Abstract
Success in competition for limiting parental resources depends on the interplay between parental decisions over allocation of care and offspring traits. Birth order, individual sex and sex of competing siblings are major candidates as determinants of success in sib–sib competition, but experimental studies focusing on the combined effect of these factors on parent–offspring communication and within-brood competitive dynamics are rare. Here, we assessed individual food intake and body mass gain during feeding trials in barn swallow chicks differing for seniority and sex, and compared the intensity of their acoustic and postural solicitation (begging) displays. Begging intensity and success in competition depended on seniority in combination with individual sex and sex of the opponent. Junior chicks begged more than seniors, independently of satiation level (which was also experimentally manipulated), and obtained greater access to food. Females were generally weaker competitors than males. Individual sex and sex of the opponent also affected duration of begging bouts. Present results thus show that competition with siblings can make the rearing environment variably harsh for developing chicks, depending on individual sex, sex of competing broodmates and age ranking within the nest. They also suggest that parental decisions on the allocation of care and response of kin to signalling siblings may further contribute to the outcome of sibling competition.
Keywords: begging, competitive asymmetry, environmental sensitivity, hatching asynchrony, sexual dimorphism, sibling competition
1. Introduction
Family life, rather than being a harmonious setting in which genetically related individuals cooperate in view of their overlapping evolutionary interests, is a stage for intense conflicts both between parents and offspring and among the offspring [1–5]. Understanding the resolution of these conflicts is crucial not just to model the dynamics of parental investment, but also to explain the evolution of behaviour when the environment contains genes that are shared among the interactors [6–8]. Despite recent progress towards a more dynamic framework of parent–offspring coadaptation [7,8], considerable uncertainty still exists concerning the role of both parental and offspring traits in shaping the resolution of these conflicts [9]. This is largely owing to the fact that a wealth of factors is expected to affect performance in the intra-familial competitive arena [5,10,11] and that the arena itself requires the consequences to be assessed on inclusive fitness [5,10–13].
Individual offspring of either sex can differ in their ability to outcompete siblings and secure parental resources, with the larger sex usually attaining a better performance in scramble interactions [14]. The two sexes can also differ in their sensitivity to rearing conditions [15,16], the larger sex being more vulnerable to food shortage because of higher energy requirements [14] and therefore expected to evolve superior competitive ability in order to prevent deterioration of body condition [17,18]. Older siblings are generally expected to benefit from size-related advantages in scramble competition [14,19–21], and this gap can even be emphasized when younger broodmates are of the weaker sex and/or receive a poorer share of maternal resources via the egg [3,21,22]. In fact, parents can influence the outcome of scrambling among the offspring through both pre- and post-natal strategies of differential resource allocation [14,16,23–25]. Parents can manipulate competitive asymmetries among progeny members by promoting variation in egg quality along the laying sequence [25,26] and/or by modulating hatching asynchrony [16], as is frequently observed in birds. Interestingly, prenatal (maternal) effects have been suggested as a mechanism for coupling offspring food soliciting and parental provisioning [7,23].
Success in competition is therefore expected to depend on the interplay between individual sex, sex of competing siblings and birth order [14]. Surprisingly, studies focusing on the combined effect of offspring sex and birth order on sibling competition and access to parental provisioning are rare [19,21,27]. This is of the utmost importance for understanding the control of parental investment, parent–offspring coadaptation and the evolution of strategies for resource and sex allocation [7–9,23,28,29].
In birds, begging behaviour is a major mediator of scramble competition among broodmates, with differences in individual begging strategies reflecting asymmetry among the offspring in quality and/or competitive ability [3,30]. Theoretical models dually depict begging as a means of scrambling, with parents passively following the outcome of sibling rivalry in their allocation decisions, or as a reliable signal of offspring need or condition (sensu [31]), with parents controlling food allocation according to honest signalling of individual quality by begging offspring [9,12,30,32].
Differential competitive ability according to age, as determined by hatching order [10,19,20,22], can result either from persistence of the effects of asynchronous hatching or through differential maternal transfer of resources over the laying sequence. Asymmetry in need between competitors results in needier chicks probably being more willing to compete for monopolizing food. However, hunger levels being equal, high-quality, larger offspring are expected to prevail over feeble siblings [22]. Accordingly, younger chicks try to compensate for their competitive disadvantage by begging more than their siblings, although their individual strategies may depend on the number, behaviour and size of competitors [12,19,20,22,33], and therefore on their resource-holding potential [33,34]. As individual sex, sex of the opponent(s) and age rank within the brood are all expected to affect individual relative resource-holding potential within the nest, experimental tests where these factors are jointly manipulated are extremely important for understanding the resolution of sib–sib and parent–offspring conflicts.
In the barn swallow, sib–sib competition can be severe and is mediated by vocalizations, gaping and posturing [17,35]. The intensity of begging increases with hunger and hatching order, and parents provide more food to the chicks that beg more intensely [35–37]. Offspring of the two sexes, although similar in size, differ in their sensitivity to the rearing conditions, as well as in their short-term competitive ability. Males prevail over short time periods but are more negatively affected than females by harsh conditions [17,38]. In addition, male and female barn swallow nestlings differ in their begging behaviour [17,39].
Here, we provide a comprehensive experimental test of the relative significance of sex and birth order for sibling rivalry and begging behaviour. To this aim, we compared the acoustic and postural begging of focal pairs of siblings differing for seniority and optionally for sex. In our framework, seniority reflects the effects of both hatching order and variation in egg quality with laying order (see electronic supplementary material, materials and methods). Then, we assessed individual success in competition by estimating food intake and body mass gain during feeding trials. In the barn swallow, parents preferentially follow a brood survival strategy [31] and hatching order negatively affects individual condition [37]. Thus, we predicted that needier, junior chicks should attain higher begging levels and obtain more food than senior siblings, particularly for males, owing to their superior competitive ability in the short term [17]. In addition, we tested whether the effects of sex and seniority on begging behaviour and success in competition varied according to the satiation level of chicks.
2. Methods
(a). General field procedures
The study was performed during spring 2008 in three breeding colonies near Milan (Italy). All nests were checked daily to mark the eggs according to laying order. Around the estimated hatching time, clutches were transferred to a Covatutto 24 Eco incubator (Novital, Italy) and replaced with dummy barn swallow eggs. The incubator was checked every 3 h from 7.00 until 19.00. Hatching order in the incubator closely paralleled laying order (r = 0.882, n = 151, p < 0.001; see also [40]). Hatchlings were individually marked and immediately brought back to their nest; a dummy egg was removed for each chick that was returned. At day 7 (day 0 = day of hatching of the first chick of the brood), we measured chick body mass and tarsus length and collected a blood sample for molecular sexing according to Saino et al. [39].
(b). Feeding trials and begging recordings
Based on sex information obtained at day 7, we identified the pairs of siblings to be used in competition tests on days 13–16. According to laying order and brood size, chicks were classified either as seniors (chicks from egg 1 or 2) or juniors (chicks from the two last-laid eggs in clutches of four to six eggs). In each nest, up to four of the following comparisons were performed: senior male versus junior male (n = 20); senior female versus junior female (n = 22); senior male versus junior female (n = 23); senior female versus junior male (n = 22). The tests confronting these four different ‘seniority by sex’ classes of siblings were performed one per day, starting on day 13, in random sequence. We did not compare between male and female chicks within seniority classes because we had already investigated the effect of sex per se on competition in a previous study [17]. Pairs were tested both before and after a short period of food deprivation [17,35]. On each test day, after temporarily removing the non-focal broodmates, we recorded begging vocalizations of each nestling while alone at the nest during feeding visits of parents (see electronic supplementary material). Recording sessions started in the morning (7.00–8.00 h). After the second chick had been recorded, we assessed the ability of each focal nestling to obtain food while competing with its opponent under normal satiation conditions (trial before food deprivation BFD; hereafter). We weighed both nestlings, individually marked them on the forehead with white markings, and put them back together into their nest for a 1.5 h feeding trial, while simultaneously video recording parental and offspring behaviour. At the end of the trial, nestlings were weighed again to record variation in body mass, reflecting individual food intake. Then, focal nestlings were placed in a cloth bag for a 2 h period of food deprivation and their non-focal siblings were returned to the nest. Food deprivation simulated a short period of starvation, similar to that naturally occurring during spells of bad weather. A second session of audio and video recording was performed after food deprivation (AFD), following the same procedure as in the first trial. Body mass was also measured before and after the second feeding trial (AFD trial). In subsequent analyses, we used the number of feedings received by each chick during each trial to assess the inherent competitive ability of the chicks in terms of number of interactions won. We also used body mass at the end of trials as a proxy of the fitness-related balance between costs and benefits of scrambling, and because our estimates of feeding rates could not account for variation in size of individual feedings.
A mean of 2.81 (1.01 s.d.) comparisons per nest was conducted. Each chick was involved in up to two comparisons (mean: 1.60 (0.49 s.d.)). The inclusion of chick identity in the analyses (see below) statistically accounted for non-independence of data from chicks used in different comparisons.
(c). Analysis of audio and video recordings
Audio recordings were analysed according to Boncoraglio et al. [17] (see electronic supplementary material). Mean bout and syllable duration (s), begging rate (number of syllables per second during begging bouts) and relative amplitude (dB) of begging calls were measured following Boncoraglio et al. [17].
Video recordings were analysed with movie editing software (Vegas Pro 9, Sony Creative Software). We randomly selected three feeding visits per trial (see electronic supplementary material and Boncoraglio et al. [35] for further details), and measured the maximum begging intensity reached by each chick during each visit on a four-level scale varying from zero (chick not begging) to three (chick standing on its tarsi and begging with fully stretched neck towards the attending parent). Postural scores of each chick were averaged within trial. The number of feedings obtained by each nestling over the whole trial was also measured. All measures were performed blindly with respect to treatments.
(d). Statistical analyses
Our main aim was to test for the independent and combined effects of sex and seniority on begging behaviour and access to food. Data from BFD were therefore first analysed separately from those from AFD trials, using linear mixed models. Chick and focal pair identity, and nest of origin together with its interactions with all fixed factors and covariates, were entered as random factors [17,35]. We analysed the effect of seniority, sex, sex of the opponent (fixed factors) and their two-way interactions on feeding rates, final body mass and begging features. For each dependent variable, we compared the Akaike's information cirterion for small samples (AICc) values of all models that could be built by linear combination of the main factors and their two-way interactions, and selected the model with the lowest AICc value (‘best model’ hereafter). All other models for which AICc values did not differ for more than two units from the best model were considered as equally explicative [41]. Except for one case (see below), these alternative models never differed from the best model for any significant effect.
To test for differential effects of satiation level on begging behaviour and access to food depending on the concomitant effects of chick sex and seniority, we ran for each variable an additional linear mixed model on the whole data sample. In these analyses, we included those terms that were significant in BFD and/or AFD best models, a two-level factor accounting for satiation level (BFD or AFD), and all two- and three-way interactions involving food deprivation that could be predicted based on the differences in significance of the terms included in BFD and AFD best models (see electronic supplementary material). All the main effects and the two-way interactions that were needed to properly test for three-way interactions were also included in these models.
Throughout the manuscript, we report mean values of the variables of interest, together with their associated standard error (s.e.) in parentheses.
3. Results
(a). Access to food
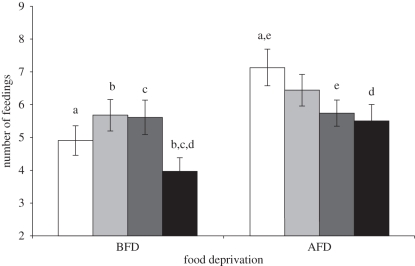
Junior chicks received more feedings than seniors in AFD trials (seniority: F1,26.2 = 7.72, p = 0.010; figure 1), while in BFD trials the effect of seniority depended on individual sex (seniority × sex: F1,120 = 6.39, p = 0.013). Senior females received less food compared with senior males (t199 = 2.28, p = 0.025) and junior females (t83.9 = −2.92, p = 0.005; figure 1). Thus, juniors obtained more feedings than seniors, and females were weaker competitors than males. In the whole sample of BFD and AFD trials, the three-way interaction between food deprivation, seniority and sex was highly significant (F1,207 = 7.18, p = 0.008; figure 1).
Figure 1.
Mean (±s.e.) number of feedings received by each nestling in AFD and BFD trials according to individual sex and seniority. Significant differences in seniority × sex × feeding trial at post hoc tests are indicated by the same letter. White bars, junior males; light grey bars, junior females; dark grey bars, senior males; black bars, senior females.
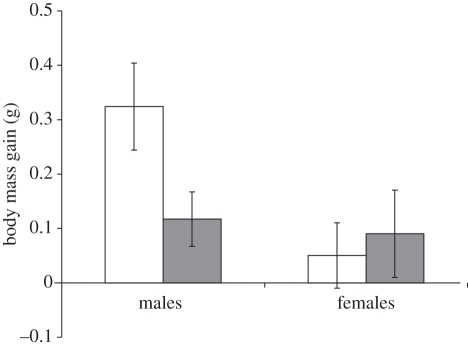
The best models of final body mass for BFD or AFD trials only included initial body mass (always p < 0.001) and seniority (BFD: F1,22.9 = 4.45, p = 0.046; AFD: F1,85.1 = 4.89, p = 0.030). Juniors gained more mass than seniors both BFD and AFD (BFD: mean body mass gain: senior, 0.08 (0.05) g; junior, 0.20 (0.05) g; AFD: senior, 0.27 (0.04) g; junior, 0.38 (0.04) g). However, two out of five equally informative models of BFD trials (see electronic supplementary material, statistical analyses) showed also that sex significantly predicted final body mass depending on the sex of the opponent (p < 0.026 in both cases). In both models, males gained more mass than females when competing with a male (p < 0.008) and males competing with a male gained more than those competing with a female (p < 0.038), irrespective of seniority (figure 2). While confirming the importance of seniority and sex, the analyses on individual mass gain thus demonstrated that access to food depended also on sex of the opponent. However, since the effect of the interaction between individual sex and sex of the opponent differed according to food deprivation, we also ran a model where we included the effect of the three-way interaction between these factors. This interaction was non-significant (F1,103 = 0.83, p = 0.36).
Figure 2.
Mean (±s.e.) body mass gain in BFD trials for male and female chicks depending on sex of the opponent. White bars, male opponent; grey bars, female opponent.
(b). Begging call features
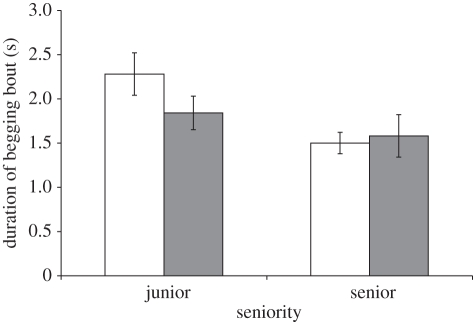
During BFD trials junior chicks uttered longer begging bouts than seniors (table 1), while in AFD trials the effect of seniority depended on both individual sex and sex of the opponent (table 1 and figure 3). Bout length was greater in junior compared with senior males (t69.4 = 3.32, p = 0.001), while it did not differ between junior and senior females (t69.4 = 0.49, p = 0.623; figure 3). In addition, junior chicks uttered longer bouts when confronted with a female compared with a male sibling (t57.9 = 3.61, p = 0.001; female opponent: 2.31 (0.24) s; male opponent: 1.90 (0.24) s). The differential effects of seniority according to individual sex did not depend on satiation level (seniority × sex × food deprivation: F1,183 = 0.07, p = 0.785). Thus, juniors begged more, particularly among male chicks and when confronted with a female sibling.
Table 1.
Best linear mixed models of duration of begging bouts in BFD and AFD trials with respect to seniority, individual sex, sex of the opponent (AFD trials only) and their interactions.
| z | F | d.f. | p | |
|---|---|---|---|---|
| before food deprivation (BFD) | ||||
| nest | 0.81 | 0.208 | ||
| seniority | 8.62 | 1, 40.0 | 0.006 | |
| sex | 2.17 | 1, 25.7 | 0.153 | |
| seniority × sex | 1.67 | 1, 55.2 | 0.202 | |
| after food deprivation (AFD) | ||||
| nest | 2.51 | 0.006 | ||
| seniority | 7.61 | 1, 42.9 | 0.009 | |
| sex | 0.03 | 1, 72.3 | 0.868 | |
| opponent's sex | 0 | 1, 49.2 | 0.974 | |
| seniority × sex | 3.97 | 1, 74.5 | 0.050 | |
| seniority × opponent's sex | 5.26 | 1, 45.9 | 0.026 | |
Figure 3.
Mean (±s.e.) duration of begging bouts in BFD trials for junior and senior chicks of either sex. White bars, males; grey bars, females.
In AFD trials chicks uttered louder calls when they had been exposed to a female (−14.51 (0.35) dB) compared with a male opponent (−15.85 (0.36) dB; F1,161 = 7.90, p = 0.006) in the BFD trial, in a model controlling for sex and seniority. This small difference, however, might be biologically meaningless.
Begging rate was higher in female chicks, independent of other factors (BFD: F1,28.6 = 5.06, p = 0.032; AFD: F1,108 = 8.23, p = 0.005). This effect was due to males uttering longer syllables than females for a given length of begging bout (cumulative analysis on BFD and AFD trials: F1,125 = 8.02, p = 0.005; males: 0.143 (0.006) s; females: 0.126 (0.006) s), consistent with previous studies [17].
Finally, postural begging was affected by seniority in BFD trials, with juniors begging more intensely than seniors (F1,40.9 = 4.52, p = 0.040; junior chicks: 1.86 (0.06); senior chicks: 1.62 (0.08)), while this was not the case in AFD trials. However, the effect of seniority did not depend on food deprivation in the overall sample of tests (F1,54.8 = 1.75, p = 0.192).
4. Discussion
In this experiment, we found that junior chicks obtained greater access to food than their senior siblings, as reflected by both feeding rate and body mass at the end of feeding trials. Access to food during the trial also depended on individual sex and sex of the opponent, with females generally being weaker competitors than males. Consistently, junior chicks were found to generally beg more intensely than seniors, independent of satiation level.
From an evolutionary point of view, begging can be interpreted either as a reliable indicator of inherent competitiveness of the chicks or as a signal of need [9,12]. The ‘honesty’ of begging is supported by experimental evidence that begging increases with hunger (e.g. [42,43]). However, evidence that begging intensity positively predicts parental provisioning is consistent with both interpretations of begging [9]. Similarly, both models predict begging to be costly [42]. Rather, the two models could be distinguished by the nature of the signal, as begging behaviour is always assumed to be honest under honest signalling, whereas this is not necessarily the case under scramble competition [9]. Interestingly, however, the level of honesty and the accuracy of the begging signal are thought to strongly depend on the context, as shaped by resource availability and the age and sex composition of the brood [9].
If begging reflects individual capability in scramble interactions, our finding that begging by juniors is more intense implies that they outcompete senior siblings. A possible explanation would then be maternal favouritism in allocation of resources to the last eggs. In the barn swallow, egg size increases along the laying sequence because of an allometric increase in the amount of protein-rich albumen, this pattern being interpreted as evidence that the parents privilege the last-hatched chicks by providing resources that are fundamental for skeletal growth [40]. Although previous studies of this species have found no variation in yolk testosterone content along the laying sequence [44], maternal favouritism towards younger chicks could also unfold via the uneven allocation of other compounds (e.g. [45,46]).
On the other hand, if begging is a reliable signal of need, last-hatched, needier chicks are expected to beg more intensely because of the occurrence of carry-over effects of hatching asynchrony. Higher begging levels would be afforded by junior offspring because of the higher potential gains [9]. Needier chicks could then be favoured by parents because of the higher marginal return from investing in disadvantaged offspring for a given effort level, and/or by better-fed, larger siblings adopting altruistic strategies in order to facilitate survival of kin. Preliminary analyses on chick mass and condition at day 12 indicated that junior chicks were in poorer condition than seniors, with seniors being around 5 per cent heavier when correcting for tarsus length at day 12 (A. Bonisoli-Alquati, G. Boncoraglio & N. Saino 2008, unpublished data). Adult barn swallows are known to adopt a brood survival strategy (sensu [31,38,47]), which implies that parents at least partly compensate for the disadvantage of junior chicks arising from asynchronous hatching. In addition, senior chicks in relatively good physiological state may refrain from monopolizing food items that would add a low marginal return for them if these could prove valuable for needier siblings, as predicted by kin selection theory [13,35]. Indeed, senior chicks have repeatedly been found to invest less in begging than junior chicks, when experiencing a competitive advantage (e.g. [20]). The conditions for interpreting begging as a signal of need are met, and our findings thus suggest that parents and older siblings might both favour juniors. On the other side, the finding that junior chicks, even with a better access to food in the short term, did not attain similar size to their senior siblings might indicate that their begging signalling comes at a greater cost than for siblings in better condition, consistent with evolutionarily stable strategy (ESS) models of animal communication [48,49].
Individual sex is a further layer of complexity in this framework. Male and female barn swallow chicks differ in their susceptibility to environmental conditions (e.g. [36]). Although sexual size dimorphism is small, differences in need and sensitivity to rearing conditions might arise from other factors (e.g. sex-specific androgen levels; [16,50]). Interestingly, the effects of sex mainly depended on seniority. Access to food, as indexed by the number of feedings received during the trial, was greater for males than for females, at least among seniors. This effect could be due to senior males being stronger competitors than senior females, but also to parents delivering more food to the nest when attending sons rather than daughters, because of generally higher begging levels by males.
Present results show that exposure to competitors of a particular sex makes the rearing environment variably harsh to the chicks, depending on individual sex and age ranking among broodmates. It has been shown that seniority and the sex ratio of siblings both affect the condition of barn swallow chicks around fledging [19,37,38]. More studies are required to validate further predictions about the effect of the covariation between sex and seniority on chick phenotype. Although the isolation of two competing chick is a common treatment in both theoretical studies (e.g. [12,51]; but see [10]) and empirical studies (e.g. [13,35]), our results should be cautiously interpreted when extrapolating to the overall dynamics of sibling rivalry, which operate on a longer time scale and with a larger number of individuals involved.
Confronting a strong competitor might have positive consequences for individual fitness if parents respond to escalating begging levels by increasing food provisioning. Here, we found that, independent of seniority, chicks from focal pairs including two males, which were likely to attain the highest signalling level of any combination of chicks, gained more mass than chicks in focal pairs including a female. The effect of the opponent's sex on acoustic begging in AFD trials is also consistent with this finding. Exposure to a female in BFD trials resulted in longer bout duration (although in juniors only). We therefore speculate that the poorer feeding effort by parents to focal pairs including females was owing to their lower signalling level compared with all-male pairs, leading to reduced satiation level of the chicks at the end of BFD trials. This may have prompted the chicks that were previously exposed to a female competitor to reach higher begging intensity in AFD trials. Overall begging intensity might be the signal to which parents are responding [52,53]. This scenario is also consistent with previous findings that a male-biased brood is beneficial for all offspring under harsh rearing conditions [21,38], and suggests offspring control over food allocation. This result has important implications for parent–offspring coadaptation dynamics [7,9,23,29]. Indeed, offspring control of provisioning has recently been shown to predict parental control on the evolution of prenatal effects, implying that selection on parents drives the coadaptation of parental and offspring traits [7,23]. The rise in parental provisioning rate can also be an indication of cooperative begging within the brood, a subject of increasing interest for both theoretical [51] and experimental studies [33,54].
Our results also confirmed that male and female nestlings have distinct begging features [17,39]. This discloses the possibility of parental and sibling favouritism, as both parents and siblings might be able to discriminate between offspring of the two sexes. In this respect, we cannot conclude whether parents and siblings respond after actively assessing chicks' need by means of their signalling level and/or their sex-specific features, or whether they are both passively accepting the outcome of sibling competition within the nest, which is in turn affected by sex-related features.
Acknowledgements
This study was performed in compliance with Italian laws on animal research. We thankfully acknowledge the help of E. Bonati, D. Cagnetti, A. Gerevini, A. Matteo and V. Pignataro in conducting fieldwork. We thank N. Bennett, A. Cockburn and three anonymous reviewers for valuable comments on an earlier draft of the manuscript. A.B.-A. was funded by a PhD grant from the Italian Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR). G.B. was funded by a grant from the University of Milan and by a Marie Curie Intra-European Fellowship (PIEF-GA-2009-252120).
References
- 1.Trivers R. L. 1974. Parent–offspring conflict. Am. Zool. 14, 249–264 [Google Scholar]
- 2.Macnair M. R., Parker G. A. 1979. Models of parent–offspring conflict. III. Intra-brood conflict. Anim. Behav. 27, 1202–1209 10.1016/0003-3472(79)90067-8 (doi:10.1016/0003-3472(79)90067-8) [DOI] [Google Scholar]
- 3.Mock D. W., Parker G. A. 1997. The evolution of sibling rivalry. New York, NY: Oxford University Press [Google Scholar]
- 4.Parker G. A., Royle N. J., Hartley I. R. 2002. Intrafamilial conflict and parental investment: a synthesis. Phil. Trans. R. Soc. Lond. B 357, 295–307 10.1098/rstb.2001.0950 (doi:10.1098/rstb.2001.0950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Royle N. J., Hartley I. R., Parker G. A. 2004. Parental investment and family dynamics: interactions between theory and empirical tests. Popul. Ecol. 46, 231–241 [Google Scholar]
- 6.Wolf J. B., Brodie E. D. 1998. The coadaptation of parental and offspring characters. Evolution 52, 299–308 10.2307/2411068 (doi:10.2307/2411068) [DOI] [PubMed] [Google Scholar]
- 7.Hinde C. A., Johnstone R. A., Kilner R. M. 2010. Parent offspring conflict and coadaptation. Science 327, 1373–1376 10.1126/science.1186056 (doi:10.1126/science.1186056) [DOI] [PubMed] [Google Scholar]
- 8.Kölliker M., Ridenhour B. J., Gaba S. 2010. Antagonistic parent–offspring co-adaptation. PLoS ONE 5, e8606. 10.1371/journal.pone.0008606 (doi:10.1371/journal.pone.0008606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royle N. J., Hartley I. R., Parker G. A. 2002. Begging for control: when are offspring solicitation behaviours honest? Trends Ecol. Evol. 17, 434–440 10.1016/S0169-5347(02)02565-X (doi:10.1016/S0169-5347(02)02565-X) [DOI] [Google Scholar]
- 10.Parker G. A., Mock D. W., Lamey T. C. 1989. How selfish should stronger sibs be? Am. Nat. 133, 846–868 10.1086/284956 (doi:10.1086/284956) [DOI] [Google Scholar]
- 11.Parker G. A., Royle N. J., Hartley I. R. 2002. Begging scrambles with unequal chicks: interactions between need and competitive ability. Ecol. Lett. 5, 206–215 10.1046/j.1461-0248.2002.00301.x (doi:10.1046/j.1461-0248.2002.00301.x) [DOI] [Google Scholar]
- 12.Godfray H. C. J. 1995. Signalling of need between parents and young: parent–offspring conflict and sibling rivalry. Am. Nat. 146, 1–24 10.1086/285784 (doi:10.1086/285784) [DOI] [Google Scholar]
- 13.Boncoraglio G., Saino N. 2008. Barn swallow chicks beg more loudly when broodmates are unrelated. J. Evol. Biol. 21, 256–262 [DOI] [PubMed] [Google Scholar]
- 14.Uller T. 2006. Sex-specific sibling interactions and offspring fitness in vertebrates: patterns and implications for maternal sex ratios. Biol. Rev. 81, 207–217 10.1017/S1464793105006962 (doi:10.1017/S1464793105006962) [DOI] [PubMed] [Google Scholar]
- 15.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348 10.1016/S0169-5347(99)01639-0 (doi:10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 16.Badyaev A. V. 2002. Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol. Evol 17, 369–378 10.1016/S0169-5347(02)02569-7 (doi:10.1016/S0169-5347(02)02569-7) [DOI] [Google Scholar]
- 17.Boncoraglio G., Martinelli R., Saino N. 2008. Sex-related asymmetry in competitive ability of sexually monomorphic barn swallow nestlings. Behav. Ecol. Sociobiol. 62, 729–738 10.1007/s00265-007-0498-8 (doi:10.1007/s00265-007-0498-8) [DOI] [Google Scholar]
- 18.Saino N., de Ayala R. M., Martinelli R., Boncoraglio G. 2008. Male-biased brood sex ratio depresses average phenotypic quality of barn swallow nestlings under experimentally harsh conditions. Oecologia 156, 441–453 10.1007/s00442-008-0971-8 (doi:10.1007/s00442-008-0971-8) [DOI] [PubMed] [Google Scholar]
- 19.Price K., Harvey H., Ydenberg R. 1996. Begging tactics of nestling yellow-headed blackbirds, Xanthocephalus xanthocephalus, in relation to need. Anim. Behav. 51, 421–435 10.1006/anbe.1996.0039 (doi:10.1006/anbe.1996.0039) [DOI] [Google Scholar]
- 20.Cotton P. A., Wright J., Kacelnik A. 1999. Chick begging strategies in relation to brood hierarchies and hatching asynchrony. Am. Nat. 153, 412–420 10.1086/303178 (doi:10.1086/303178) [DOI] [PubMed] [Google Scholar]
- 21.Oddie K. R. 2000. Size matters: competition between male and female great tit offspring. J. Anim. Ecol. 69, 903–912 [DOI] [PubMed] [Google Scholar]
- 22.Price K., Ydenberg R., Daust D. 2002. State-dependent begging with asymmetries and costs: a genetic algorithm approach. In The evolution of begging (eds Wright J., Leonard M. L.), pp. 21–42 Dordrecht, The Netherlands: Kluwer [Google Scholar]
- 23.Hinde C. A., Buchanan K. L., Kilner R. M. 2009. Prenatal environmental effects match offspring begging to parental provisioning. Proc. R. Soc. B 276, 2787–2794 10.1098/rspb.2009.0375 (doi:10.1098/rspb.2009.0375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stearns S. C. 1992. The evolution of life-histories. Oxford, UK: Oxford University Press [Google Scholar]
- 25.Müller W., Lessells M., Korsten P., von Engelhardt N. 2007. Manipulative signals in family conflict? On the function of maternal yolk hormones in birds. Am. Nat. 169, E84–E96 10.1086/511962 (doi:10.1086/511962) [DOI] [PubMed] [Google Scholar]
- 26.Groothuis T. G. G., Müller W., von Engelhardt N., Carere C., Eising C. 2005. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 29, 329–352 10.1016/j.neubiorev.2004.12.002 (doi:10.1016/j.neubiorev.2004.12.002) [DOI] [PubMed] [Google Scholar]
- 27.Bortolotti G. R. 1986. Influence of sibling competition on nestling sex ratios of sexually dimorphic birds. Am. Nat. 127, 495–507 10.1086/284498 (doi:10.1086/284498) [DOI] [Google Scholar]
- 28.Hardy I. C. W. 2002. Sex ratios: concepts and research methods. Cambridge, UK: Cambridge University Press [Google Scholar]
- 29.Hinde C. A., Kilner R. M. 2007. Negotiations within the family over the supply of parental care. Proc. R. Soc. B 274, 53–60 10.1098/rspb.2006.3692 (doi:10.1098/rspb.2006.3692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright J., Leonard M. L. 2002. The evolution of begging. Dordrecht, The Netherlands: Kluwer [Google Scholar]
- 31.Saino N., Calza S., Ninni P., Møller A. P. 1999. Barn swallows trade survival against offspring condition and immunocompetence. J. Anim. Ecol. 68, 999–1009 10.1046/j.1365-2656.1999.00350.x (doi:10.1046/j.1365-2656.1999.00350.x) [DOI] [Google Scholar]
- 32.Godfray H. C. J. 1991. Signalling of need by offspring to their parents. Nature 352, 328–330 10.1038/352328a0 (doi:10.1038/352328a0) [DOI] [Google Scholar]
- 33.Roulin A., Dreiss A., Fioravanti C., Bize P. 2009. Vocal sib–sib interactions: how siblings adjust signalling level to each other. Anim. Behav. 77, 717–725 10.1016/j.anbehav.2008.12.004 (doi:10.1016/j.anbehav.2008.12.004) [DOI] [Google Scholar]
- 34.Johnstone R. A, Roulin A. 2003. Sibling negotiation. Behav. Ecol. 14, 780–786 10.1093/beheco/arg024 (doi:10.1093/beheco/arg024) [DOI] [Google Scholar]
- 35.Boncoraglio G., Caprioli M., Saino N. 2009. Fine-tuned modulation of competitive behaviour according to kinship in barn swallow nestlings. Proc. R. Soc. Lond. B 276, 2117–2123 10.1098/rspb.2009.0085 (doi:10.1098/rspb.2009.0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lotem A. 1998. Differences in begging behaviour between barn swallow, Hirundo rustica, nestlings. Anim. Behav. 55, 809–818 10.1006/anbe.1997.0675 (doi:10.1006/anbe.1997.0675) [DOI] [PubMed] [Google Scholar]
- 37.Saino N., Incagli M., Martinelli R. 2001. Immunity, growth and begging behaviour of nestling Barn Swallows Hirundo rustica in relation to hatching order. J. Avian Biol. 32, 263–270 10.1111/j.0908-8857.2001.320309.x (doi:10.1111/j.0908-8857.2001.320309.x) [DOI] [Google Scholar]
- 38.Bonisoli-Alquati A., Martinelli R., Rubolini D., Saino N. 2008. Sex-specific effects of albumen removal and nest environment manipulation on barn swallow nestlings. Ecology 89, 2315–2324 10.1890/07-1066.1 (doi:10.1890/07-1066.1) [DOI] [PubMed] [Google Scholar]
- 39.Saino N., de Ayala R. M., Martinelli R., Boncoraglio G. 2008. Sex difference in mouth coloration and begging calls of barn swallow nestlings. Anim. Behav. 75, 1375–1382 10.1016/j.anbehav.2007.09.011 (doi:10.1016/j.anbehav.2007.09.011) [DOI] [Google Scholar]
- 40.Ferrari R. P., Martinelli R., Saino N. 2006. Differential effects of egg albumen content on barn swallow nestlings in relation to hatch order. J. Evol. Biol. 19, 981–993 10.1111/j.1420-9101.2005.01030.x (doi:10.1111/j.1420-9101.2005.01030.x) [DOI] [PubMed] [Google Scholar]
- 41.Burnham K. P., Anderson D. R. 2002. Model selection and multi-model inference: a practical information-theoretic approach. New York, NY: Springer [Google Scholar]
- 42.Kilner R. M., Johnstone R. A. 1997. Begging the question: are offspring solicitation behaviours signals of need? Trends Ecol. Evol. 12, 11–15 10.1016/S0169-5347(96)10061-6 (doi:10.1016/S0169-5347(96)10061-6) [DOI] [PubMed] [Google Scholar]
- 43.Leonard M. L., Horn A. G. 2001. Dynamics of calling by tree swallow (Tachycineta bicolor) nestmates. Behav. Ecol. Sociobiol. 50, 430–435 10.1007/s002650100380 (doi:10.1007/s002650100380) [DOI] [Google Scholar]
- 44.Safran R. J., Pilz K. M., McGraw K. J., Correa S. M., Schwabl H. 2008. Are yolk androgens and carotenoids in barn swallow eggs related to parental quality? Behav. Ecol. Sociobiol. 62, 427–438 10.1007/s00265-007-0470-7 (doi:10.1007/s00265-007-0470-7) [DOI] [Google Scholar]
- 45.Badyaev A. V., Acevedo Seaman D., Navara K. J., Hill G. E., Mendonça M. T. 2006. Evolution of sex-biased maternal effects in birds: III. Adjustment of ovulation order can enable sex-specific allocation of hormones, carotenoids, and vitamins. J. Evol. Biol. 19, 1044–1057 10.1111/j.1420-9101.2006.01106.x (doi:10.1111/j.1420-9101.2006.01106.x) [DOI] [PubMed] [Google Scholar]
- 46.Badyaev A. V., Young R. L., Hill G. E., Duckworth R. A. 2008. Evolution of sex-biased maternal effects in birds: IV. Intra-ovarian growth dynamics can link sex-determination and sex-specific acquisition of resources. J. Evol. Biol. 21, 449–460 10.1111/j.1420-9101.2007.01498.x (doi:10.1111/j.1420-9101.2007.01498.x) [DOI] [PubMed] [Google Scholar]
- 47.Clark A. B., Wilson D. S. 1981. Avian breeding adaptations: hatching asynchrony, brood reduction and nest failure. Q. Rev. Biol. 56, 253–277 10.1086/412316 (doi:10.1086/412316) [DOI] [Google Scholar]
- 48.Grafen A. 1990. Biological signals as handicaps. J. Theor. Biol. 144, 517–546 10.1016/S0022-5193(05)80088-8 (doi:10.1016/S0022-5193(05)80088-8) [DOI] [PubMed] [Google Scholar]
- 49.Johnstone R. A. 1999. Signalling of need, sibling competition and the cost of honesty. Proc. Natl Acad. Sci. USA 96, 12 644–12 649 10.1073/pnas.96.22.12644 (doi:10.1073/pnas.96.22.12644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheldon B. C., Merilä J., Lindgren G., Ellegren H. 1998. Gender and environmental sensitivity in nestling collared flycatchers. Ecology 79, 1939–1948 10.1890/0012-9658(1998)079[1939:GAESIN]2.0.CO;2 (doi:10.1890/0012-9658(1998)079[1939:GAESIN]2.0.CO;2) [DOI] [Google Scholar]
- 51.Johnstone R. A. 2004. Begging and sibling competition: how should offspring respond to their rivals? Am. Nat. 163, 388–406 10.1086/375541 (doi:10.1086/375541) [DOI] [PubMed] [Google Scholar]
- 52.Lotem A., Wagner R. H., Balshine-Earn S. 1999. The overlooked signalling component of nonsignaling behavior. Behav. Ecol. 10, 209–212 10.1093/beheco/10.2.209 (doi:10.1093/beheco/10.2.209) [DOI] [Google Scholar]
- 53.Rodríguez-Gironés M. A. 1999. Sibling competition stabilizes signalling resolution models of parent–offspring conflict. Proc. R. Soc. Lond. B 266, 2399–2402 10.1098/rspb.1999.0937 (doi:10.1098/rspb.1999.0937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madden J. R., Kunc H. P., English S., Manser M. B., Clutton-Brock T. H. 2009. Calling in the gap: competition or cooperation in littermates' begging behaviour? Proc. R. Soc. B 276, 1255–1262 10.1098/rspb.2008.1660 (doi:10.1098/rspb.2008.1660) [DOI] [PMC free article] [PubMed] [Google Scholar]