Abstract
Transcranial magnetic stimulation (TMS), a tool that allows noninvasive modulation of cortical neural activity, has become an important tool in cognitive neuroscience and is being increasingly explored in neurotherapeutics. Amongst the factors that are likely to influence its efficacy, the importance of the baseline cortical activation state on the impact of TMS has not received much attention. However, this state-dependency is important as the neural impact of any external stimulus represents an interaction with the ongoing brain activity at the time of stimulation. The effects of any external stimulus are therefore not only determined by the properties of that stimulus but also by the activation state of the brain. Here we review the existing evidence on the state-dependency of TMS and propose how its systematic study can provide unique insights into brain function and significantly enhance the effectiveness of TMS in investigations on the neural basis of perception and cognition. We also describe novel approaches based on this state-dependency which can be used to investigate the properties of distinct neural subpopulations within the stimulated region. Furthermore, we discuss how state-dependency can explain the functional mechanisms through which TMS impairs perception and behavior.
Keywords: Transcranial magnetic stimulation, State-dependency, Adaptation, Human brain cortical function
Introduction
Noninvasive brain stimulation techniques such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), which can induce transient changes in neural activity, have become widely used tools in cognitive and clinical neuroscience (Cowey 2005; Walsh and Pascual-Leone 2003). In cognitive neuroscience, TMS is generally used with the objective of disrupting neural activity associated with cognitive processes by inducing random neuronal activity that is uncorrelated with the ongoing activity (i.e., “virtual lesions”). Although this “virtual lesion” approach has become a widely used paradigm in determining the necessity of cortical regions in cognitive functions, it has certain limitations. One important challenge results from the fact that the effects of brain stimulation are not limited to the targeted brain region, but can spread ortho- and anti-dromically along neural connections. Studies in animal models demonstrate that TMS might in fact be best conceptualized as modulating activity across bi-hemispheric cortico-subcortical networks reached from the directly targeted brain region (Valero-Cabré et al. 2005, 2007). In humans, studies combining TMS with brain imaging methods such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) confirm such distributed network effects (Paus et al. 1997; Sack et al. 2007; Bestmann et al. 2008). Therefore, interpretation of the effects of TMS or tDCS and establishment of causal relations between activity in the targeted brain area and a given behavioral effect is complicated. Even within the targeted brain region, TMS studies have had very little to say about how distinct neural populations within the stimulated area interact to give rise to perception and behavior. Here we review evidence on how understanding of the interaction between the baseline cortical activation state and the effects of brain stimulation significantly enhance the resolution of TMS, enabling it to be used to investigate receptive field properties in the stimulated region, and provide valuable insights into fundamental aspects of brain function.
The Neural Effects of TMS
In online paradigms, single-pulse TMS (or brief pulse trains) is applied concurrently with a behavioral task, and an induced behavioral impairment is interpreted as evidence that the stimulated region is necessary for performing that task (Cowey 2005; Walsh and Pascual-Leone 2003). The neural impact of single-pulse TMS was revealed in a seminal study by Moliadze et al. (2003) who studied its effect on single-unit activity in the cat primary visual cortex. Distinct episodes of enhanced and suppressed activity were observed. A facilitation of neural activity was found during the first 500 ms, followed thereafter by a suppression of activity lasting up to a few seconds. This early period of facilitation was dependent on the stimulation intensity: stimulation at intensities exceeding 50% of maximal stimulator output lead to an early suppression of activity during the first 100–200 ms, followed by a period of facilitation. These patterns of early suppression and facilitation of activity were proposed to be related to a more or less direct stimulation of inhibitory and excitatory interneurons, probably with different thresholds. The late, long-lasting suppression is more likely to be related to metabotropic or metabolic processes, or even vascular responses. The neural basis of effects induced by repetitive TMS (rTMS) is likely to be very different from those of online stimulation. rTMS has a prolonged effect on brain activity, suppressing cortical excitability for up to 30–60 min (see Ridding and Rothwell 2007 for a review), depending on variables such TMS intensity, stimulation frequency, and the number of pulses. It is believed to act by modulating long-term depression (LTD) and long-term potentiation (LTP) between synaptic connections (see Ridding and Rothwell (2007) for a more detailed discussion; in this review we concentrate mainly on the effects induced by single-pulse and brief pulse trains of TMS in online paradigms).
The importance of a number of factors in determining the effects of TMS is still unknown. For example, it is not clear which cortical layers are primarily affected by the stimulation, and whether axons or dendrites are primarily affected; what is the role of the orientation of neural elements, and what is the impact of bends in fiber bundles? All of the above are likely to play an important role in the determining the impact of TMS. A further critical factor, which is the focus of the present article, involves the context at which TMS is applied. The neural impact of an external stimulus is not determined only by the properties of that stimulus but also on the initial state of the activated brain region. This applies to the neural responses elicited by visual or tactile stimulation, and direct brain stimulation is no different. We argue here that TMS effects need to be conceptualized as an interaction between the stimulus applied and the level of activity of the affected brain region.
State-Dependent Effects of TMS
Although state-dependency is an influential concept in cognitive neuroscience (for example in the study of visual attention), it has been a largely ignored issue in the TMS literature. The interaction between the effects of TMS and the initial state of the stimulated region is important, however, because, as was mentioned above, any induced neural activity occurs in the context of a baseline neural activity. In other words, the neural impact of an external stimulus is not only determined by the properties of that stimulus but also on the susceptibility of the stimulated brain region to being activated by the stimulus. Recent studies have shown this also to be the case for the effects of TMS.
Silvanto et al. (2007) investigated the state-dependency of TMS by manipulating initial cortical activation state of functionally distinct neural populations by the use of adaptation, a phenomenon in which changes in neural tuning and excitability induced by prolonged exposure to sensory stimulation bias the perception of subsequently presented stimuli (Gibson and Radner 1937; Mather et al. 1998; Grill-Spector et al. 2006). The interaction between the neural activation state and the effects of TMS was assessed by TMS-induced visual percepts (phosphenes) as well as using a psychophysical task. The key finding was that, after adaptation to a color stimulus, phosphenes induced from the early visual cortex took on the color qualities of the adapting stimulus. In the psychophysical task in which TMS was applied at an intensity below the phosphene threshold, TMS similarly facilitated the perception of the adapted attributes. As neurons encoding the adapted attribute were made less active/excitable by adaptation, the finding that phosphenes took on the color of the adapting stimulus implies that TMS perceptually/behaviorally facilitates the less active/excitable neural populations relative to the more active neural populations. Similar findings have been obtained in motion perception after adaptation to simple translational motion, with TMS facilitating the detection of the adapted direction and impairing the detection of the opposite direction (Cattaneo and Silvanto 2008a, b). Finally, similar state-dependent effects of TMS have also been found in the context of multisensory interaction. Romei et al. (2007) found that single-pulse TMS over the occipital pole produced opposing behavioral effects during a simple reaction time task to visual and auditory stimuli, with TMS slowing down reaction times to visual stimuli but facilitating reaction times to auditory stimuli.
In addition to adaptation, another psychophysical method for manipulating the initial state of the visual system prior to the presentation of a target is priming, a phenomenon in which repetition of an object's feature or spatial position facilitates subsequent detection or identification of that object (e.g., Maljkovic and Nakayama 1994, 1996; Campana et al. 2002, 2006, 2007; Magnussen and Greenlee 1999). On the neural level, various theories have been put forward to account for the effects of priming (see Grill-Spector et al. 2006; Schacter and Buckner 1998; Wiggs and Martin 1998). One theory is that priming occurs because neurons activated by the prime are still active when the test stimulus is presented; this elevated activity level facilitates target detection if those pre-activated neurons are involved in encoding the target stimulus. Priming has also been proposed to reflect changes in neural tuning. In this view, neurons that code features irrelevant to identification of a stimulus become less responsive to that stimulus, leading to a sparser representation of stimuli (Desimone 1996; Wiggs and Martin 1998). Because tuning curves become narrower, neurons become more sensitive to change, enabling more efficient or faster processing of repeated stimuli (Schacter and Buckner 1998; Wiggs and Martin 1998).
Cattaneo et al. (in press) investigated the state-dependency of TMS in the context of priming, and the findings support the conclusion that TMS preferentially facilitates the attributes encoded by the less active neural populations. Specifically, Cattaneo et al. (in press) compared the state-dependent effects of TMS when the initial activation states had been modulated either with adaptation or priming. Consistent with previous studies, in the adaptation experiment, TMS facilitated the detection of the adapted attributes. In the priming paradigm, TMS facilitated the detection of nonprimed targets. As the activity level of neurons encoding the nonprimed targets was lower at the time of TMS application than the activity level of neurons encoding primed targets, this finding provides further evidence for the view that that TMS preferentially facilitates the less active neural populations. This is an important finding as it demonstrates that the principle of TMS facilitating less active neural populations is not simply restricted to adaptation but can also be observed in other paradigms.
State-dependent effects of online TMS have also been observed when the stimulated region has been uniformly suppressed by 1 Hz repetitive TMS prior to application of online TMS. In a recent study by Silvanto et al. (2008), when TMS was applied over the motion-selective region V5/MT during a simple motion detection task, subjects’ motion detection ability was impaired. Similarly, suppression of V5/MT activity using offline 1 Hz rTMS disrupted performance in a subsequent motion detection task. However, paradoxically, online V5/MT TMS facilitated motion detection if V5/MT had been suppressed by offline 1 Hz rTMS prior to the motion detection task. These results demonstrate that online TMS can have an unexpected facilitatory effect on behavior when the targeted neural population is in a suppressed state. This finding provides further evidence for the view that the effects of TMS are modulated by the initial activation state of the targeted neural population.
These findings demonstrate that the behavioral effects of TMS depend on the relative activity state of functionally distinct neural populations within the stimulated region. Furthermore, the finding that TMS behaviorally facilitates the less active neural populations suggests that, at the behavioral level, the effects of TMS are akin to microstimulation of the less active neural populations. The conceptualization of TMS as a tool for disrupting cognitive function by inducing reversible “lesions” does not do justice to these subtle effects.
State-dependency is an important factor in determining the impact of TMS on brain areas that are anatomically connected to the stimulated region. Specifically, TMS can either facilitate or inhibit cortico-cortical functional connectivity depending on the initial states of these regions. Bestmann et al. (2008) used TMS concurrently with event-related fMRI to determine how the impact of left dorsal premotor cortex (PMd) TMS upon contralateral (right) motor areas depends on the current state of the motor system. This was achieved by applying short pulse trains of high- or low-intensity TMS to left PMd during single isometric left-hand grips or during rest. During active left-hand grip, high (vs. low)-intensity TMS led to activity increases in contralateral right PMd and M1, whereas TMS produced an activity decrease during no-grip rest. These findings demonstrate that the distal effect of aTMS pulse-train applied over the left PMd on the contralateral hemisphere depends on the context in which the stimulation is applied.
State-dependent effects have also been observed with repetitive TMS (rTMS) paradigms, in which low- or high-frequency stimulation is applied with the objective of inducing a longer lasting suppression of neural activity (cf. Ridding and Rothwell 2007). In a study by Brighina et al. (2002), 1 Hz rTMS over the occipital cortex led to an increase visual cortex excitability in subjects affected by migraine with aura. In contrast, in normal subjects a decrease in visual cortex excitability (inferred from phosphene thresholds) was observed. This study shows that changes in cortical excitability induced by neurological conditions such as migraine can have a major impact on the efficacy of TMS. There is also physiological evidence for the state-dependency of offline TMS. Siebner et al. (2004) and Lang et al. (2004) showed that preconditioning motor cortical excitability using transcranial direct current stimulation (tDCS) modulates the direction of effects induced by subsequent repetitive TMS. When the excitability of the corticospinal projection was increased, a subsequent period of 1 Hz rTMS produced a lasting reduction in corticospinal excitability (Siebner et al. 2004). Conversely, when corticospinal excitability was reduced prior to application of rTMS, the same 1 Hz rTMS caused a sustained increase in corticospinal excitability. Lang et al. (2004) observed similar physiological state-dependent effects of preconditioning with high frequency rTMS.
Neural Basis of State-Dependency
The suggestion that the less active neural populations are more susceptible to TMS may seem counterintuitive. However, it is consistent with findings obtained in another domain, epileptic seizures. There are many case studies suggesting that when parts of the neural network are recruited to subserve normal sensation and cognition this normal activity can interfere with the spread of an epileptic discharge (Wilkins 1986; Wilkins et al. 2004). For example, Hughlings Jackson described a patient who discovered he could sometimes prevent the “march” of a seizure from the extremities of a limb to the remainder of his body by vigorously rubbing the affected limb above the part that was involved in the seizure (Wilkins 1986; Wilkins et al. 2004). The view that high level of neural activity also “protects” from the effects of TMS and that less active neurons are more susceptible to TMS is consistent with this. The fact that for less active neural populations there is more scope for firing rate to be increased before a ceiling effect is reached is also likely to be important.
A recent study combining TMS with electroencephalography (EEG) has provided the first neural evidence for the state-dependency of TMS. Romei et al. (2008) investigated how fluctuations of oscillatory brain activity in the alpha-frequency band (8–14 Hz) modulate the impact of brain stimulation. Decreased oscillatory activity in the alpha-frequency band is thought to reflect a state of enhanced cortical excitability, and increased activity to reflect a state of cortical idling or inhibition in which excitability is reduced. Romei et al. (2008) determined the relationship between the alpha-band resting oscillatory activity and the efficacy of TMS in inducing visual percepts (phoshenes) and found a correlation between subjects’ occipital alpha-band power and the stimulation intensity required for inducing phosphenes. The authors concluded that the highly synchronous volleys of activity elicited by TMS may be more likely to be perceptually effective during desynchronized cortical activity than during synchronous volleys of alpha-oscillations because the synchronous neural activity induced by TMS contrasts more strongly with low than high alpha-activity.
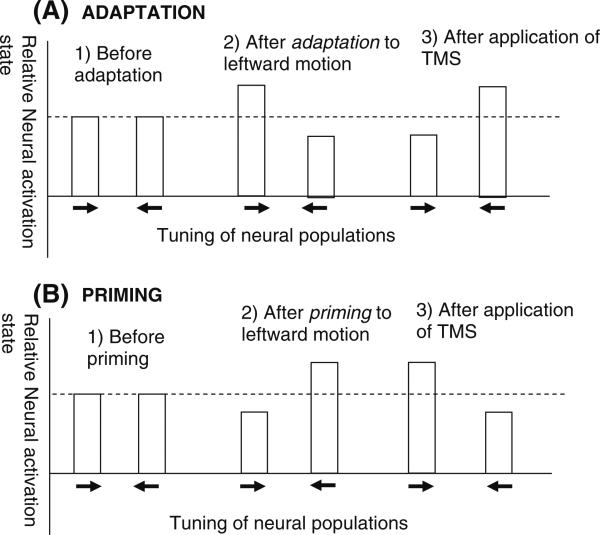
What is the neural basis of the state-dependent TMS effects found in adaptation and priming paradigms? In the present manuscript we have conceptualized these effects in terms of TMS preferentially activating (i.e., increasing the firing rate) of less active neural populations; this may occur because for less active neurons there is a greater range for firing rate to be increased. Of course any detailed explanation of these effects relies on assumptions on the neural mechanism underlying priming and adaptation. The present explanation assumes that priming occurs because neurons activated by the prime remain more active than neurons not activated by the prime (e.g., the “Facilitation model”; cf. James and Gauthier 2007, see also Grill-Spector et al. 2006). In adaptation, the assumption is that the baseline level of activity of neurons tuned to the adapted neurons is lower than that of other neurons (see the “Fatigue model”; cf. Li et al. 1993). These mechanisms are schematically depicted in Fig. 1. However, as discussed above, priming and adaptation have also been proposed to reflect changes in neural tuning. In the “Sharpening models” that have been used to explain priming (see Grill-Spector et al. 2006 for a review), neurons optimally tuned to the primed stimulus show little or no response reductions whereas neurons not optimally tuned show reduced activation. This model also leads to the conclusion that TMS preferentially facilitates less active neural populations: as priming selectively reduces activation of neurons not ideally tuned to the primed stimulus, the fact that TMS selectively improves the detection of nonprimed attributes suggest that less active neural populations are preferentially facilitated.
Fig. 1.
A schematic of the state-dependency of TMS effects, depicting the principle of TMS preferentially facilitating attributes encoded by the less active neural populations. The same outcome is observed when the initial cortical activation state has been manipulated by either adaptation or priming prior to application of TMS. (a) After adaptation to leftward motion, neurons tuned to this direction are less active than neurons tuned to rightward motion. Perceptually, this adaptation is manifested as a bias to perceive a subsequent moving stimulus moving in the opposite, rightward direction (panel 2). Application of TMS over the visual area V5/MT reverses this bias, with subjects more likely to report a test stimulus moving in the adapted direction. (b) After priming to leftward motion, neurons tuned to this direction are more active than neurons tuned to rightward motion. Perceptually, this priming is manifested as a bias to perceive a subsequent motion stimulus to move in the primed direction (panel 2). Application of TMS over the visual area V5/MT reverses this bias, with subjects now more likely to report a test stimulus moving in the nonadapted (rightward) direction
The state-dependent effects of repetitive TMS have been explained in terms of homeostatic plasticity that stabilizes corticospinal excitability within a physiologically useful range (Sejnowski 1977). Homeostatic mechanisms are believed to play an important role in activity-dependent synaptic plasticity, such as long-term potentiation (LTP) and long-term depression (LTD). Activity-driven synaptic plasticity carries the risk of destabilizing the properties of neuronal networks (Abbott and Nelson 2000), and the homeostatic model enables stabilization of neuronal activity by adaptation of the level of postsynaptic response that leads to synaptic plasticity. In this view, a sustained reduction in postsynaptic activity (induced by tDCS or visual adaptation) would lead to a reduction in the modification threshold, favoring the induction of LTP, whereas a sustained increase in postsynaptic activity would increase the modification threshold, favoring the induction of LTD.
State-Dependency in Cognitive Neuroscience: The TMS-Adaptation and TMS-Priming Paradigms
An understanding of state-dependency allows one to predict the various outcomes of TMS when neurons in the stimulated region are in different neural activation states. As briefly discussed above, one example of how the initial activation state can have a profound impact on subsequent information processing is adaptation, a phenomenon in which prolonged exposure to sensory stimulation induces changes in the perception of subsequently presented stimuli (Gibson and Radner 1937). An example of this is the motion aftereffect: after prolonged viewing of a motion stimulus in one direction (Mather et al. 1998), a subsequently viewed static stimulus appears to move in the opposite direction to the adapting stimulus. Adaptation can also occur at higher perceptual level: after adaptation to female faces, subsequent faces look more male (Webster et al. 2004).
The behavioral effects of adaptation reveal that the visual system consists of subunits of neurons with different receptive field properties. For instance, the motion aftereffect demonstrates that the visual system contains subunits of neurons tuned to different directions of motion, with adaptation of neurons tuned to one direction biasing the observer's percept towards the opposite direction of motion, encoded by a distinct subpopulation of neurons. In psychophysics, there is a long history of exploiting these phenomena in uncovering the mechanisms that underlie perceptual processes. More recently, adaptation has been combined with functional magnetic resonance imaging (fMRI) to study the neural basis of perceptual representations (Tootell et al. 1998). At the neural level, the behavioral manifestations of adaptation may arise due to a decrease in neural firing in response to the adapting stimulus (Albrecht et al. 1984; Movshon and Lennie 1979; Carandini et al. 1988; Engel 2005), specifically by lowering of the neuron's resting potential (Carandini and Ferster 1997; Sanchez-Vives et al. 2000). This biases neural activity in favour of neurons encoding nonadapted attributes. Tuning changes have also been associated with adaptation (e.g., Dragoi et al. 2000). For example, in the motion-selective visual area V5/MT responsiveness to the adapted direction is maintained whereas it is strongly reduced for nearby directions, leading to a sharpening of the tuning curve (Kohn and Movshon 2003; see Grill-Spector et al. 2006, for detailed discussion of various neural changes associated with stimulus repetition). However, the neural substrate for the adaptation and the hypothesized shift in neuronal firing rates in unclear. For example, it is conceivable that astrocytes may play critical roles in modulating extracellular calcium and thus contributing the shifts in neuronal resting potentials (Schummers et al. 2008).
As it offers a tool for manipulating initial cortical excitability, adaptation is a useful tool for exploiting the phenomenon that TMS facilitates the attributes encoded by the less active/excitable neural populations (Silvanto and Muggleton 2008a, b). By using adaptation to systematically manipulate neural activation states prior to application of TMS, one can control which neural populations are behaviorally/perceptually facilitated by TMS (see Fig. 2 for an example of the use of TMS-adaptation paradigm). This paradigm (depicted in Fig. 2) can reveal neural tuning in the stimulated region: if TMS facilitates the perception of the adapted attribute, this indicates that neurons in the stimulated region were suppressed by adaptation and thus tuned to the adapted attribute. This paradigm provides more information than the conventional “virtual lesion” approach to TMS which can reveal the necessity of cortical regions in cognitive functions, but not the neural properties of the stimulated region. In conventional experiments, TMS impairs behavior if the stimulated region in any way contributes to the perceptual or cognitive function under investigation. For instance, TMS applied over the primary visual cortex (V1) disrupts face detection (Camprodon et al. under review), even though this region does not contain face-selective neurons. This occurs because V1 is critical in providing input to face-selective regions such as the occipital face area (e.g., Pitcher et al. 2007). However, with the TMS-adaptation paradigm, a state-dependent effect would only be observed when TMS is applied over the OFA, as neurons in this region (but not in V1) are tuned to faces and thus affected by the adapting stimulus. In other words, face adaptation would induce a differential neural activation in OFA with neurons tuned to the adapting face suppressed (and neurons with other tunings unaffected) and this differential activation would interact with the effects of TMS. In contrast, no such differential activation would take place in V1. In this manner the TMS-adaptation paradigm allows one to tease apart the different contribution of various cortical regions in stimulus encoding (Silvanto and Muggleton 2008a, b). The most informative component of this paradigm is that it can reveal receptive properties of subunits of neurons in the stimulated region: if TMS does not reverse the behavioral effect of adaptation, neurons in the stimulated region were not suppressed by and thus not strongly driven by the adapting stimulus.
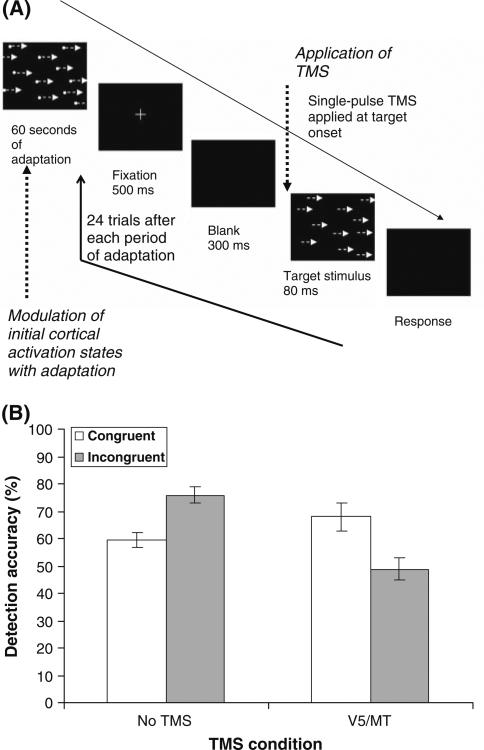
Fig. 2.
The TMS-adaptation paradigm. (a) Each test block begins with a period of adaptation, with the objective of differentially affecting the initial states of functionally distinct neural populations. In this study by Cattaneo and Silvanto (2008b), subjects were adapted to either leftward or rightward moving motion stimulus. Adaptation is followed by a block of experimental trials; in this study subjects were presented with motion stimuli moving either in the adapted direction (i.e., congruent targets) or targets moving in the opposite targets (i.e., incongruent trials). At least 24 experimental trials can be run after each period of adaptation without TMS significantly weakening the strength of adaptation (Cattaneo and Silvanto 2008b). (b) The state-dependent effect of TMS. In the No TMS condition, subjects were worse in detecting the adapted direction (i.e. congruent trials) relative to the nonadapted direction (i.e., incongruent trials), demonstrating that adaptation was behaviorally effective. This effect was reversed when TMS was applied over the motion-selective area V5/MT (a region known to contain motion-selective neurons), with TMS facilitating the detection of the adapted stimuli relative to the nonadapted stimuli. As neurons encoding the adapted direction of motion were less active than neurons encoding other attributes at the time of stimulation, this finding implies that TMS behaviorally facilitates the less active neural populations
This TMS-adaptation paradigm is useful for investigations on a wide range of higher-level visual stimuli. An example can be made of face perception. One question that the state-dependency of TMS enables one to study is whether the cortical representation of objects and faces is independent of the position from which the stimulus is viewed. If this is the case, TMS applied after adaptation to a face or an object viewed from a specific angle should facilitate the detection of that stimulus independent of the angle from which it is subsequently viewed. In contrast, if object/face processing in a given cortical area is view-dependent, TMS would only facilitate detection when the stimulus is viewed from the same angle as during adaptation. In this manner TMS can be used to reveal the tuning of neurons in the stimulated region. This paradigm is useful for studying not only visual perception but also higher-level cognitive functions; for instance, adaptation to letters and words can be used to study the selectivity and tuning of neurons involved language processing (Cattaneo et al. in press).
In the TMS-adaptation paradigm a period of adaptation is followed by a block of psychophysical trials (see Fig. 2). An important requirement for this paradigm is that the state of adaptation is consistent over the whole block: if adaptation decays, state-dependent effects of TMS cannot be induced. As TMS has been shown to reduce the duration of motion after-effects (Stewart et al. 1999; Theoret et al. 2002) potential confounding with this approach is that TMS may itself speed up the decay of adaptation. A recent study has shown that this is not a major concern: statistically significant state-dependent single-pulse TMS effects can be obtained for throughout a block of 24 post-adaptation trials, implying that single-pulse does not weaken the state of adaptation (Cattaneo and Silvanto 2008b). This is consistent with the evidence discussed above showing that the neural effects of single-pulse TMS last up to a few 100 ms (Moliadze et al. 2003); therefore, as long as individual trials are separated by a sufficient temporal window, the effects of TMS may occur at a trial-by-trial basis without a carry-over to the subsequent trials.
As discussed above, another method of manipulating initial activation states prior to target presentation and TMS application is priming. The state-dependent effects of TMS observed with priming can also be used to investigate neural properties of the stimulated region. The principle is the same as in the TMS-adaptation paradigm: an activity imbalance between functionally distinct neural populations is induced prior to application of TMS. Whether TMS reverses the behavioral effect of priming reveals whether neurons in the stimulated region were affected by priming. As the state-dependent effect of TMS in priming paradigms is similar to that observed in TMS-adaptation (Cattaneo et al. in press), the two paradigms are complementary. Figure 3 depicts the TMS-priming paradigm.
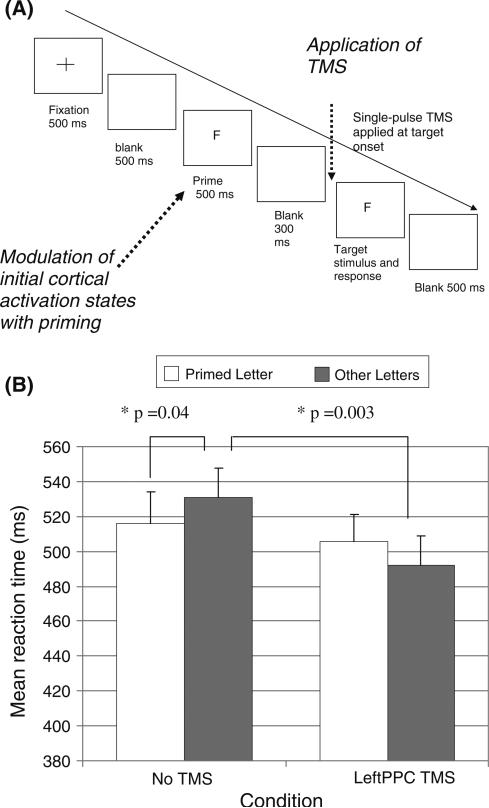
Fig. 3.
TMS-priming paradigm (adapted from Cattaneo et al. in press). On each experimental trial, a priming stimulus is presented with the objective of differentially affecting the initial states of functionally distinct neural populations prior to application of TMS and presentation of the test stimulus. In this study by Cattaneo et al. (in press), subjects were primed to one of four letters (either V, A, E, or F) and one of these letters was also presented as the target stimulus, Subjects were asked to indicate whether the target letter was a vowel or a consonant. A single-pulse of TMS was applied at stimulus onset on each experimental trial. (b) The mean reaction times (n = 12) for primed vs. unprimed letters in the letter discrimination task (Error bars depict standard error of the means). In the No TMS condition, subjects were significantly faster in detecting primed letters than to unprimed letters, demonstrating that priming was effective. Left PPC TMS reversed the effects of priming: subjects’ RTs to the unprimed letters (i.e., congruent trials) were faster than their RTs to the primed letters (i.e., incongruent trials). This reversal occurred by TMS facilitating the detection of the unprimed letters (rather than by impairing the detection of the primed letters). The critical statistical comparisons are indicated with asterisks. As neurons tuned to the nonprimed targets were less active during application of TMS than neurons encoding the primed targets, this result shows that, as in the TMS-adaptation paradigm, TMS perceptually facilitates the attributes encoded by the less active neural populations
State-Dependency and the Functional Mechanisms of TMS
State-dependency can account for various TMS phenomena and explain how online TMS impairs in cognitive and perceptual functions (see Silvanto and Muggleton 2008a, b for a detailed discussion). One such phenomenon is the importance of the time point of stimulation. Whereas disruptions are generally induced when TMS is applied during a cognitive process (Cowey 2005; Walsh and Pascual-Leone 2003), TMS can facilitate behavior when single-pulse TMS is applied shortly before the onset of a cognitive process (Töpper et al. 1998; Grosbras and Paus 2003). These increases in sensitivity to subsequent sensory stimulation have been interpreted to result from an increase in cortical excitability induced by TMS.
The importance of the time point of stimulation can be explained in terms of state-dependency. When TMS is applied before the onset of a cognitive process, all neural populations are at their baseline level of activity and thus the attributes encoded by all neural populations are equally facilitated. This results in a general increase in cortical excitability, reflected as a heightened sensitivity to subsequent sensory stimulation (Töpper et al. 1998; Grosbras and Paus 2003). However, when TMS is applied during the cognitive process, functionally distinct neural populations in the stimulated region are differentially activated: neurons not involved in that process are less active than neurons that are critical to it. Such an activity imbalance, as discussed above, modulates the effects of TMS: attributes encoded by neurons that are not involved in the cognitive process are preferentially facilitates, as these neurons are relatively inactive. This adds noise to the neural processing and can produce a behavioral disruption.
This mechanism can be illustrated in the context of motion detection. The presentation of a moving stimulus induces an activity imbalance in motion-selective areas of the cortex, with neurons tuned to presented direction strongly activated whereas neurons tuned to the opposite direction are less active or even inhibited. In a conventional online paradigm TMS interacts with this activity imbalance, with neurons tuned the opposite direction preferentially facilitated, as these neurons are less active than neurons tuned to the presented direction. As a result, the observer becomes more likely to report the opposite direction of motion that was presented.
State-Dependency of Brain Function
The purpose of this review has been to propose a conceptualization of TMS as an interaction between stimulation and the stimulated region. We believe it is logical to adopt such a viewpoint because state-dependency is central feature of all neural activity; any input (internal or external) into a given brain region will exert a behavioral and neural impact depending on the susceptibility of that region to be activated. That neural activation induced by normal sensation and cognition interferes with the spread of epileptic seizures is an example of this (Wilkins 1986) and there are many others. One area of research in cognitive neuroscience in which state-dependency has been very influential is the study of visual attention. By attending to a feature or an object, the observer can detect that item at a lower threshold than would be the case without attention. This perceptual benefit occurs because the cortical region encoding the target stimulus is in a state that allows input relating to the attended target to reach perceptual threshold more efficiently (Kastner and Ungeleider 2003). Another well-known example of how manipulation of initial activation state modulates stimulus encoding and detection is priming, a phenomenon in which repetition of an object's feature or spatial position facilitates subsequent detection or identification of that object (e.g., Maljkovic and Nakayama 1994, 1996). On the neural level, priming is likely to occur because neurons activated by the prime are still active when the test stimulus is presented; this elevated activity level facilitates target detection if those pre-activated neurons are involved in encoding the target stimulus. These examples demonstrate that understanding the interaction between the initial activation state and the impact of an external stimulus is critical for understanding not only TMS effects but brain functions in general.
Conclusions
As discussed above, the neural impact of an external stimulus is not determined only by the properties of that stimulus but also on the initial activation state of the activated brain region; this state-dependency is a general feature of cortical neural processing. In this light, the recent findings on the impact of the initial cortical activation state on the efficacy of TMS are hardly surprising. We have argued here that rather than conceptualizing TMS as a tool for inducing “virtual lesions”, its effects need to be considered in the neural context in which stimulation is applied. In addition to explaining how online TMS impairs cognitive and perceptual functions, we believe that state-dependency can be exploited to substantially increase the spatial resolution of TMS and even reveal receptive properties in the stimulated region. One example of this is the TMS-adaptation paradigm which can potentially inform us of the receptive field properties of the stimulated region.
Acknowledgments
We wish thank Arnold Wilkins for his insightful comments on this work. Supported in part by the Harvard-Thorndike Clinical Research Center at Beth Israel Deaconess Medical Center part of the Harvard Clinical and Translational Science Center (UL1 RR025758) and a grant from the National Institutes of Health (K24 RR018875).
Contributor Information
Juha Silvanto, Department of Psychology, University of Essex, Wivenhoe Park, Colchester C04 3SQ, UK; Berenson-Allen Center for Noninvasive Brain Stimulation, Harvard Medical School and Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA, UK.
Alvaro Pascual-Leone, Berenson-Allen Center for Noninvasive Brain Stimulation, Harvard Medical School and Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA, UK.
References
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3:1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- Albrecht DG, Farrar SB, Hamilton DB. Spatial contrast adaptation characteristics of neurones recorded in the cat's visual cortex. J Physiol. 1984;347:713–739. doi: 10.1113/jphysiol.1984.sp015092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Swayne O, Blankenburg F, Ruff CC, Haggard P, Weiskopf N, Josephs O, Driver J, Rothwell JC, Ward NS. Dorsal premotor cortex exerts state-dependent causal influences on activity in contralateral primary motor and dorsal premotor cortex. Cereb Cortex. 2008;18(6):1281–1291. doi: 10.1093/cercor/bhm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighina F, Piazza A, Daniele O, Fierro B. Modulation of visual cortical excitability in migraine with aura: effects of 1 Hz repetitive transcranial magnetic stimulation. Exp Brain Res. 2002;145(2):177–181. doi: 10.1007/s00221-002-1096-7. [DOI] [PubMed] [Google Scholar]
- Campana G, Cowey A, Casco C, Oudsen I, Walsh V. Left frontal eye field remembers “where” but not “what”. Neuropsychologia. 2007;45(1):2340–2345. doi: 10.1016/j.neuropsychologia.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Campana G, Cowey A, Walsh V. Priming of motion direction and area V5/MT: a test of perceptual memory. Cereb Cortex. 2002;12:663–669. doi: 10.1093/cercor/12.6.663. [DOI] [PubMed] [Google Scholar]
- Campana G, Cowey A, Walsh V. Visual area V5/MT remembers “what” but not “where”. Cereb Cortex. 2006;16:1766–1770. doi: 10.1093/cercor/bhj111. [DOI] [PubMed] [Google Scholar]
- Camprodon JC, Romei V, Halligan E, Shih MC, Pascual-Leone A. Different times of striate cortex contribution to face recognition. J Cogn Neurosci. under review. [Google Scholar]
- Carandini M, Ferster D. A tonic hyperpolarization underlying contrast adaptation in cat visual cortex. Science. 1997;276:949–952. doi: 10.1126/science.276.5314.949. [DOI] [PubMed] [Google Scholar]
- Carandini M, Movshon JA, Ferster D. Pattern adaptation and cross-orientation interactions in the primary visual cortex. Neuropharmacology. 1988;37:501–511. doi: 10.1016/s0028-3908(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z, Silvanto J. Investigating visual motion perception using the TMS-adaptation paradigm. Neuroreport. 2008a;19(14):1423–1427. doi: 10.1097/WNR.0b013e32830e0025. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z, Silvanto J. Time course of the state-dependent effect of transcranial magnetic stimulation in the TMS-adaptation paradigm. Neurosci Lett. 2008b doi: 10.1016/j.neulet.2008.07.051. Available online. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z, Rota F, Vecchi T, Silvanto J. Using state-dependency of TMS to investigate letter selectivity in the left posterior parietal cortex: a comparison of TMS-priming and TMS-adaptation paradigms. Eur J Neurosci. doi: 10.1111/j.1460-9568.2008.06466.x. in press. [DOI] [PubMed] [Google Scholar]
- Cowey A. The Ferrier Lecture 2004 what can transcranial magnetic stimulation tell us about how the brain works? Philos Trans R Soc Lond B Biol Sci. 2005;360:1185–1205. doi: 10.1098/rstb.2005.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci USA. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi V, Sharma J, Sur M. Adaptation-induced plasticity of orientation tuning in adult visual cortex. Neuron. 2000;28:287–298. doi: 10.1016/s0896-6273(00)00103-3. [DOI] [PubMed] [Google Scholar]
- Engel SA. Adaptation of oriented and unoriented color-selective neurons in human visual areas. Neuron. 2005;45:613–623. doi: 10.1016/j.neuron.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Gibson JJ, Radner M. Adaptation, aftereffect and contrast in the perception of tilted lines: I. Quantitative studies. J Exp Psychol. 1937;20:453–467. [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Paus T. Transcranial magnetic stimulation of the human frontal eye field facilitates visual awareness. Eur J Neurosci. 2003;18:3121–3126. doi: 10.1111/j.1460-9568.2003.03055.x. [DOI] [PubMed] [Google Scholar]
- James TW, Gauthier I. Repetition-induced changes in BOLD response reflect accumulation of neural activity. Hum Brain Mapp. 2006;27(1):37–46. doi: 10.1002/hbm.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Ungeleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2003;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kohn A, Movshon JA. Neuronal adaptation to visual motion in area MT of the macaque. Neuron. 2003;39:681–691. doi: 10.1016/s0896-6273(03)00438-0. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry. 2004;56:634–635. doi: 10.1016/j.biopsych.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Li L, Miller EK, Desimone R. The representation of stimulus familiarity in anterior inferior temporal cortex. J Neurophysiol. 1993;69:1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- Magnussen S, Greenlee MW. The psychophysics of perceptual memory. Psychol Res. 1999;62:81–92. doi: 10.1007/s004260050043. [DOI] [PubMed] [Google Scholar]
- Maljkovic V, Nakayama K. Priming of pop-out: I. Role of features. Mem Cognit. 1994;22:657–672. doi: 10.3758/bf03209251. [DOI] [PubMed] [Google Scholar]
- Maljkovic V, Nakayama K. Priming of pop-out: II. The role of position. Percept Psychophys. 1996;58:977–991. doi: 10.3758/bf03206826. [DOI] [PubMed] [Google Scholar]
- Mather G, et al. The motion after-effect—a modern perspective. The MIT Press; Boston, MA: 1998. [Google Scholar]
- Moliadze V, Zhao Y, Eysel U, Funke K. Effect of transcranial magnetic stimulation on single-unit activity in the cat primary visual cortex. J Physiol. 2003;553:665–679. doi: 10.1113/jphysiol.2003.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon JA, Lennie P. Pattern-selective adaptation in visual cortical neurones. Nature. 1979;1278:850–852. doi: 10.1038/278850a0. [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci. 1997;17:3178–3184. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Yovel G, Duchaine B. TMS evidence for the involvement of the right occipital face area in early face processing. Curr Biol. 2007;17:1568–1573. doi: 10.1016/j.cub.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Is there a future of therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007;8:559–567. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- Romei V, Murray MM, Merabet LB, Thut G. Occipital transcranial magnetic stimulation has opposing effects on visual and auditory stimulus detection: implications for multisensory interactions. J Neurosci. 2007;27:11465–11472. doi: 10.1523/JNEUROSCI.2827-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Rihs T, Brodbeck V, Thut G. Resting electroencephalogram alpha-p7wer over posterior sites indexes baseline visual cortex excitability. Neuroreport. 2008;19:203–208. doi: 10.1097/WNR.0b013e3282f454c4. [DOI] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Bestmann S, Linden DE, Dechent P, Goebel R, Baudewig J. Imaging the brain activity changes underlying impaired visuospatial judgments: simultaneous FMRI, TMS, and behavioral studies. Cereb Cortex. 2007;17(12):2841–2852. doi: 10.1093/cercor/bhm013. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Nowak LG, McCormick DA. Membrane mechanisms underlying contrast adaptation in cat area 17 in vivo. J Neurosci. 2000;20:4267–4285. doi: 10.1523/JNEUROSCI.20-11-04267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Sejnowski TJ. Statistical constraints on synaptic plasticity. J Theor Biol. 1977;69:385–389. doi: 10.1016/0022-5193(77)90146-1. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Cattaneo Z, Battelli L, Pascual-Leone A. Baseline cortical excitability determines whether TMS disrupts or facilitates behavior. J Neurophysiol. 2008;99:2725–2730. doi: 10.1152/jn.01392.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG. New light through old windows: moving beyond the virtual lesion approach to transcranial magnetic stimulation. Neuroimage. 2008a;39:549–552. doi: 10.1016/j.neuroimage.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG. Testing the validity of the TMS state-dependency approach: targeting functionally distinct motion-selective neural populations in visual areas V1/V2 and V5/MT?. Neuroimage. 2008b;40:1841–1848. doi: 10.1016/j.neuroimage.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG, Cowey A, Walsh V. Neural adaptation reveals state-dependent effects of transcranial magnetic stimulation. Eur J Neurosci. 2007;25:1874–1881. doi: 10.1111/j.1460-9568.2007.05440.x. [DOI] [PubMed] [Google Scholar]
- Stewart L, Battelli L, Walsh V, Cowey A. Motion perception and perceptual learning studied by magnetic stimulation. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:334–350. [PubMed] [Google Scholar]
- Theoret H, Kobayashi M, Ganis G, Di Capua P, Pascual-Leone A. Repetitive transcranial magnetic stimulation of human area MT/V5 disrupts perception and storage of the motion aftereffect. Neuropsychologia. 2002;40:2280–2287. doi: 10.1016/s0028-3932(02)00112-4. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Hadjikhani NK, Vanduffel W, Liu AK, Mendola JD, Sereno MI, Dale AM. Functional analysis of primary visual cortex (V1) in humans. Proc Natl Acad Sci USA. 1998;95:811–817. doi: 10.1073/pnas.95.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpper R, Mottaghy FM, Brügmann M, Noth J, Huber W. Facilitation of picture naming by focal transcranial magnetic stimulation of Wernicke's area. Exp Brain Res. 1998;121:371–378. doi: 10.1007/s002210050471. [DOI] [PubMed] [Google Scholar]
- Valero-Cabré A, Payne BR, Pascual-Leone A. Opposite impact on 14C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Exp Brain Res. 2007;176:603–615. doi: 10.1007/s00221-006-0639-8. [DOI] [PubMed] [Google Scholar]
- Valero-Cabré A, Payne BR, Rushmore J, Lomber SG, Pascual-Leone A. Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity: a 14C-2DG tracing study in the cat. Exp Brain Res. 2005;163:1–12. doi: 10.1007/s00221-004-2140-6. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial magnetic stimulation: a neurochronometrics of mind. MIT Press; Boston, MA: 2003. [Google Scholar]
- Webster MA, Kaping D, Mizokami Y, Duhamel P. Adaptation to natural facial categories. Nature. 2004;428:557–561. doi: 10.1038/nature02420. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Curr Opin Neurobiol. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ. On the manner in which sensory and cognitive processes contribute to epileptogenesis and are disrupted by it. Acta Neurol Scand. 1986;74:91–95. doi: 10.1111/j.1600-0404.1986.tb04867.x. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ, Bonanni P, Porciatti V, Guerrini R. Physiology of human photosensitivity. Epilepsia. 2004;45(suppl 1):1–7. doi: 10.1111/j.0013-9580.2004.451009.x. [DOI] [PubMed] [Google Scholar]