Abstract
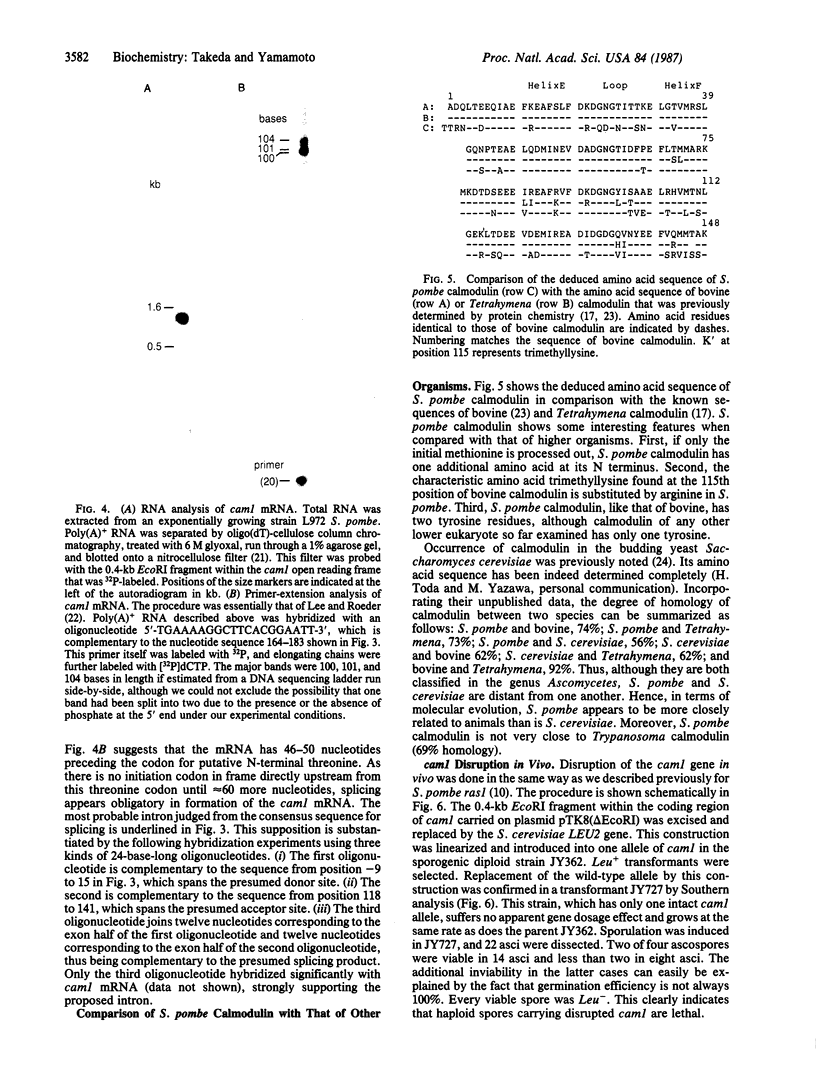
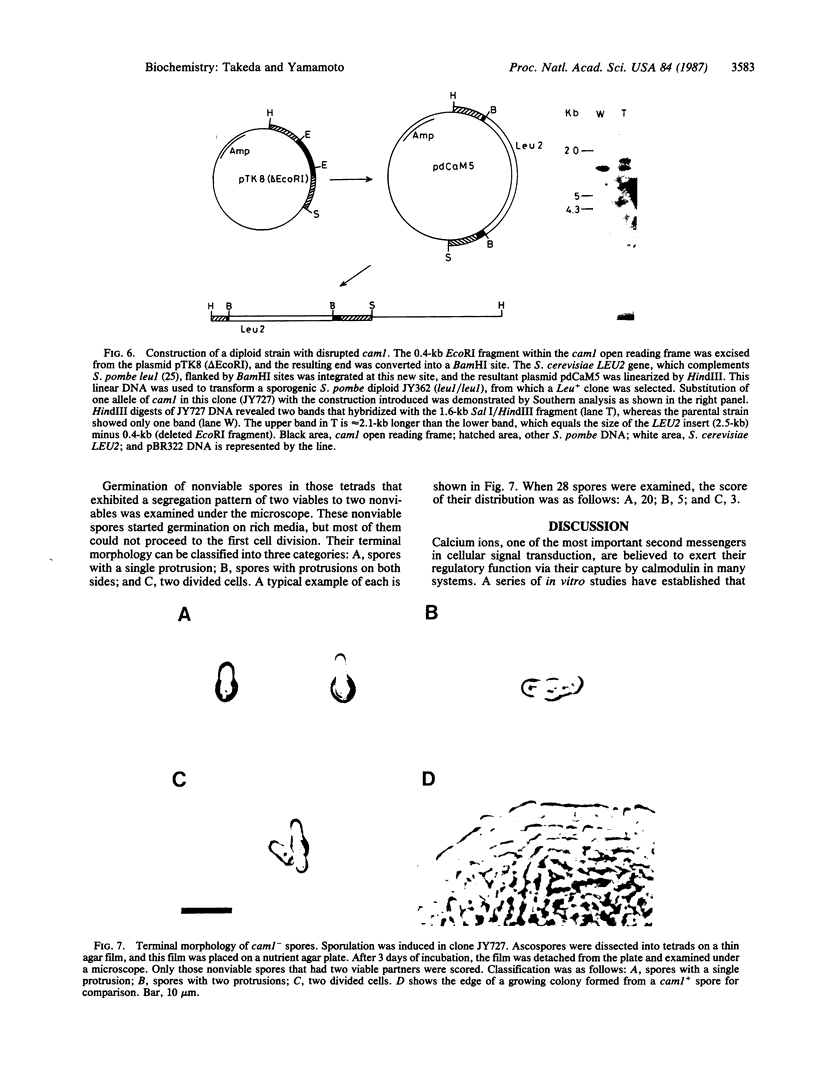
Calmodulin is a low molecular weight calcium-binding protein that modulates many enzyme systems in eukaryotes. We have cloned the gene encoding calmodulin from the fission yeast, Schizosaccharomyces pombe, by using synthetic oligonucleotide probes that correspond to three distinct regions of Tetrahymena calmodulin. A 1.6-kilobase (kb) DNA fragment that hybridized to all of them contains a gene whose deduced product possesses 74% amino acid homology with bovine calmodulin. This gene, which is unique in the S. pombe genome and is named cam1, encodes 149 amino acids excluding the first methionine and is transcribed into mRNA of 1.2-kb length. It has an intron that apparently starts immediately after the initiation codon and is 126 bp long. S. pombe calmodulin exhibits more homology to vertebrate calmodulin than to that of the budding yeast, Saccharomyces cerevisiae. Gene disruption experiments revealed that cam1 gene function is essential for vegetative growth of S. pombe. Spores bearing disrupted cam1 halt growth soon after germination and rarely carry out the first cell division, indicating that calmodulin does not exist in excess in those cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazari W. L., Clarke M. Characterization of a novel calmodulin from Dictyostelium discoideum. J Biol Chem. 1981 Apr 10;256(7):3598–3603. [PubMed] [Google Scholar]
- Chafouleas J. G., Bolton W. E., Hidaka H., Boyd A. E., 3rd, Means A. R. Calmodulin and the cell cycle: involvement in regulation of cell-cycle progression. Cell. 1982 Jan;28(1):41–50. doi: 10.1016/0092-8674(82)90373-7. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Lagacé L., Bolton W. E., Boyd A. E., 3rd, Means A. R. Changes in calmodulin and its mRNA accompany reentry of quiescent (G0) cells into the cell cycle. Cell. 1984 Jan;36(1):73–81. doi: 10.1016/0092-8674(84)90075-8. [DOI] [PubMed] [Google Scholar]
- Cox J. A., Ferraz C., Demaille J. G., Perez R. O., van Tuinen D., Marmé D. Calmodulin from neurospora crassa. General properties and conformational changes. J Biol Chem. 1982 Sep 25;257(18):10694–10700. [PubMed] [Google Scholar]
- Davis T. N., Urdea M. S., Masiarz F. R., Thorner J. Isolation of the yeast calmodulin gene: calmodulin is an essential protein. Cell. 1986 Nov 7;47(3):423–431. doi: 10.1016/0092-8674(86)90599-4. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Kozasa T., Kaziro Y., Takeda T., Yamamoto M. Role of a ras homolog in the life cycle of Schizosaccharomyces pombe. Cell. 1986 Jan 31;44(2):329–336. doi: 10.1016/0092-8674(86)90767-1. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Nairn A. C., Perry S. V. The preparation of calmodulins from barley (Hordeum sp.) and basidiomycete fungi. Biochem J. 1980 Mar 1;185(3):755–760. doi: 10.1042/bj1850755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley J., Phear G. A. Sequence of the cell division gene CDC2 from Schizosaccharomyces pombe; patterns of splicing and homology to protein kinases. Gene. 1984 Nov;31(1-3):129–134. doi: 10.1016/0378-1119(84)90203-8. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hubbard M., Bradley M., Sullivan P., Shepherd M., Forrester I. Evidence for the occurrence of calmodulin in the yeasts Candida albicans and Saccharomyces cerevisiae. FEBS Lett. 1982 Jan 11;137(1):85–88. doi: 10.1016/0014-5793(82)80320-7. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- Lagacé L., Chandra T., Woo S. L., Means A. R. Identification of multiple species of calmodulin messenger RNA using a full length complementary DNA. J Biol Chem. 1983 Feb 10;258(3):1684–1688. [PubMed] [Google Scholar]
- Lee D. C., Roeder R. G. Transcription of adenovirus type 2 genes in a cell-free system: apparent heterogeneity of initiation at some promoters. Mol Cell Biol. 1981 Jul;1(7):635–651. doi: 10.1128/mcb.1.7.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak D. R., Clarke M., Roberts D. M., Watterson D. M. Structural and functional properties of calmodulin from the eukaryotic microorganism Dictyostelium discoideum. Biochemistry. 1984 Jun 19;23(13):2891–2899. doi: 10.1021/bi00308a007. [DOI] [PubMed] [Google Scholar]
- Means A. R., Dedman J. R. Calmodulin--an intracellular calcium receptor. Nature. 1980 May 8;285(5760):73–77. doi: 10.1038/285073a0. [DOI] [PubMed] [Google Scholar]
- Munjaal R. P., Chandra T., Woo S. L., Dedman J. R., Means A. R. A cloned calmodulin structural gene probe is complementary to DNA sequences from diverse species. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2330–2334. doi: 10.1073/pnas.78.4.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. M., Rowe P. M., Siegel F. L., Lukas T. J., Watterson D. M. Trimethyllysine and protein function. Effect of methylation and mutagenesis of lysine 115 of calmodulin on NAD kinase activation. J Biol Chem. 1986 Feb 5;261(4):1491–1494. [PubMed] [Google Scholar]
- Russell P. R., Hall B. D. Structure of the Schizosaccharomyces pombe cytochrome c gene. Mol Cell Biol. 1982 Feb;2(2):106–116. doi: 10.1128/mcb.2.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher M., Lukas T. J., Watterson D. M. Isolation and characterization of calmodulin from the motile green alga Chlamydomonas reinhardtii. Arch Biochem Biophys. 1984 Feb 15;229(1):33–42. doi: 10.1016/0003-9861(84)90127-9. [DOI] [PubMed] [Google Scholar]
- Simmen R. C., Tanaka T., Ts'ui K. F., Putkey J. A., Scott M. J., Lai E. C., Means A. R. The structural organization of the chicken calmodulin gene. J Biol Chem. 1985 Jan 25;260(2):907–912. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Adachi Y., Hiraoka Y., Yanagida M. Identification of the pleiotropic cell division cycle gene NDA2 as one of two different alpha-tubulin genes in Schizosaccharomyces pombe. Cell. 1984 May;37(1):233–242. doi: 10.1016/0092-8674(84)90319-2. [DOI] [PubMed] [Google Scholar]
- Tschudi C., Young A. S., Ruben L., Patton C. L., Richards F. F. Calmodulin genes in trypanosomes are tandemly repeated and produce multiple mRNAs with a common 5' leader sequence. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3998–4002. doi: 10.1073/pnas.82.12.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik L. J., Piperno G., Watterson D. M. Similarities and dissimilarities between calmodulin and a Chlamydomonas flagellar protein. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4779–4783. doi: 10.1073/pnas.77.8.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson D. M., Sharief F., Vanaman T. C. The complete amino acid sequence of the Ca2+-dependent modulator protein (calmodulin) of bovine brain. J Biol Chem. 1980 Feb 10;255(3):962–975. [PubMed] [Google Scholar]
- Yazawa M., Yagi K., Toda H., Kondo K., Narita K., Yamazaki R., Sobue K., Kakiuchi S., Nagao S., Nozawa Y. The amino acid sequence of the Tetrahymena calmodulin which specifically interacts with guanylate cyclase. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1051–1057. doi: 10.1016/0006-291x(81)90725-7. [DOI] [PubMed] [Google Scholar]










