Abstract
The role of neuroinflammation is increasingly being recognised in a diverse range of cerebral pathologies, including traumatic brain injury (TBI). We used cerebral microdialysis and paired arterial and jugular bulb plasma sampling to characterise the production of 42 cytokines after severe TBI in 12 patients over 5 days. We compared two microdialysis perfusates in six patients: central nervous system perfusion fluid and 3.5% human albumin solution (HAS); 3.5% HAS has a superior fluid recovery (95.8 versus 83.3%), a superior relative recovery in 18 of 42 cytokines (versus 8 of 42), and a qualitatively superior recovery profile. All 42 cytokines were recovered from the human brain. Sixteen cytokines showed a stereotyped temporal peak, at least twice the median value for that cytokine over the monitoring period; day 1: tumour necrosis factor, interleukin (IL)7, IL8, macrophage inflammatory protein (MIP)1α, soluble CD40 ligand, GRO, IL1β, platelet derived growth factor (PDGF)-AA, MIP1β, RANTES; day 2: IL1 receptor antagonist (ra). IL6, granulocyte-colony stimulating factor (G-CSF), chemokine CXC motif ligand 10 (IP10); days 4 to 5: IL12p70, IL10. Brain extracellular fluid concentrations were significantly higher than plasma concentrations for 19 cytokines: basic fibroblast growth factor (FGF2), G-CSF, IL1α, IL1β, IL1ra, IL3, IL6, IL8, IL10, IL12p40, IL12p70, IP10, monocyte chemotactic protein (MCP)1, MCP3, MIP1α, MIP1β, PDGF-AA, transforming growth factor (TGF)α and vascular endothelial growth factor. No clear arterio-jugular venous gradients were apparent. These data provide evidence for the cerebral production of these cytokines and show a stereotyped temporal pattern after TBI.
Keywords: arterio-jugular venous difference, brain trauma, chemokines, cytokine, inflammation, microdialysis
Introduction
Traumatic brain injury (TBI) is a complex pathology with numerous mechanisms of neuronal injury, including blood–brain barrier (BBB) breakdown, cerebral oedema (Unterberg et al, 2004), excitotoxicity (Yi and Hazell, 2006), altered cerebrovascular reactivity (Czosnyka et al, 2009), and mitochondrial dysfunction (Robertson et al, 2009). The role of the cerebral inflammatory response has received increasing interest because it has become apparent that the central nervous system is not immunologically privileged as believed previously (Denes et al, 2009). Empirical evidence for the role of neuroinflammation after TBI has come from a number of findings, including microgial activation (Hailer, 2008), local invasion of circulating immune cells (Gentleman et al, 2004), local cytokine production (Goodman et al, 2008; Hutchinson et al, 2007), and astrocytic activation and proliferation (Schultzberg et al, 2007). Cytokines are a diverse group of small proteins synthesised by a wide variety of cell types, which act as autocrine and paracrine mediators of the inflammatory response to injury or infection. Cytokines may provide a promising therapeutic target because they are likely to have a mechanistic role in the injurious and subsequent reparative processes triggered by neurotrauma (Morganti-Kossmann et al, 2007; Schmidt et al, 2005). Cytokines are expressed by a wide variety of cell types and have pleiotropic actions depending on the exact context in which they are expressed. Therefore, it is important to accurately define the specific cerebral component of the inflammatory response to trauma because it may differ, both quantitatively and qualitatively, from systemic inflammatory responses.
Cerebral microdialysis is a technique that is unique in its capability to continuously sample brain parenchymal concentrations of numerous molecules of interest in vivo over a period of several days (Helmy et al, 2007a). We have previously shown a reliable methodology for the recovery of cytokines using microdialysis in vitro, specifically, overcoming the combined difficulties of low absolute concentration (pg/mL range), small volume of sample (μL range), and low relative recovery (RR) (Helmy et al, 2009).
This study is divided into two parts. The first section of the study is methodological, building on our previous study of cytokine microdialysis in vitro, comparing a crystalloid solution with a 3.5% human albumin solution (3.5% HAS) as microdialysis catheter perfusates. This was achieved by placing two microdialysis catheters in six human patients, side by side, using the same cranial access device to compare both cytokine proportional recovery (PR) and fluid recovery (FR) between the two perfusates (see the ‘Materials and methods' section for definitions).
We concluded that the 3.5% HAS perfusate was superior, and in the second section of the study, we recruited a further 6 patients, using a single microdialysis catheter in each patient perfused with 3.5% HAS solution, to characterise the cytokine response to human TBI using cerebral microdialysis in a total of 12 patients. A 42-cytokine panel (Supplementary Table 1) was chosen to provide the widest currently commercially available multiplex analysis platform, incorporating numerous cytokines of interest. The final aim was to define the relationship between the systemic and the central inflammatory responses by incorporating simultaneous plasma sampling (arterial and jugular venous) alongside microdialysis sampling in all patients.
Materials and methods
Clinical Aspects
The study protocol was approved by the ‘Cambridgeshire (2) Local Research Ethics Committee'. The study recruited patients with severe TBI defined as patients presenting with cranial trauma, a computed tomography scan consistent with TBI, and a postresuscitation Glasgow coma score (GCS) ⩽8. All patients were treated with paralysis, sedation, endotracheal intubation, and ventilation throughout the study period. Patients were included in the study for a maximum of 5 days or until monitoring was discontinued for clinical reasons.
In the first six patients, intracerebral monitoring using a triple lumen cranial access device (Technicam, Newton Abbot, UK) comprised an intracranial pressure (ICP) monitor (Codman, Raynham, MA, USA) and two microdialysis catheters (CMA 71, 100 kDa molecular weight cutoff) placed in adjacent ports within the access device. One catheter was perfused with central nervous system perfusion fluid (CMA Microdialysis AB, Solna, Sweden) (‘crystalloid perfusate') and the other with 3.5% (w/v) HAS (Pharmacy Manufacturing Unit, Ipswich Hospital NHS Trust, Ipswich, UK) composed in central nervous system perfusion fluid. In the second six patients, a single microdialysis catheter perfused with 3.5% HAS was used alongside a brain tissue oxygen monitor (Licox Neurosciences, Andover, UK) and an ICP monitor. All microdialysis catheters were perfused at 0.3 μL/min using CMA 106 microinfusion pumps. The microdialysis vials were kept at the same height as the microdialysis pump to negate any additional hydrostatic forces that could affect FR. The location of all monitoring devices within the brain was confirmed on computed tomography scans. All patients were treated using standardised clinical protocols for severe TBI, as described previously, including jugular bulb oximetry (Helmy et al, 2007b).
Microdialysis Methodology
Microdialysis vials were changed hourly in all catheters and analysed on an ISCUS (CMA Microdialysis AB) bedside analyser as per standard clinical protocols. The remainder of each sample was then stored at −80°C.
Microdialysis is a technique that relies on the diffusion of solutes between the extracellular space and the perfusing fluid within the catheter. Water also diffuses freely across the microdialysis membrane and a fluid shift into or out of the microdialysis catheter can potentially alter the biology of the extracellular space. Fluid recovery is defined as the volume of fluid collected in the microdialysis vial as a percentage of the fluid pumped into the microdialysis catheter, and it provides a measure of the net shift of water across the microdialysis membrane. Ideally, there should be no net flux of water across the microdialysis membrane, i.e., FR=100%. In the first six patients, microdialysis vials were labelled and weighed before and after use to calculate FR: both perfusing solutions had a density of 1 mg/μL (Helmy et al, 2009). An allowance was made for the volume removed by the bedside ISCUS analyser (2.7 μL) and for any variations in the fluid collection time; 100% FR was calculated as 18 μL/h (0.3 μL/min).
Relative recovery, also termed ‘extraction efficiency,' is a key concept in microdialysis: it is defined as the concentration of a species in the microdialysate divided by the concentration of that species in the extracellular space/external solution (Ungerstedt, 1991). It is frequently estimated in vitro to allow a correction for the efficiency by which the microdialysis catheter recovers substances from the external milieu (Helmy et al, 2009). In this manner, microdialysis concentrations of a species can be used to calculate ‘true' external concentrations. Using this methodology, it is not possible to determine RR for any molecular species, because there is no definitive measure of extracellular concentration. However, it is possible to compare the absolute concentration recovered by crystalloid and 3.5% HAS-perfused catheters using PR, defined as concentration of cytokine recovered with 3.5% HAS perfusate divided by the concentration of cytokine recovered with crystalloid perfusate. A PR>1 implies that the 3.5% HAS perfusate recovers cytokines more efficiently than does the crystalloid perfusate, and therefore has a higher RR. A high RR is desirable because it makes the microdialysis technique more sensitive at detecting molecules that are at low concentration in the extracellular space.
Intracranial pressure was measured continuously during the monitoring period in each patient. To explore the relationship between FR and ICP, a mean ICP was calculated over the time period during which each individual vial was collecting fluid, using the ICMplus software (University of Cambridge, Cambridge, UK) (Czosnyka et al, 1994). This allows a direct comparison of the FR in each vial with the mean ICP over the time the vial was collecting fluid.
Plasma Sampling
Blood samples were limited to a maximum of twice daily to minimise the risk of iatrogenic anaemia. Paired samples were collected simultaneously from the arterial and jugular bulb catheters in all patients. The first 1 mL of sample from the dead space within the catheter was discarded and 5 mL was subsequently collected and placed into an ethylenediaminetetraacetic acid tube. Samples were collected at 0800 and 1800 hours notwithstanding periods when this was not possible for clinical reasons (such as patient transfers to the operating theatre). Samples were immediately centrifuged for 15 minutes at 5,000 r.p.m., at 4°C. The supernatant (plasma) was decanted and divided into 150 μL aliquots before storage at −80°C.
Cytokine Analysis
All samples were analysed using the Milliplex MultiAnalyte Profiling Human Cytokine/Chemokine 42 analyte premixed kit (Millipore, St Charles, MI, USA) using the manufacturer's instructions as described previously (Helmy et al, 2009). Owing to the ultralow flow rates inherent in microdialysis, the volume recovered in 1 hour is insufficient for cytokine analysis. Therefore, microdialysates from a 6-hour time period were pooled immediately before analysis in all patients. Plasma samples had sufficient volume for analysis without dilution or pooling. All samples were assayed in duplicate wells (25 μL per well) and the mean of the ensuing results was collected. The plates were read using a Luminex 200 analyser (Luminex Corporation, Austin, TX, USA) running STarStation software (Applied Cytometry Systems, Sheffield, UK). Cytokine concentrations were calculated by reference to an eight-point five-parameter logistic standard curve for each cytokine. Microdialysate and plasma samples were run on separate plates as the buffers and background (control) wells for these samples differ within the assay protocol.
Temporal Profile Cytokine Production in Microdialysate
To allow for a valid comparison of temporal cytokine profiles between patients, an allowance was made for the varying time period between injury and starting of monitoring (Supplementary Table 2). In this manner, a generic temporal cytokine profile was built up, using data obtained from all patients, related to the absolute time of injury. We elected to look for ‘peaks' for each of the cytokines in each patient using the data from the 3.5% HAS-perfused catheters, therefore incorporating data obtained from all 12 patients. The peak is defined as two consecutive time points at which these time point values are at least twice the median value in that patient. The timing of the peak is the time point at which the highest value is found.
Statistical Analysis
All statistical analyses were carried out using the SPSS15 (SPSS, Chicago, IL, USA) for Windows. In all cases in which paired microdialysate or plasma samples were available, samples were only compared with those collected concurrently (microdialysate or plasma samples) because this provides a direct comparison in each patient at a specific time point. The Kolmogorov–Smirnov test was used to determine whether the various parameters measured were normally distributed. On this basis, Student's paired two-tailed t-test (P⩽0.05) was used to compare FR between catheters perfused with crystalloid and 3.5% HAS. The Wilcoxon signed rank test (two tailed, P⩽0.05) was used to compare the cytokine concentrations in paired crystalloid- and 3.5% HAS-perfused catheters, in paired microdialysate and plasma samples, and in plasma arterial and jugular venous samples. Median values are quoted for PR and the ratio between brain extracellular and plasma concentrations because these values are also not normally distributed. The relationship between FR and ICP was explored using a linear regression model and ANOVA (analysis of variance) was used to calculate an F-ratio and determine statistical significance (P⩽0.05). A linear regression model was used to predict RR from previous in vitro data (Supplementary Figure 4) (Helmy et al, 2009).
Results
Patient Demographics and Clinical Course
A total of 12 patients were recruited into the study, all with severe diffuse TBI, of which 6 had two microdialysis catheters inserted (crystalloid perfusate and 3.5% HAS perfusate) and 6 had a single microdialysis catheter inserted (3.5% HAS perfusate). Baseline demographics (Supplementary Table 2) and catheter positions on computed tomography scan (Supplementary Figure 1) were recorded. Patient 6 required a bifrontal decompressive craniectomy, for refractory raised ICP, on day 2 after the beginning of the study. At the time of operation, the microdialysis catheters and ICP monitor were replaced through the craniectomy defect rather than through a triple lumen cranial access device. Supplementary Figure 1 illustrates the repositioned catheters in patient 6. One catheter is clearly seen within the frontal horn of the right lateral ventricle, although it is not possible to definitively determine which of the two catheters is which solely on the scan appearance.
Fluid Recovery
As reported previously in vitro (Helmy et al, 2009), at some time points, minimal (defined as FR<2%) or no fluid was recovered from the microdialysis catheters in vitro. Overall, 16 of 429 (3.7%) of the crystalloid-perfused catheter vials recovered minimal fluid, whereas 12 of 429 (2.8%) 3.5% HAS-perfused catheters recovered minimal fluid. These catheters were excluded from any subsequent analysis. Table 1 shows the mean FR±s.e.m. for each of the catheters, divided by patient and pooled for the first six patients.
Table 1. Comparison of fluid recovery between crystalloid and 3.5% HAS perfusate.
| Patient identifier | n | Fluid recovery mean%±s.e.m. (crystalloid) | Fluid recovery mean%±s.e.m. (3.5% HAS) | Paired t-test P-value |
|---|---|---|---|---|
| 1 | 59 | 93.6±2.5 | 90.2±2.3 | 0.162 |
| 2 | 75 | 73.7±1.2 | 92.1±2.7 | <0.0001 |
| 3 | 61 | 89.8±4.7 | 103.7±5.4 | <0.0001 |
| 4 | 57 | 92.7±2.3 | 96.7±2.8 | 0.038 |
| 5 | 63 | 83.6±3.9 | 99.9±4.5 | <0.0001 |
| 6 | 83 | 85.3±4.5 | 154.8±7.0 | <0.0001 |
| 6 (before craniectomy) | 22 | 83.0±3.2 | 87.3±3.6 | <0.0001 |
| 6 (after craniectomy) | 61 | 96.4±4.9 | 179.2±7.1 | <0.0001 |
| Pooled† | 337 | 83.3±1.5 | 95.8±1.5 | <0.0001 |
FR, fluid recovery; ICP, intracranial pressure; HAS, human albumin solution.
The mean FR±s.e.m. for each of the patients for each perfusate is listed above. ‘n' refers to the total number of pairs of microdialysis vials from which the mean is derived in that patient. The final column is the significance value using a paired, two-tailed t-test comparing the FR in the two catheters. Patient 6 had two sets of catheters inserted for monitoring, one pair before and one pair after decompressive craniectomy, for refractory raised ICP. The postcraniectomy FR values in the 3.5% HAS perfusate catheter may be artefactual as a result of aberrant placement within the cerebral ventricle. The final row shows the pooled (†) values excluding the postcraniectomy values from patient 6.
The mean FR in patient 6 using 3.5% HAS perfusate was strikingly larger than any of the other catheters in the study. There was a marked increase in FR between the initial catheter (87.3% FR) and the repositioned catheter after craniectomy (179.2% FR). This large increase in FR was not mirrored in the crystalloid-perfused catheter in the same patient, suggesting that patient factors, such as ICP, could not have been responsible for the change: in fact, mean ICP was lower after decompressive craniectomy (16.2 mm Hg postcraniectomy versus 20.7 mmHg). We speculated that the large jump in FR in the 3.5% HAS-perfused catheter is a result of inadvertent placement in the ventricle (Supplementary Figure 1).
We also explored the relationship between ICP and FR as a source of potential variation in FR. Supplementary Figure 2 shows a scatter plot of paired ICP versus FR values for both crystalloid and 3.5% HAS perfusates showing that for the crystalloid perfusate, there is a statistically significant relationship between ICP and FR, whereas with the 3.5% HAS perfusate, this relationship is abolished, i.e., FR is constant despite variations in ICP.
Proportional Recovery of Cytokines
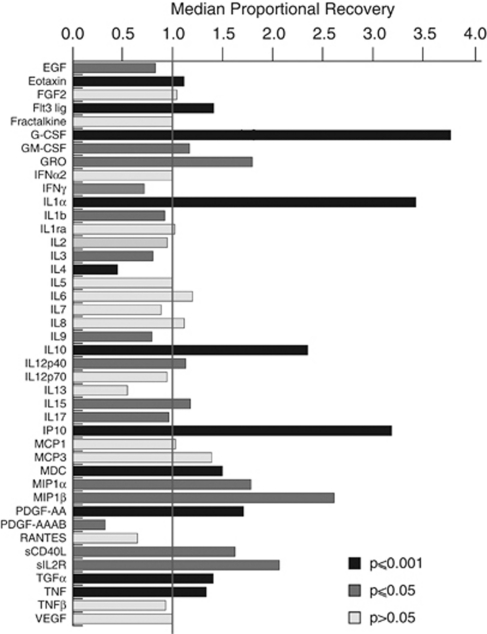
Table 2 summarises the data obtained from the first six patients: the percentage of samples in which the concentration of cytokine could be determined and the median cytokine concentrations recovered with each perfusate (i.e., crystalloid or 3.5% HAS perfusate). Proportional recovery was used to compare the relative performance of the crystalloid and 3.5% HAS perfusates in recovering cytokines (Table 2): the median PR for each cytokine is illustrated in Figure 1. To aid in distinguishing which cytokine had a significant difference in paired cytokine concentrations (Wilcoxon signed rank test, two tailed, P⩽0.05 and P⩽0.001) between the two catheters, these cytokines have been highlighted with darker coloured bars. A total of 18 cytokines had a significantly higher recovery using 3.5% HAS perfusate (PR>1), whereas 8 cytokines had a significantly higher recovery with the crystalloid perfusate (PR<1). For a number of cytokines, a proportion of samples analysed decreased below the lower limit of sensitivity of the relevant assay. There is considerable variation (Supplementary Figure 3) over time in the absolute cytokine level recovered in each patient; therefore, comparisons were made only between catheters in the same patient.
Table 2. Cytokine concentrations and proportional recovery in the first 6 patients.
| Cytokine |
Crystalloid perfusate concentrations |
3.5% HAS perfusate concentrations |
Proportional recovery | ||
|---|---|---|---|---|---|
| % Samples recovering cytokine | Median (pg/mL) | % Samples recovering cytokine | Median (pg/mL) | Median (pg/mL) | |
| EGF | 88.6 | 4.17 | 76.3 | 3.00 | 0.83* |
| Eotaxin | 100.0 | 3.48 | 99.0 | 3.49 | 1.12** |
| FGF2 | 94.9 | 5.73 | 99.0 | 5.92 | 1.04 |
| Flt3 lig | 82.4 | 4.22 | 80.4 | 7.13 | 1.42*** |
| Fractalkine | 69.0 | 9.21 | 83.5 | 20.39 | 1.00 |
| G-CSF | 83.5 | 10.82 | 99.0 | 72.83 | 3.80*** |
| GM-CSF | 65.0 | 3.57 | 79.4 | 3.99 | 1.17* |
| GRO | 80.4 | 31.61 | 99.0 | 53.77 | 1.80* |
| IFNα2 | 58.8 | 8.81 | 53.6 | 11.60 | 1.00 |
| IFNγ | 24.7† | 7.61 | 41.2† | 6.07 | 0.72 |
| IL1α | 83.5 | 19.25 | 86.6 | 80.21 | 3.45*** |
| IL1β | 61.9 | 5.75 | 51.6 | 4.27 | 0.92* |
| IL1ra | 97.9 | 90.60 | 99.0 | 73.23 | 1.02 |
| IL2 | 68.0 | 0.89 | 61.9 | 0.94 | 0.95 |
| IL3 | 59.8 | 49.83 | 55.7 | 26.26 | 0.80* |
| IL4 | 40.2† | 2.59 | 21.7† | 0.97 | 0.45** |
| IL5 | 80.4 | 1.16 | 79.4 | 1.17 | 1.00 |
| IL6 | 92.8 | 384.61 | 99.0 | 570.44 | 1.20 |
| IL7 | 40.2† | 12.77 | 56.7 | 6.41 | 0.89 |
| IL8 | 88.7 | 317.59 | 99.0 | 339.09 | 1.12 |
| IL9 | 72.2 | 0.65 | 69.0 | 0.52 | 0.80* |
| IL10 | 66.0 | 2.80 | 95.9 | 6.64 | 2.36*** |
| IL12p40 | 56.7 | 2.45 | 51.6 | 3.91 | 1.13* |
| IL12p70 | 100.0 | 1.48 | 99.0 | 1.43 | 0.95 |
| IL13 | 35.0† | 0.76 | 48.4† | 0.27 | 0.55 |
| IL15 | 70.1 | 1.79 | 60.8 | 2.05 | 1.18* |
| IL17 | 80.4 | 0.93 | 78.4 | 0.89 | 0.97* |
| IP10 | 85.6 | 851.34 | 97.9 | 2834.09 | 3.20*** |
| MCP1 | 81.4 | 2562.55 | 74.2 | 2521.35 | 1.03 |
| MCP3 | 100.0 | 6.69 | 99.0 | 23.01 | 1.40 |
| MDC | 87.6 | 8.71 | 97.9 | 12.45 | 1.50*** |
| MIP1α | 87.6 | 26.94 | 93.8 | 38.10 | 1.79* |
| MIP1β | 99.0 | 35.55 | 99.0 | 66.53 | 2.63* |
| PDGF-AA | 97.9 | 397.15 | 99.0 | 518.75 | 1.72*** |
| PDGF-AAAB | 41.2† | 15.87 | 17.5† | 1.81 | 0.32* |
| RANTES | 66.0 | 49.09 | 84.5 | 22.01 | 0.65 |
| sCD40L | 51.6 | 11.36 | 47.4† | 39.02 | 1.63* |
| sIL2R | 40.2† | 1.66 | 71.1 | 1.53 | 2.07* |
| TGFα | 89.7 | 6.70 | 90.7 | 17.93 | 1.41*** |
| TNF | 73.2 | 0.95 | 96.9 | 1.03 | 1.34*** |
| TNFβ | 45.4† | 1.00 | 46.4† | 0.96 | 0.93 |
| VEGF | 74.2 | 11.89 | 78.4 | 11.89 | 1.00 |
EGF, epidermal growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; PR, proportional recovery; TGF, transforming growth factor; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor; HAS, human albumin solution; FGF2, basic fibroblast growth factor; Flt3 lig, Fms-related tyrosine kinase 3 ligand; IP10, chemokine CXC motif ligand 10; MCP, monocyte chemoattractant protein; MDC, macrophage derived chemoattractant; MIP, monocyte inflammatory protein; PDGF, platelet derived growth factor; sIL2R, soluble interleukin 2 receptor; sCD40L, soluble CD40 ligand.
The % samples recovering cytokine columns show the number of samples in which a given cytokine could be quantified for the two perfusates. The cytokines that could only be quantified in less than half the samples are marked with a †. The median values are shown for each of the two perfusates and the PR values. The Wilcoxon signed rank test (two tailed) was used to determine whether there was a statistical difference between the crystalloid and 3.5% HAS-perfused cytokine concentrations recovered by microdialysis. The stars in the median proportional recovery column indicate the level of significance (*P<0.05, **P<0.001, ***P<0.0001).
Figure 1.
Proportional cytokine recovery. The histogram plots the median proportional recovery for each cytokine, defined as the cytokine concentration (3.5% HAS perfusate)/cytokine concentration (crystalloid perfusate). A Wilcoxon signed rank test two-tailed was used to compare the cytokine values derived from the two catheters. Only paired samples were used for this calculation. The dark coloured bars indicate the cytokines that had a statistically significant difference between the two perfusates (grey bars P⩽0.05, black bars P⩽0.001). HAS, human albumin solution.
Qualitative Comparison of Cytokine Production Profiles Between Perfusates
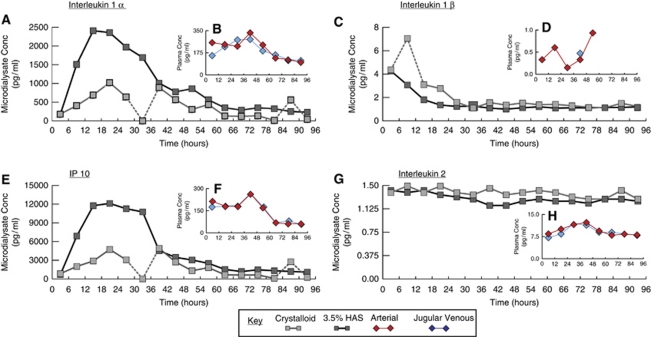
In addition to differences in cytokine RR between the two perfusates, a qualitative difference in temporal cytokine profiles between the two perfusates is also apparent. Figure 2 shows representative examples of the cytokine temporal profiles recovered with crystalloid and 3.5% HAS perfusates from patient 1. There are a number of time epochs in which the crystalloid-perfused catheter seems to recover cytokines at aberrant concentrations, e.g., interleukin (IL)1α time point 30 to 36 hours and IL1β time point 6 to 12 hours. These anomalous values are unlikely to be caused by patient- or catheter-related factors, as the anomalous values only occur for a given cytokine. For example, IL2, has a consistent concentration throughout the monitoring period. These apparently aberrant values occur sporadically throughout crystalloid-perfused catheter data but not in the 3.5% HAS data set.
Figure 2.
Representative cytokine temporal profiles in microdialysate and plasma. The line graphs illustrate representative data obtained from patient 1. The larger panels (A, C, E, G) illustrate the microdialysate-derived values with 3.5% HAS and crystalloid perfusates. The inset panels (B, D, F, H) show the paired arterial and jugular venous plasma values. Sampling frequency differs between the microdialysate (6 hourly) and the plasma (12 hourly). Timescales on horizontal axes are relative to the beginning of microdialysis. Dashed lines link time points that appear aberrant. For IL1α, IL1β, and IP10, their concentrations in microdialysates are several fold higher than in the corresponding plasma samples. Contrastingly, for IL2, plasma concentrations are higher than for microdialysates. Microdialysate cytokine temporal profiles appear independent of those in plasma. These cytokines are chosen to illustrate that the temporal profiles may show a well-defined peak (IL1α and IP10), high initial values that then decrease, suggesting the downstroke of an earlier peak (ILβ), or stability over the entire monitoring period (IL2), suggesting consistent catheter performance. IL, interleukin; HAS, human albumin solution.
Temporal Profile Cytokine Production in Microdialysate
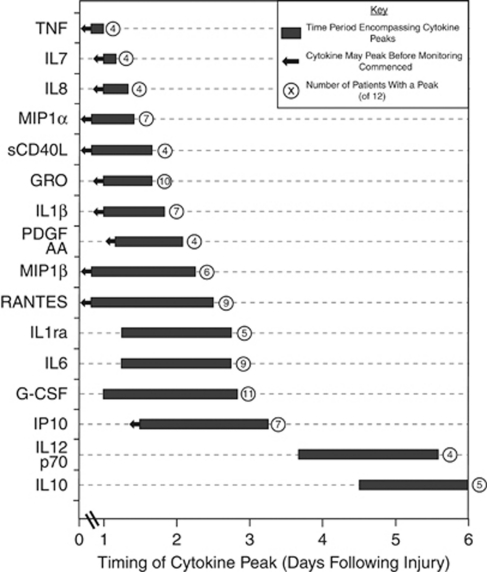
Supplementary Table 3 lists the times at which peaks occurred for each of the cytokines in each patient. If a peak value occurs in the first sample and falls off with time (e.g., Figure 2C, 3.5% HAS-perfused catheter), it is not possible to determine whether this is the true peak time point in the patient or a downstroke of an earlier peak that occurred before monitoring started. In this case, a ‘<' has been used to indicate that the peak occurred at or before this point. Figure 3 summarises the data for all patients by showing a bar encompassing all the time points at which at least four patients had peaks that coincided over a 48-hour period. An arrow has been used to indicate those peaks that occurred in the first monitoring sample. A total of 16 cytokines showed such peaks and they are ordered with the first cytokine having the earliest peak.
Figure 3.
Timing of cytokine peaks in microdialysates. A peak is defined as two consecutive values greater than twice the median concentration for each cytokine recovered with 3.5% HAS perfusate. For further explanation, see the ‘Discussion' section. For any cytokine with peaks in four or more patients clustered within a 48-hour period, a box is plotted on the timeline encompassing these peak time points. The cytokine with the earliest peaks is plotted at the top of the figure. Some peaks occurred in the first sample in the monitoring period. In this circumstance, it is not possible to distinguish whether this is the ‘true' peak or a downstroke of an earlier peak that occurred before the start of monitoring (e.g., Figure 2C, IL1β with 3.5% HAS perfusate). In such cases, an arrow is used to indicate that the ‘true' peak may precede the box plotted on the timeline. Not all patients had peaks for every cytokine. The number following the box on the timeline indicates how many patients (out of 12) had a peak within the box. Cytokines that only had peaks in four or fewer patients are not plotted. G-CSF, granulocyte colony-stimulating factor; IL, interleukin; TNF, tumour necrosis factor; HAS, human albumin solution.
Arterio-Jugular Venous Concentration Gradient
The arterio-jugular (AJ) difference (arterial concentration minus jugular venous concentration) was calculated for each individual pair of samples in each patient. The absolute differences in cytokine concentration between arterial and jugular venous samples are small (few pg/mL) and are therefore difficult to reliably resolve. Therefore, we grouped data obtained from all 12 patients by calculating individual AJ gradients for each pair of samples and taking the mean for each day after injury, to build up a more accurate picture of AJ gradients (Supplementary Table 4). Grouping by day after injury is required as monitoring started at differing times. For this reason, the earliest mean AJ difference is at day 2. A negative value indicates net cytokine production across the cerebrovascular bed. No clear pattern was resolved from the AJ gradient data.
Comparison of Microdialysis and Arterial Plasma Concentration
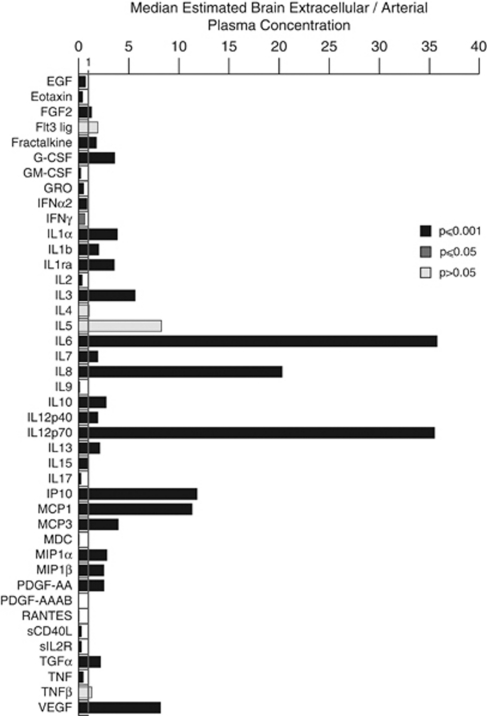
Pairs of microdialysis and arterial plasma samples collected simultaneously were also compared. The RR for each cytokine varies; hence, a correction to the microdialysate concentration is required to allow a valid comparison with the plasma: we termed this ‘estimated brain extracellular concentration.' The RR was either determined in vitro, where available, or estimated from the molecular weight and oligomerisation characteristics of each of the cytokines (Supplementary Figure 4) (Helmy et al, 2009). Figure 4 shows the median brain extracellular/arterial plasma ratio for each of the cytokines. Table 3 shows the median values for the brain extracellular/arterial plasma ratios and microdialysate/arterial plasma ratios. These data show that for 23 cytokines, there is a significantly higher concentration in the brain compared with the plasma, suggesting cerebral production of these cytokines. These include IL1α, IL1β, and their natural inhibitor IL1ra, the median concentrations of which are 3.87-, 2.01-, and 3.55-fold higher, respectively, in the brain extracellular space than in the corresponding arterial plasma. Even more marked elevations occur, e.g., for IL6, IL8, IP-10, IL12p70, and MCP1, the brain extracellular median concentrations of which are each more than 10-fold higher than in the plasma. This is consistent with the concept of a central inflammatory cytokine cascade occurring within the injured brain.
Figure 4.
Estimated brain extracellular/plasma arterial concentration. The histogram illustrates the median ratio of estimated brain extracellular concentration (calculated from 3.5% HAS-perfused catheter values and RR values in Table 3) divided by the paired plasma arterial concentration. The paired samples were used to calculate the ratio at each time point, and the median of these values plotted. A ratio >1 indicates that the estimated brain extracellular concentration is higher than the arterial plasma concentration. A Wilcoxon signed rank two-tailed test was used to compare the cytokine concentrations in brain extracellular space and arterial plasma. The dark coloured bars indicate the cytokines that had a statistically significant difference between the two perfusates (grey bars P⩽0.05, black bars P⩽0.001). EGF, epidermal growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; RR, relative recovery; TGF, transforming growth factor; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor; HAS, human albumin solution.
Table 3. Brain extracellular/arterial plasma ratios.
| Cytokine | In vitro relative recovery (%) | Calculated relative recovery (%) | Median [brain extracellular]/[arterial plasma] ratio | Median [microdialysate]/[arterial plasma] ratio |
|---|---|---|---|---|
| EGF | 55.9 | 0.69*** | 0.38 | |
| Eotaxin | 54.1 | 0.39*** | 0.21 | |
| FGF2 | 46.5 | 1.29*** | 0.60 | |
| Flt3 lig | 46.2 | 1.91 | 0.88 | |
| Fractalkine | 54.0 | 1.77*** | 0.96 | |
| G-CSF | 45.2 | 3.61*** | 1.63 | |
| GM-CSF | 48.7 | 0.21*** | 0.10 | |
| GRO | 54.6 | 0.50*** | 0.27 | |
| IFNα2 | 44.8 | 0.83** | 0.37 | |
| IFNγ | 46.9 | 0.63* | 0.30 | |
| IL1α | 48.0 | 3.87*** | 1.86 | |
| IL1β | 38.4 | 2.01** | 0.77 | |
| IL1ra | 38.4 | 3.55*** | 1.36 | |
| IL2 | 52.8 | 0.35*** | 0.19 | |
| IL3 | 28.6 | 5.64*** | 2.73 | |
| IL4 | 56.7 | 1.06** | 0.60 | |
| IL5 | 39.0 | 8.26 | 3.22 | |
| IL6 | 25.5 | 35.75*** | 9.12 | |
| IL7 | 46.3 | 1.91** | 0.89 | |
| IL8 | 73.4 | 20.30*** | 14.90 | |
| IL9 | 49.2 | 0.09*** | 0.04 | |
| IL10 | 8.7 | 2.75*** | 0.24 | |
| IL12p40 | 27.0 | 1.91*** | 0.52 | |
| IL12p70 | 1.3 | 35.49*** | 0.44 | |
| IL13 | 50.5 | 2.12*** | 1.07 | |
| IL15 | 49.2 | 1.00** | 0.49 | |
| IL17 | 43.7 | 0.23*** | 0.10 | |
| IP10 | 54.0 | 11.81*** | 6.37 | |
| MCP1 | 53.9 | 11.33** | 6.10 | |
| MCP3 | 53.5 | 3.94*** | 2.11 | |
| MDC | 54.4 | 0.06*** | 0.03 | |
| MIP1α | 55.6 | 2.81*** | 1.56 | |
| MIP1β | 50.1 | 2.51*** | 1.26 | |
| PDGF-AA | 36.8 | 2.53*** | 0.93 | |
| PDGF-AAAB | 39.9 | 0.01*** | 0.00 | |
| RANTES | 54.6 | 0.05*** | 0.03 | |
| sCD40L | 45.8 | 0.27** | 0.12 | |
| sIL2R | 34.7 | 0.27*** | 0.09 | |
| TGFα | 56.5 | 2.18*** | 1.23 | |
| TNF | 31.2 | 0.46*** | 0.14 | |
| TNFβ | 13.4 | 1.30 | 0.18 | |
| VEGF | 4.2 | 8.16*** | 0.34 |
EGF, epidermal growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; RR, relative recovery; TGF, transforming growth factor; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor; HAS, human albumin solution; FGF2, basic fibroblast growth factor; Flt3 lig, Fms-related tyrosine kinase 3 ligand; IP10, chemokine CXC motif ligand 10; MCP, monocyte chemoattractant protein; MDC, macrophage derived chemoattractant; MIP, monocyte inflammatory protein; PDGF, platelet derived growth factor; sIL2R, soluble interleukin 2 receptor; sCD40L, soluble CD40 ligand.
The 3.5% HAS microdialysis catheter concentrations have been used to calculate a ratio of microdialysate to arterial plasma ratio. As this does not take into account the fact that the microdialysis sample recovers only a fraction of the true extracellular concentration, we have also quoted the estimated brain extracellular/arterial plasma ratio. We have used our previous in vitro measurements of RR (Helmy et al, 2009) where available, and for those cytokines where there is no published data for RR, we have estimated the RR based on apparent molecular weight (Supplementary figure 4). This provides the best estimate for RR available from previously published data; however, we have also included the uncorrected median microdialysate/arterial plasma concentration ratio for transparency. The brain extracelluar/arterial concentration ratio was only calculated for those samples that were taken concurrently and the median quoted for this distribution. The Wilcoxon signed rank test (two-tailed) was used to determine whether there was a statistical difference between the plasma and estimated brain extracellular concentration. The stars in the median [brain extracellular]/[arterial plasma] ratio indicate the level of significance (*P<0.05, **P<0.001, ***P<0.0001). The ‘raw' median [microdialysate]/[arterial plasma] ratio has also been included to show that even without a correction for RR, many of the cytokines have a several-fold higher concentration in microdialysate than in arterial plasma. The square brackets are used to denote concentrations throughout.
Discussion
In this study, we have shown a reliable methodology for the recovery of a wide range of cytokines from the human brain in vivo using cerebral microdialysis and 3.5% HAS perfusate. We established that the 3.5% HAS perfusate has a superior FR and PR to crystalloid perfusate, for the majority of cytokines, and we therefore used this perfusate in six subsequent patients to further explore the temporal profile of cytokine production after TBI and its relationship to plasma cytokines. Cytokine production is highly compartmentalised, with quantitative and qualitative differences between brain parenchymal and systemic cytokine concentrations.
Factors Affecting Fluid Recovery
The evidence from in vitro microdialysis studies is that the key variables affecting FR are hydrostatic (Hamrin et al, 2002; Hillman et al, 2005) and oncotic pressure gradients (Helmy et al, 2009). However, in vitro FR data cannot be easily extrapolated to the in vivo situation because both ICP (affecting hydrostatic pressure) and protein concentration in the extracellular space (affecting oncotic pressure) may both vary between patients and over time within the same patient. We have shown that 3.5% HAS has a superior FR in vivo (95.8%±1.5% versus 83.3%±1.5% pooled across the first 6 patients, Table 1) and over the range of pressures encountered clinically, ICP does not appreciably affect FR.
For a small minority of the microdialysis collection time points, no fluid was recovered from the catheter. Our previously published in vitro data showed that this occurred in 56% of crystalloid-perfused catheters but in none of the 3.5% HAS-perfused catheters (Helmy et al, 2009). In this study, crystalloid-perfused catheters recovered fluid in 96% of hourly epochs approximating catheter performance with the 3.5% HAS perfusate (97% of hourly epochs). We hypothesise that the improved performance of the crystalloid perfusate in vivo compared with that in vitro reflects protein within the brain extracellular fluid diffusing into the microdialysis catheter effectively modifying the perfusate into one that more closely approximates a colloid, such as 3.5% HAS (Maurer et al, 2003). This endogenously derived protein may counterbalance the oncotic pressure across the microdialysis membrane or may interact directly with the membrane substrate.
The mean FR values for the 3.5% HAS-perfused catheter in patient 6, after craniectomy, appear to be ∼1.5-fold higher than in other patients, and we speculate that it is this catheter that is most likely to be in an intraventricular location after craniectomy (Supplementary Figure 1). This has two important implications. First, we excluded the FR values from both postcraniectomy catheters when calculating a mean FR across all patients. Second, it seems that microdialysis catheter performance is related to the interface between the catheter and the surrounding tissue, specifically brain/catheter versus cerebrospinal fluid (CSF)/catheter. We hypothesise that diffusion of fluid within the brain extracellular space is limited by the tortuous path between cells and the interactions with extracellular matrix as compared with the diffusion of fluid within CSF (Sykova and Nicholson, 2008). In this manner, water in the CSF can more easily pass into a microdialysis catheter in the ventricle and result in a higher FR value. This highlights a further important limitation of in vitro estimations of microdialysis catheter performance, as they rely on the placement of a catheter into a fluid-filled container.
Cytokine Proportional Recovery
We have previously shown that in vitro, the 3.5% HAS perfusate has a higher RR than the crystalloid perfusate for a range of cytokines (Helmy et al, 2009). It is not possible to calculate RR using the current methodology; therefore, to allow a comparison between perfusates, we calculated PR. The underlying assumption is that adjacent regions of the brain in which the paired microdialysis catheters reside have equivalent levels of cytokine production. For the majority of cytokines in which a significant difference exists (18 of 42 cytokines in Figure 1), the 3.5% HAS perfusate resulted in a higher RR than did the crystalloid perfusate (i.e., PR>1). However, for 8 of 42 cytokines (namely epidermal growth factor, interferon-γ, IL1β, IL3, IL4, IL9, IL17, and PDGF-AA) the crystalloid perfusate achieved a significantly higher mean recovery than did 3.5% HAS.
The median cytokine concentrations recovered with crystalloid- and 3.5%HAS-perfused catheters are listed in Table 2 and the percentage of samples that recovered quantifiable cytokine. For those cytokines at a concentration at the limit of sensitivity of the assay, there were samples that fell below this threshold and the cytokine concentration could not be measured. For a minority of the cytokines (6 of 42), less than half the samples could be quantified using the 3.5% HAS perfusate (indicated with †). We suggest that the microdialysis methodology used is not suitable for reliable recovery of these cytokines. This difficulty in recovering these cytokines is likely to be a combination of low ‘true' extracellular concentration and/or a poor RR. In the majority of cases, a cytokine concentration could not be quantified in every sample. This reflects, in part, the dramatic changes in cytokine concentrations over the period of monitoring, such that baseline levels of the cytokine may be close to the limit of sensitivity of the assay, particularly during the latter part of the monitoring period, although peak values may be much higher. For example, in Figure 2, the highest/lowest values in pg/mL of IL1α (2,500/500), IL1β (4/1), and IP10 (12,000/3,000) vary by 4- to 5-fold. In addition to a quantitative difference in cytokine recovery with crystalloid and 3.5% HAS perfusate (PR), there is also a qualitative difference between the perfusates in that 3.5%HAS-perfused catheters seem to recover cytokine more consistently. Although this finding is empirical, we believe that it is worth highlighting because it has implications for the methodology of future studies.
Temporal Patterns of Cerebral Extracellular Cytokine Production
A key aim of this study is to provide a description of the sequential production of cytokines in the human brain after TBI. We have presented the data set of cytokine concentrations recovered by microdialysis from the first six patients in Supplementary Figure 3, showing a several-fold variation in the cytokine concentrations recovered between patients, even though the patients have radiologically similar diffuse TBI. This variation causes a fundamental problem in interpreting and presenting microdialysis data, which has already been shown in the microdialysis literature (Hutchinson et al, 2007; Mellergard et al, 2008). This is compounded by the fact that the time at which monitoring starts varies between patients (Supplementary Table 1), such that if maximal cytokine production occurs at an early time point and then decreases to a baseline level, this peak may not be apparent in patients in whom monitoring starts later than this peak (e.g., patients 1, 4, and 7). The variation in absolute microdialysis cytokine concentrations between patients may reflect differences in the severity of the patient's injury, the time after injury at which monitoring starts, or the specific locality in which the microdialysis catheter resides. For example, in patient 2, many of the highest cytokine concentrations are recovered. Supplementary Figure 1 shows that both catheters in this patient reside in the pericontusional brain as evidenced by hypodensity on computed tomography scan. Patient 2 also started monitoring within 24 hours of injury, potentially leading to high concentrations of cytokines that peak early after injury. This combination of factors might contribute to the high concentrations of cytokines recovered, although firm conclusions cannot be drawn on the basis of a single patient's data.
In view of the inherent variations in absolute cytokine concentrations between patients, to make a direct comparison of the timing of cytokine production between patients, we defined a ‘peak' time point for each cytokine in each patient. This is based on the assumption that whatever the underlying severity of injury in the volume of brain that is sampled, and therefore the absolute magnitude of cytokine produced, we would expect each cytokine to be expressed in a stereotyped temporal sequence in relation to the injury. We deliberately used conservative criteria to identify peaks: namely two consecutive time points, at which the level of the cytokine in question is twice that of its median level in the whole of that patient's period of monitoring, to identify genuine peaks among the intrinsically ‘noisy' microdialysis data. The time corresponding to the higher of these two concentrations is judged as the ‘peak time point.' In this manner, we can be confident that any peaks identified are true changes from baseline values, although at the cost of missing potentially genuine but smaller peaks. We stipulated that two consecutive values must be twice the median value (rather than a single data point) to avoid wrongly ascribing a peak to a single outlier. We chose the median concentration as the measure of central tendency because of the skewed nature of the concentration values. We realise that this description of a peak is arbitrary, but we believe it provides a robust narrative of the timing of cytokine production.
Other approaches such as calculating the area under the curve of concentration versus time are not suitable because this is the equivalent of taking a mean of the cytokine concentrations (sampling time points are evenly spaced). A mean value would discard much of the useful information in the temporal profile as the baseline values simply dilute the large, but brief, period of increased cytokine production. This is particularly relevant when the time that monitoring starts varies, as the period over which baseline concentrations are found also varies.
Supplementary Table 3 lists all the cytokine peaks for each of the 12 patients using the 3.5% HAS microdialysis data. In Figure 3, the peaks are grouped into boxes with each box covering the time period in which at least four separate patient's peaks occurred within 48 hours of each other. The cytokines fitting these criteria were then ordered with those peaking earliest first to build up a temporal profile of cytokine production after TBI. This provides a novel description of the cerebral inflammatory response encompassing several molecules of interest. These observational data provide direct empirical evidence, in human patients, for a number of interrelationships shown in preclinical studies. For example, IL1β has been shown to regulate IL6 and IP10 release in primary neuronal cell culture (Tsakiri et al, 2008), IL1β increases rapidly in microdialysis studies of rodent models of TBI (Fassbender et al, 2000), and IL1β release precedes that of IL6 at the level of both mRNA and protein (Folkersma et al, 2008; Yan et al, 1992). Interleukin-1β is a promising target for modulating the inflammatory response to neurotrauma, leading to the fascinating possibility of selectively inhibiting its action with IL1ra and using downstream cytokines as a measure of biologic efficacy.
Compartmentalisation of Cytokine Production
Simultaneous plasma and microdialysis sampling allows a direct comparison between brain extracellular concentration and arterial plasma concentration of cytokines (Table 3, Figure 4). We used cytokine's RR values determined empirically in vitro or estimated based on the relationship between RR and molecular weight (Helmy et al, 2009) to estimate ‘true' extracellular concentrations. For clarity, we also listed a ‘raw' median microdialysate concentration/arterial plasma ratio in Table 3, without any correction for RR. A number of cytokines have a concentration several fold higher in brain extracellular space than in the plasma even when no correction is made for RR. If Figures 3 and 4 are compared, it can be seen that cytokines that showed peak values beyond the first day (namely IL1ra, IL6, G-CSF, IP10, IL12p70, IL10) and six of the cytokines that may have peaked on the first day (IL7, IL8, MIP1α, IL1β, PDGF-AA, MIP1β) all had significantly higher brain extracellular concentrations than in arterial plasma. Furthermore, as this calculation uses median values, it tends to underestimate the even larger gradients that exist when a cytokine peak occurs in the microdialysate samples, especially if this putative peak has occurred before monitoring starts, such as for IL1β (e.g., if concentrations at the earliest time points are compared in Figures 2C and 2D). The interactions between immune mediators and the BBB is complex (Banks and Erickson, 2010); however, the most parsimonious explanation is that these high concentration gradients between arterial plasma and the brain extracellular fluid compartments are attributed to cerebral production of cytokines. Therefore, we conclude that plasma cytokine levels are a poor reflection of intracerebral inflammation. Even if plasma cytokine levels correlate with outcome after TBI (Berger et al, 2009), there are multiple mechanisms by which plasma markers can be confounded by stronger predictors of outcome (Helmy et al, 2010).
Concurrent sampling of jugular venous and arterial blood allows the calculation of the AJ difference, which has been used extensively for determining net production/uptake of substances from the intravascular space into the brain, based on the reverse Fick principle (Macmillan and Andrews, 2000). We could not show any clear pattern in the AJ gradient for any cytokine and the small absolute AJ differences may reflect poor BBB permeability, despite the large gradient across the BBB for many cytokines. Therefore, we conclude that the AJ gradient is a poor gauge of intracerebral cytokine production, as previous studies have also struggled to show a reliable AJ gradient for cytokines known to be expressed after TBI. For example, McKeating et al (1997) could only show an AJ difference for IL6 but not for IL1β, IL8, and tumour necrosis factor, whereas Goodman et al (2008) found no overt AJ differences for IL1β, IL6, IL8, IL10, IL12, and tumour necrosis factor.
Cerebrospinal fluid is often used as a surrogate of intracerebral concentrations of cytokines, particularly in pharmacokinetic studies (Clark et al, 2008). It has the advantage of providing a global measure of cytokine production, it does not require pooling/dilution for analysis, and it is easier to sample. Previous studies have shown the differences between CSF and the peripheral inflammatory responses to trauma (Buttram et al, 2007; Morganti-Kossmann et al, 2001). However, a comparison of CSF cytokine levels with our microdialysis data, suggests that for a number of cytokines (IL1β, IL1ra, IL6, tumour necrosis factor), CSF concentrations are considerably higher than their brain extracellular concentrations (Chiaretti et al, 2008; Graetz et al, 2009; Shiozaki et al, 2005). This may reflect differences between the BBB and blood–CSF barrier such that the CSF compartment and the brain extracellular fluid compartment cannot be regarded as equivalent. Therefore, CSF sampling and microdialysis-derived cytokines should be considered as complementary techniques. As these cytokines are produced within the brain, are secreted into the brain extracellular space, and act on cell-surface receptors without the need for any exchange with the CSF space, we would suggest that the biologically relevant compartment for study is the brain extracellular space and not the CSF space.
Limitations
A key issue within the microdialysis literature is whether insertion of a microdialysis catheter in itself traumatises the tissue in which it resides and results in a response that is artefactual or a composite of underlying physiology overlaid with catheter microtrauma. Cerebral microdialysis has been widely used as a monitor of metabolic intermediaries, and consensus guidelines suggest ignoring samples taken in the first hour after catheter insertion (Bellander et al, 2004). Our current data are not consistent with catheter trauma artefact for two reasons. First, the timings of the cytokine peaks we identified clustered between patients when the time from injury was considered rather than the time from the beginning of monitoring. If cytokine production was related to catheter trauma, we would expect cytokines to reliably peak a given time after catheter insertion. Patients 1,4, and 7, who started monitoring ∼2 to 4 days after injury had many more peaks in the first sampling time points (suggesting a downstroke of an earlier peak) than in the remainder of the patients who started monitoring at ∼1 day after injury. The magnitude of these peaks (at least twice the median value) suggests that these are biologically relevant changes that reflect the underlying pathophysiology. For this reason, we did not exclude the data from our earliest samples as has been suggested previously (Hutchinson et al, 2007; Mellergard et al, 2008). Second, the temporal profile of a number of cytokines shows no peaks at all. This stable concentration recovery, suggests that peaks in cytokine levels are cytokine specific and that the microdialysis catheter performance is not altering over time owing to haemorrhage, encapsulation, or biofouling. It is therefore reasonable to conclude that the observed changes in cytokine concentration are a genuine reflection of the cerebral inflammatory response to trauma.
This study is limited by the small number of patients recruited and the wide variation in the absolute concentrations of cytokine recovered using microdialysis. This makes it difficult to analyse a complex data set in a heterogeneous pathology such as TBI and limits us to a largely descriptive approach. At present, this is the most wide-ranging study of cytokine production after TBI in any species and has allowed us to measure cytokines not previously recovered from the human brain using microdialysis (e.g., tumour necrosis factor, IL7, MIP-1α, sCD40L, GRO, PDGF-AA, MIP1β, G-CSF, IP10, and IL12p70, all of which showed cytokine peaks) as well as allowing direct comparisons of temporal profiles. The expense of the multiplex technology required to simultaneously assay a wide range of molecules combined with the logistical challenges in recruiting human patients makes it difficult to expand the number of patients dramatically. Our approach has been to summarise the data as succinctly as possible and to avoid multiple comparisons that may throw up spurious associations. Human research of this type is nevertheless a powerful complement to preclinical studies because it provides the first step in translating our understanding of basic science into the clinical domain.
Conclusions
This study has shown a robust and reliable methodology, using the 3.5% HAS perfusate, for the systematic recovery of cytokines from the brain extracellular space using microdialysis. Compared with the crystalloid perfusate, the 3.5% HAS perfusate has a more reliable FR, which is not markedly affected by ICP and it improves recovery of cytokines in vivo increasing the absolute sensitivity of the technique.
Using the data from the 3.5% HAS-perfused catheter, we showed a temporally stereotyped cytokine response to TBI for a number of cytokines: these cytokines, together with those that are expressed at high concentration in the cerebral extracellular fluid are promising candidates for further study into TBI pathophysiology. These cytokine production patterns mimic data from animal models and provide a powerful validation of these data in humans. As the temporal production of these cytokines extends for many hours or days after injury, modulation of their actions may provide a credible therapeutic time window that is amenable to clinical intervention.
Finally, we have shown that there is a poor correlation between the systemic inflammatory response and the central inflammatory response. This is an important finding, because this compartmentalisation has often been overlooked, and previous studies of cytokine release in neurologic conditions have relied on plasma markers or CSF. Our findings are particularly relevant to drug studies because the site of action of many of these powerful mediators is at the parenchymal level.
Acknowledgments
The authors acknowledge the helpful comments provided by Professor Nancy Rothwell, Faculty of Life Sciences, University of Manchester. The authors also thank Dr Chris Palmer, Centre for Applied Medical Statistics, University of Cambridge for his statistical advice.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
AH is supported by a joint Medical Research Council/Royal College of Surgeons of England Clinical Research Training Fellowship and a Raymond and Beverly Sackler Fellowship. KLHC is supported by the National Institute of Health Research Biomedical Research Centre, Cambridge and by the Medical Research Council (Acute Brain Injury Programme Grant). PJAH is supported by the Academy of Medical Sciences/Health Foundation Senior Surgical Scientist Fellowship. Study support was provided by the Medical Research Council (grant numbers G9439390 ID 65883 and G0600986 ID 79068).
Supplementary Material
References
- Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Bellander BM, Cantais E, Enblad P, Hutchinson P, Nordstrom CH, Robertson C, Sahuquillo J, Smith M, Stocchetti N, Ungerstedt U, Unterberg A, Olsen NV. Consensus meeting on microdialysis in neurointensive care. Intensive Care Med. 2004;30:2166–2169. doi: 10.1007/s00134-004-2461-8. [DOI] [PubMed] [Google Scholar]
- Berger RP, Táasan S, Rand A, Lokshin A, Kochanek P. Multiplex assessment of serum biomarker concentrations in well-appearing children with inflicted traumatic brain injury. Pediatr Res. 2009;65:97–102. doi: 10.1203/PDR.0b013e31818c7e27. [DOI] [PubMed] [Google Scholar]
- Buttram SD, Wisniewski SR, Jackson EK, Adelson PD, Feldman K, Bayir H, Berger RP, Clark RS, Kochanek PM. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: effects of moderate hypothermia. J Neurotrauma. 2007;24:1707–1717. doi: 10.1089/neu.2007.0349. [DOI] [PubMed] [Google Scholar]
- Chiaretti A, Antonelli A, Mastrangelo A, Pezzotti P, Tortorolo L, Tosi F, Genovese O. Interleukin-6 and nerve growth factor upregulation correlates with improved outcome in children with severe traumatic brain injury. J Neurotrauma. 2008;25:225–234. doi: 10.1089/neu.2007.0405. [DOI] [PubMed] [Google Scholar]
- Clark SR, McMahon CJ, Gueorguieva I, Rowland M, Scarth S, Georgiou R, Tyrrell PJ, Hopkins SJ, Rothwell NJ. Interleukin-1 receptor antagonist penetrates human brain at experimentally therapeutic concentrations. J Cereb Blood Flow Metab. 2008;28:387–394. doi: 10.1038/sj.jcbfm.9600537. [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Whitehouse H, Smielewski P, Kirkpatrick P, Guazzo EP, Pickard JD. Computer supported multimodal bed-side monitoring for neuro-intensive care. Int J Clin Monit Comput. 1994;11:223–232. doi: 10.1007/BF01139874. [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care. 2009;10:373–386. doi: 10.1007/s12028-008-9175-7. [DOI] [PubMed] [Google Scholar]
- Denes A, Thornton P, Rothwell NJ, Allan SM. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun. 2009;24:708–723. doi: 10.1016/j.bbi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Schneider S, Bertsch T, Schlueter D, Fatar M, Ragoschke A, Kuhl S, Kischka U, Hennerici M. Temporal profile of release of interleukin-1beta in neurotrauma. Neurosci Lett. 2000;284:135–138. doi: 10.1016/s0304-3940(00)00977-0. [DOI] [PubMed] [Google Scholar]
- Folkersma H, Breve JJ, Tilders FJ, Cherian L, Robertson CS, Vandertop WP.2008Cerebral microdialysis of interleukin (IL)-1beta and IL-6: extraction efficiency and production in the acute phase after severe traumatic brain injury in rats Acta Neurochir (Wien) 1501277–1284.discussion 84 [DOI] [PubMed] [Google Scholar]
- Gentleman SM, Leclercq PD, Moyes L, Graham DI, Smith C, Griffin WS, Nicoll JA. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci Int. 2004;146:97–104. doi: 10.1016/j.forsciint.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Goodman JC, Van M, Gopinath SP, Robertson CS. Pro-inflammatory and pro-apoptotic elements of the neuroinflammatory response are activated in traumatic brain injury. Acta Neurochir Suppl. 2008;102:437–439. doi: 10.1007/978-3-211-85578-2_85. [DOI] [PubMed] [Google Scholar]
- Graetz D, Nagel A, Schlenk F, Sakowitz O, Vajkoczy P, Sarrafzadeh A.2009High ICP as trigger of proinflammatory IL-6 cytokine activation in aneurysmal subarachnoid hemorrhage Neurol Res13 August 2010 (e-pub ahead of print) [DOI] [PubMed]
- Hailer NP. Immunosuppression after traumatic or ischemic CNS damage: it is neuroprotective and illuminates the role of microglial cells. Prog Neurobiol. 2008;84:211–233. doi: 10.1016/j.pneurobio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Hamrin K, Rosdahl H, Ungerstedt U, Henriksson J. Microdialysis in human skeletal muscle: effects of adding a colloid to the perfusate. J Appl Physiol. 2002;92:385–393. doi: 10.1152/jappl.2002.92.1.385. [DOI] [PubMed] [Google Scholar]
- Helmy A, Carpenter KL, Hutchinson PJ. Microdialysis in the human brain and its potential role in the development and clinical assessment of drugs. Curr Med Chem. 2007a;14:1525–1537. doi: 10.2174/092986707780831113. [DOI] [PubMed] [Google Scholar]
- Helmy A, Vizcaychipi M, Gupta AK. Traumatic brain injury: intensive care management. Br J Anaesth. 2007b;99:32–42. doi: 10.1093/bja/aem139. [DOI] [PubMed] [Google Scholar]
- Helmy A, Carpenter KL, Skepper JN, Kirkpatrick PJ, Pickard JD, Hutchinson PJ. Microdialysis of cytokines: methodological considerations, scanning electron microscopy, and determination of relative recovery. J Neurotrauma. 2009;26:549–561. doi: 10.1089/neu.2008.0719. [DOI] [PubMed] [Google Scholar]
- Helmy A, Timofeev I, Palmer CR, Gore A, Menon DK, Hutchinson PJ. Hierarchical log linear analysis of admission blood parameters and clinical outcome following traumatic brain injury. Acta Neurochir (Wien) 2010;152:953–957. doi: 10.1007/s00701-009-0584-y. [DOI] [PubMed] [Google Scholar]
- Hillman J, Aneman O, Anderson C, Sjogren F, Saberg C, Mellergard P.2005A microdialysis technique for routine measurement of macromolecules in the injured human brain Neurosurgery 561264–1268.discussion 8–70 [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, O'Connell MT, Rothwell NJ, Hopkins SJ, Nortje J, Carpenter KL, Timofeev I, Al-Rawi PG, Menon DK, Pickard JD. Inflammation in human brain injury: intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J Neurotrauma. 2007;24:1545–1557. doi: 10.1089/neu.2007.0295. [DOI] [PubMed] [Google Scholar]
- Macmillan CS, Andrews PJ. Cerebrovenous oxygen saturation monitoring: practical considerations and clinical relevance. Intensive Care Med. 2000;26:1028–1036. doi: 10.1007/s001340051315. [DOI] [PubMed] [Google Scholar]
- Maurer MH, Berger C, Wolf M, Futterer CD, Feldmann RE, Jr, Schwab S, Kuschinsky W. The proteome of human brain microdialysate. Proteome Sci. 2003;1:7. doi: 10.1186/1477-5956-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeating EG, Andrews PJ, Signorini DF, Mascia L. Transcranial cytokine gradients in patients requiring intensive care after acute brain injury. Br J Anaesth. 1997;78:520–3. doi: 10.1093/bja/78.5.520. [DOI] [PubMed] [Google Scholar]
- Mellergard P, Aneman O, Sjogren F, Pettersson P, Hillman J.2008Changes in extracellular concentrations of some cytokines, chemokines, and neurotrophic factors after insertion of intracerebral microdialysis catheters in neurosurgical patients Neurosurgery 62151–157.discussion 7–8 [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T. Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock. 2001;16:165–177. doi: 10.1097/00024382-200116030-00001. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T. Modulation of immune response by head injury. Injury. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Scafidi S, McKenna MC, Fiskum G. Mitochondrial mechanisms of cell death and neuroprotection in pediatric ischemic and traumatic brain injury. Exp Neurol. 2009;218:371–380. doi: 10.1016/j.expneurol.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt OI, Heyde CE, Ertel W, Stahel PF. Closed head injury—an inflammatory disease. Brain Res Brain Res Rev. 2005;48:388–399. doi: 10.1016/j.brainresrev.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Schultzberg M, Lindberg C, Aronsson AF, Hjorth E, Spulber SD, Oprica M. Inflammation in the nervous system—physiological and pathophysiological aspects. Physiol Behav. 2007;92:121–128. doi: 10.1016/j.physbeh.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Shiozaki T, Hayakata T, Tasaki O, Hosotubo H, Fuijita K, Mouri T, Tajima G, Kajino K, Nakae H, Tanaka H, Shimazu T, Sugimoto H. Cerebrospinal fluid concentrations of anti-inflammatory mediators in early-phase severe traumatic brain injury. Shock. 2005;23:406–410. doi: 10.1097/01.shk.0000161385.62758.24. [DOI] [PubMed] [Google Scholar]
- Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiri N, Kimber I, Rothwell NJ, Pinteaux E. Differential effects of interleukin-1 alpha and beta on interleukin-6 and chemokine synthesis in neurones. Mol Cell Neurosci. 2008;38:259–265. doi: 10.1016/j.mcn.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Microdialysis—principles and applications for studies in animals and man. J Intern Med. 1991;230:365–373. doi: 10.1111/j.1365-2796.1991.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129:1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Banos MA, Herregodts P, Hooghe R, Hooghe-Peters EL. Expression of interleukin (IL)-1 beta, IL-6 and their respective receptors in the normal rat brain and after injury. Eur J Immunol. 1992;22:2963–2971. doi: 10.1002/eji.1830221131. [DOI] [PubMed] [Google Scholar]
- Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.