Abstract
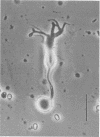
It is well-established morphologically that bipolar cells, the second-order neurons in the vertebrate retina, make reciprocal synapses with amacrine cells in the inner plexiform layer. However, neither the property nor the physiological function of the feedback synapse is understood. Autoradiographic and immunohistochemical studies suggest the presence of gamma-aminobutyric acid (GABA)-ergic amacrine cells, and therefore the bipolar cells are thought to receive GABAergic inputs from amacrine cells. This possibility was investigated in the present study, in which we used solitary bipolar cells dissociated from the goldfish retina enzymatically. Dissociated solitary bipolar cells showed a large variety in morphology. In the present study, we selected the bipolar cells with a huge bulbous axon terminal. Bipolar cells of this subtype were identical in morphology to the on-center cells with rod-dominant inputs as revealed in earlier studies by intracellular staining. Membrane currents were measured under voltage clamp with a patch pipette in the whole cell configuration. In some experiments, GABA-sensitive membrane was excised as an outside-out patch from the axon terminal bulb of solitary bipolar cells. All cells of this type responded to GABA. The highest sensitivity was located at the axon terminal. The minimal effective dose was on the order of 10(-7) M. GABA increased the chloride conductance and evoked a membrane hyperpolarization. Partial desensitization was observed during the application of GABA. The bipolar cells had GABA type A receptors. These results are consistent with the idea that the rod-dominant on-center bipolar cells receive negative feedback inputs from GABAergic amacrine cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayoub G. S., Lam D. M. The release of gamma-aminobutyric acid from horizontal cells of the goldfish (Carassius auratus) retina. J Physiol. 1984 Oct;355:191–214. doi: 10.1113/jphysiol.1984.sp015414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Kaneko A., Tachibana M. Neuronal architecture of on and off pathways to ganglion cells in carp retina. Science. 1977 Dec 23;198(4323):1267–1269. doi: 10.1126/science.73223. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. A voltage-clamp analysis of membrane currents in solitary bipolar cells dissociated from Carassius auratus. J Physiol. 1985 Jan;358:131–152. doi: 10.1113/jphysiol.1985.sp015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Blocking effects of cobalt and related ions on the gamma-aminobutyric acid-induced current in turtle retinal cones. J Physiol. 1986 Apr;373:463–479. doi: 10.1113/jphysiol.1986.sp016058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Effects of gamma-aminobutyric acid on isolated cone photoreceptors of the turtle retina. J Physiol. 1986 Apr;373:443–461. doi: 10.1113/jphysiol.1986.sp016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H., Toyoda J. GABA and glycine effects on the bipolar cells of the carp retina. Vision Res. 1983;23(11):1259–1264. doi: 10.1016/0042-6989(83)90101-3. [DOI] [PubMed] [Google Scholar]
- Kujiraoka T., Saito T. Electrical coupling between bipolar cells in carp retina. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4063–4066. doi: 10.1073/pnas.83.11.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M., Steinman L. The uptake of ( - 3 H) aminobutyric acid in the goldfish retina. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2777–2781. doi: 10.1073/pnas.68.11.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M., Su Y. Y., Swain L., Marc R. E., Brandon C., Wu J. Y. Immunocytochemical localisation of L-glutamic acid decarboxylase in the goldfish retina. Nature. 1979 Apr 5;278(5704):565–567. doi: 10.1038/278565a0. [DOI] [PubMed] [Google Scholar]
- Lam D. M. The biosynthesis and content of gamma-aminobutyric acid in the goldifsh retina. J Cell Biol. 1972 Aug;54(2):225–231. doi: 10.1083/jcb.54.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E., Ripps H. Pharmacological properties of isolated horizontal and bipolar cells from the skate retina. J Neurosci. 1984 Aug;4(8):1966–1975. doi: 10.1523/JNEUROSCI.04-08-01966.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc R. E., Stell W. K., Bok D., Lam D. M. GABA-ergic pathways in the goldfish retina. J Comp Neurol. 1978 Nov 15;182(2):221–244. doi: 10.1002/cne.901820204. [DOI] [PubMed] [Google Scholar]
- Miller A. M., Schwartz E. A. Evidence for the identification of synaptic transmitters released by photoreceptors of the toad retina. J Physiol. 1983 Jan;334:325–349. doi: 10.1113/jphysiol.1983.sp014497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Shimoda Y. Intracellular double staining: localization of recording site in single retinal neurons. Brain Res. 1978 Apr 7;144(1):164–168. doi: 10.1016/0006-8993(78)90444-4. [DOI] [PubMed] [Google Scholar]
- Saito T., Kujiraoka T. Physiological and morphological identification of two types of on-center bipolar cells in the carp retina. J Comp Neurol. 1982 Feb 20;205(2):161–170. doi: 10.1002/cne.902050207. [DOI] [PubMed] [Google Scholar]
- Saito T., Kujiraoka T., Yonaha T., Chino Y. Reexamination of photoreceptor-bipolar connectivity patterns in carp retina: HRP-EM and Golgi-EM studies. J Comp Neurol. 1985 Jun 8;236(2):141–160. doi: 10.1002/cne.902360202. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Calcium-independent release of GABA from isolated horizontal cells of the toad retina. J Physiol. 1982 Feb;323:211–227. doi: 10.1113/jphysiol.1982.sp014069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. Ionic currents of solitary horizontal cells isolated from goldfish retina. J Physiol. 1983 Dec;345:329–351. doi: 10.1113/jphysiol.1983.sp014981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M., Kaneko A. gamma-Aminobutyric acid acts at axon terminals of turtle photoreceptors: difference in sensitivity among cell types. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7961–7964. doi: 10.1073/pnas.81.24.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. Permeability changes induced by L-glutamate in solitary retinal horizontal cells isolated from Carassius auratus. J Physiol. 1985 Jan;358:153–167. doi: 10.1113/jphysiol.1985.sp015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda J., Fujimoto M. Application of transretinal current stimulation for the study of bipolar-amacrine transmission. J Gen Physiol. 1984 Dec;84(6):915–925. doi: 10.1085/jgp.84.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Dowling J. E. Synaptic relationships in the plexiform layers of carp retina. Z Zellforsch Mikrosk Anat. 1969;100(1):60–82. doi: 10.1007/BF00343821. [DOI] [PubMed] [Google Scholar]
- Yazulla S., Kleinschmidt J. Carrier-mediated release of GABA from retinal horizontal cells. Brain Res. 1983 Mar 14;263(1):63–75. doi: 10.1016/0006-8993(83)91201-5. [DOI] [PubMed] [Google Scholar]
- Yazulla S. Stimulation of GABA release from retinal horizontal cells by potassium and acidic amino acid agonists. Brain Res. 1983 Sep 19;275(1):61–74. doi: 10.1016/0006-8993(83)90417-1. [DOI] [PubMed] [Google Scholar]
- Yazulla S., Studholme K., Wu J. Y. Comparative distribution of 3H-GABA uptake and GAD immunoreactivity in goldfish retinal amacrine cells: a double-label analysis. J Comp Neurol. 1986 Feb 8;244(2):149–162. doi: 10.1002/cne.902440203. [DOI] [PubMed] [Google Scholar]
- Zucker C., Yazulla S., Wu J. Y. Non-correspondence of [3H]GABA uptake and GAD localization in goldfish amacrine cells. Brain Res. 1984 Apr 23;298(1):154–158. doi: 10.1016/0006-8993(84)91160-0. [DOI] [PubMed] [Google Scholar]