephrine, NPY and VIP in the ovarian artery. Peptides. 1991;12:831–837. doi: 10.1016/0196-9781(91)90142-c. [DOI] [PubMed] [Google Scholar]
- 30.Jun J. Savransky V. Nanayakkara A. Bevans S. Li J. Smith PL. Polotsky VY. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1274–R1281. doi: 10.1152/ajpregu.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy B. Shen GH. Ziegler MG. Neuropeptide Y-mediated pressor responses following high-frequency stimulation of the rat sympathetic nervous system. J Pharmacol Exp Ther. 1997;281:291–296. [PubMed] [Google Scholar]
- 32.Khan SA. Nanduri J. Yuan G. Kinsman B. Kumar GK. Joseph J. Kalyanaraman B. Prabhakar NR. NADPH oxidase 2 mediates intermittent hypoxia-induced mitochondrial complex I inhibition: relevance to blood pressure changes in rats. Antioxid Redox Signal. 2011;14:533–542. doi: 10.1089/ars.2010.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kline DD. Peng YJ. Manalo DJ. Semenza GL. Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. Proc Natl Acad Sci U S A. 2002;99:821–826. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar GK. Rai V. Sharma SD. Ramakrishnan DP. Peng YJ. Souvannakitti D. Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larhammar D. Ericsson A. Persson H. Structure expression of the rat neuropeptide Y gene. Proc Natl Acad Sci U S A. 1987;84:2068–2072. doi: 10.1073/pnas.84.7.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SN. Prodhomme E. Lindberg I. Prohormone convertase 1 (PC1) processing and sorting: effect of PC1 propeptide and proSAAS. J Endocrinol. 2004;182:353–364. doi: 10.1677/joe.0.1820353. [DOI] [PubMed] [Google Scholar]
- 37.Leuenberger UA. Brubaker D. Quraishi S. Hogeman CS. Imadojemu VA. Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci. 2005;121:87–93. doi: 10.1016/j.autneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Levenson CW. Moore JB. Response of rat adrenal neuropeptide Y and tyrosine hydroxylase mRNA to acute stress and enhance by long-term voluntary exercise. Neurosci Lett. 1998;242:177–179. doi: 10.1016/s0304-3940(98)00064-0. [DOI] [PubMed] [Google Scholar]
- 39.Li NF. Yao XG. Zhu J. Yang J. Liu KJ. Wang YC. Wang XL. Zu FY. Higher levels of plasma TNF-alpha and neuropeptide Y in hypertensive patients with obstructive sleep apnea syndrome. Clin Exp Hypertens. 2010;32:54–60. doi: 10.3109/10641960902993087. [DOI] [PubMed] [Google Scholar]
- 40.Lundberg JM. Terenius L. Hökfelt T. Goldstein M. High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci Lett. 1983;42:167–172. doi: 10.1016/0304-3940(83)90401-9. [DOI] [PubMed] [Google Scholar]
- 41.Maubert E. Tramu G. Croix D. Beauvillain JC. Dupouy JP. Co-localization of vasoactive intestinal polypeptide and neuropeptide Y immunoreactivities in the nerve fibers of the rat adrenal gland. Neurosci Lett. 1990;113:121–126. doi: 10.1016/0304-3940(90)90290-p. [DOI] [PubMed] [Google Scholar]
- 42.Mills PJ. Kennedy BP. Loredo JS. Dimsdale JE. Ziegler MG. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol. 2006;100:343–348. doi: 10.1152/japplphysiol.00494.2005. [DOI] [PubMed] [Google Scholar]
- 43.Minth CD. Dixon JE. Expression of the human neuropeptide Y gene. J Biol Chem. 1990;265:12933–12939. [PubMed] [Google Scholar]
- 44.Mormède P. Castagné V. Rivet JM. Gaillard R. Corder R. Involvement of neuropeptide Y in neuroendocrine stress responses: central and peripheral studies. J Neural Transm Suppl. 1990;29:65–75. doi: 10.1007/978-3-7091-9050-0_8. [DOI] [PubMed] [Google Scholar]
- 45.Mueller GP. Driscoll WJ. Eipper BA. In vivo inhibition of peptidylglycine-alpha-hydroxylating monooxygenase by 4-phenyl-3-butenoic acid. J Pharmacol Exp Ther. 1999;290:1331–1336. [PubMed] [Google Scholar]
- 46.Murthy AS. Mains RE. Eipper BA. Purification and characterization of peptidylglycine alpha-amidating monooxygenase from bovine neurointermediate pituitary. J Biol Chem. 1986;261:1815–1822. [PubMed] [Google Scholar]
- 47.Nankova B. Kvetnansky R. Hiremagalur B. Sabban B. Rusnak M. Sabban EL. Immobilization stress elevates gene expression for catecholamine biosynthetic enzymes and some neuropeptides in rat sympathetic ganglia: effects of adrenocorticotropin and glucocorticoids. Endocrinology. 1996;137:5597–5604. doi: 10.1210/endo.137.12.8940389. [DOI] [PubMed] [Google Scholar]
- 48.Nieto FJ. Young TB. Lind BK. Shahar E. Samet JM. Redline S. D'Agostino RB. Newman AB. Lebowitz MD. Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 49.Oldham CD. Li C. Feng J. Scott RO. Wang WZ. Moore AB. Girard PR. Huang J. Caldwell RB. Caldwell RW. May SW. Amidative peptide processing and vascular function. Am J Physiol. 1997;273:C1908–C1914. doi: 10.1152/ajpcell.1997.273.6.C1908. [DOI] [PubMed] [Google Scholar]
- 50.Paquet L. Massie B. Mains RE. Proneuropeptide Y processing in large dense-core vesicles: manipulation of prohormone convertase expression in sympathetic neurons using adenoviruses. J Neurosci. 1996;16:964–973. doi: 10.1523/JNEUROSCI.16-03-00964.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng YJ. Overholt JL. Kline D. Kumar GK. Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng YJ. Yuan G. Ramakrishnan D. Sharma SD. Bosch-Marce M. Kumar GK. Semenza GL. Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng YJ. Nanduri J. Yuan G. Wang N. Deneris E. Pendyala S. Natarajan V. Kumar GK. Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci. 2009;29:4903–4910. doi: 10.1523/JNEUROSCI.4768-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Periyasamy SM. Liu J. Tanta F. Kabak B. Wakefield B. Malhotra D. Kennedy DJ. Nadoor A. Fedorova OV. Gunning W. Xie Z. Bagrov AY. Shapiro JI. Salt loading induces redistribution of the plasmalemmal Na/K-ATPase in proximal tubule cells. Kidney Int. 2005;67:1868–1877. doi: 10.1111/j.1523-1755.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 55.Prabhakar NR. Kumar GK. Nanduri J. Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1397–1403. doi: 10.1089/ars.2007.1732. [DOI] [PubMed] [Google Scholar]
- 56.Rabah N. Gauthier D. Dikeakos JD. Reudelhuber TL. Lazure C. The C-terminal region of the proprotein convertase 1/3 (PC1/3) exerts a bimodal regulation of the enzyme activity in vitro. FEBS J. 2007;274:3482–3491. doi: 10.1111/j.1742-4658.2007.05883.x. [DOI] [PubMed] [Google Scholar]
- 57.Raghuraman G. Rai V. Peng YJ. Prabhakar NR. Kumar GK. Pattern-specific sustained activation of tyrosine hydroxylase by intermittent hypoxia: role of reactive oxygen species-dependent downregulation of protein phosphatase 2A and upregulation of protein kinases. Antioxid Redox Signal. 2009;11:1777–1789. doi: 10.1089/ars.2008.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramanathan L. Gozal D. Siegel JM. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J Neurochem. 2005;93:47–52. doi: 10.1111/j.1471-4159.2004.02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhodes CH. Honsinger C. Structure-activity relationships among inhibitors of peptidylglycine amidating monooxygenase. Ann N Y Acad Sci. 1993;22:663–666. doi: 10.1111/j.1749-6632.1993.tb55622.x. , 689, [DOI] [PubMed] [Google Scholar]
- 60.Rosmaninho-Salgado J. Araújo IM. Alvaro AR. Duarte EP. Cavadas C. Intracellular signaling mechanisms mediating catecholamine release upon activation of NPY Y1 receptors in mouse chromaffin cells. J Neurochem. 2007;103:896–903. doi: 10.1111/j.1471-4159.2007.04899.x. [DOI] [PubMed] [Google Scholar]
- 61.Rosmaninho-Salgado J. Araújo IM. Alvaro AR. Mendes AF. Ferreira L. Grouzmann E. Mota A. Duarte EP. Cavadas C. Regulation of catecholamine release and tyrosine hydroxylase in human adrenal chromaffin cells by interleukin-1beta: role of neuropeptide Y and nitric oxide. J Neurochem. 2009;109:911–922. doi: 10.1111/j.1471-4159.2009.06023.x. [DOI] [PubMed] [Google Scholar]
- 62.Schalling M. Dagerlind A. Brene S. Hallman H. Djurfeld M. Persson H. Terenius L. Golstein M. Schlesinger D. Hokfelt T. Coexistence and gene expression of phenylethanolamine N-methyltransferase, tyrosine hydroxylase and neuropeptide Y in the rat and bovine adrenal gland: effect of reserpine. Proc Natl Acad Sci U S A. 1988;85:8306–8310. doi: 10.1073/pnas.85.21.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schalling M. Franc-Cereceda A. Hemsen A. Dagerling A. Seroogy K. Persson H. Hokfelt T. Lundberg JM. Neuropeptide Y and catecholamines synthesizing enzymes and their mRNAs in rat sympathetic neurons and adrenal gland: studies on expression, synthesis and axonal transport after pharmacological and experimental manipulations using hybridisation techniques and radioimmunoassay. Neuroscience. 1991;41:753–766. doi: 10.1016/0306-4522(91)90365-u. [DOI] [PubMed] [Google Scholar]
- 64.Seidah NG. Mayer G. Zaid A. Rousselet E. Nassoury N. Poirier S. Essalmani R. Prat A. The activation and physiological functions of the proprotein convertases. Int J Biochem Cell Biol. 2008;40:1111–1125. doi: 10.1016/j.biocel.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 65.Sharma SD. Raghuraman G. Lee MS. Prabhakar NR. Kumar GK. Intermittent hypoxia activates peptidylglycine {alpha}-amidating monooxygenase in rat brainstem via reactive oxygen species-mediated proteolytic processing. J Appl Physiol. 2009;106:12–19. doi: 10.1152/japplphysiol.90702.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Souvannakitti D. Kumar GK. Fox A. Prabhakar NR. Neonatal intermittent hypoxia leads to long-lasting facilitation of acute hypoxia-evoked catecholamine secretion from rat chromaffin cells. J Neurophysiol. 2009;101:2837–2846. doi: 10.1152/jn.00036.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Souvannakitti D. Kuri B. Yuan G. Pawar A. Kumar GK. Smith C. Fox AP. Prabhakar NR. Neonatal intermittent hypoxia impairs neuronal nicotinic receptor expression and function in adrenal chromaffin cells. Am J Physiol Cell Physiol. 2010;299:C381–C388. doi: 10.1152/ajpcell.00530.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki YJ. Jain V. Park AM. Day RM. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med. 2006;40:1683–1692. doi: 10.1016/j.freeradbiomed.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tatemoto K. Carlquist M. Mutt V. Neuropeptide Y: a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 70.Veasey SC. Davis CW. Fenik P. Zhan G. Hsu YJ. Pratico D. Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- 71.Walker P. Grouzmann E. Burnier M. Waeber B. The role of neuropeptide Y in cardiovascular regulation. Trends Pharmacol Sci. 1991;12:111–115. doi: 10.1016/0165-6147(91)90518-w. [DOI] [PubMed] [Google Scholar]
- 72.Westfall TC. Neuropeptide Y, and sympathetic control of vascular tone in hypertension. EXS. 2006;95:89–103. doi: 10.1007/3-7643-7417-9_6. [DOI] [PubMed] [Google Scholar]
- 73.Young T. Palta M. Dempsey J. Skatrud S. Weber S. Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 74.Yuan G. Adhikary G. McCormick AA. Holcroft JJ. Kumar GK. Prabhakar NR. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J Physiol. 2004;557:773–783. doi: 10.1113/jphysiol.2003.058503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang LP. Wang L. Changes of brain neuropeptide Y and its receptors in rats with flurazepam tolerance and dependence. Acta Pharmacol Sin. 2005;26:1290–1296. doi: 10.1111/j.1745-7254.2005.00179.x. [DOI] [PubMed] [Google Scholar]
- 76.Zhan G. Serrano F. Fenik P. Hsu R. Kong L. Pratico D. Klann E. Veasey SC. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med. 2005;172:921–929. doi: 10.1164/rccm.200504-581OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ziegler MG. Mills PJ. Loredo JS. Ancoli-Israel S. Dimsdale JE. Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest. 2001;120:887–893. doi: 10.1378/chest.120.3.887. [DOI] [PubMed] [Google Scholar]
- 78.Zoccal DB. Bonagamba LG. Antunes-Rodrigues J. Machado BH. Plasma corticosterone level is elevated in rats submitted to chronic intermittent hypoxia. Auton Neurosci. 2007;134:115–117. doi: 10.1016/j.autneu.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Zukowska-Grojec Z. Neuropeptide Y: a novel sympathetic stress hormone and more. Ann NY Acad Sci. 1995;771:219–233. doi: 10.1111/j.1749-6632.1995.tb44683.x. [DOI] [PubMed] [Google Scholar]