Abstract
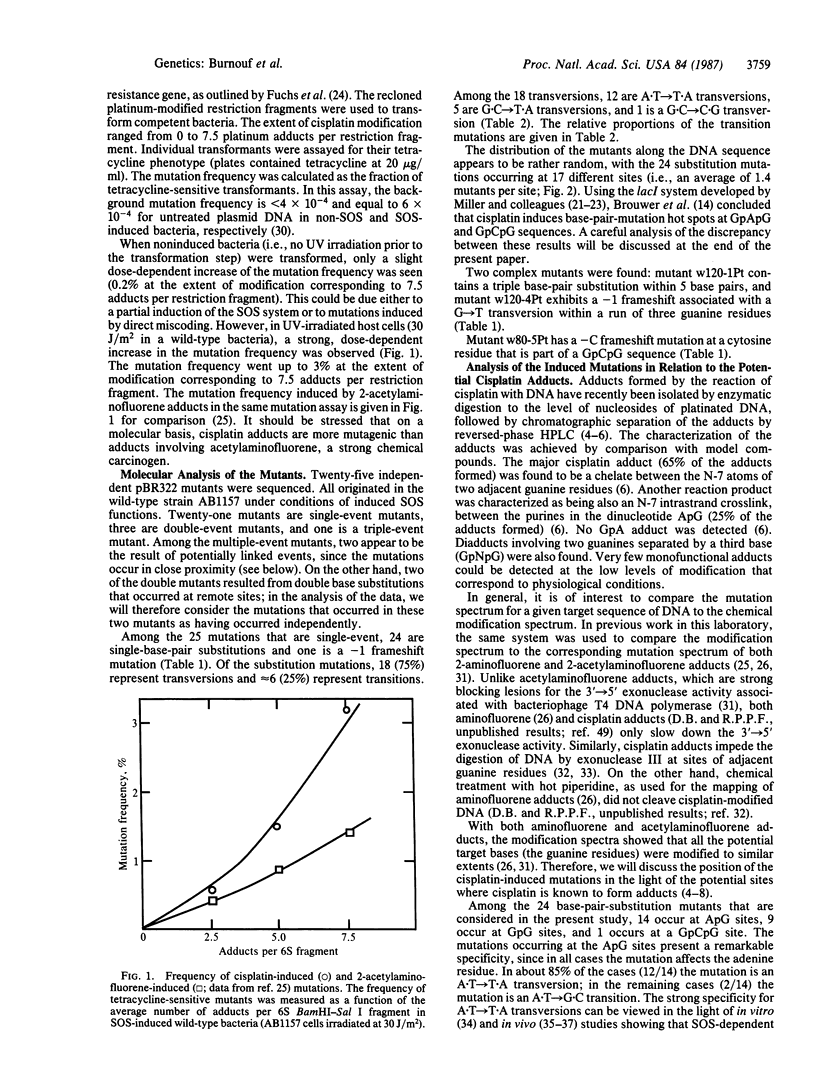
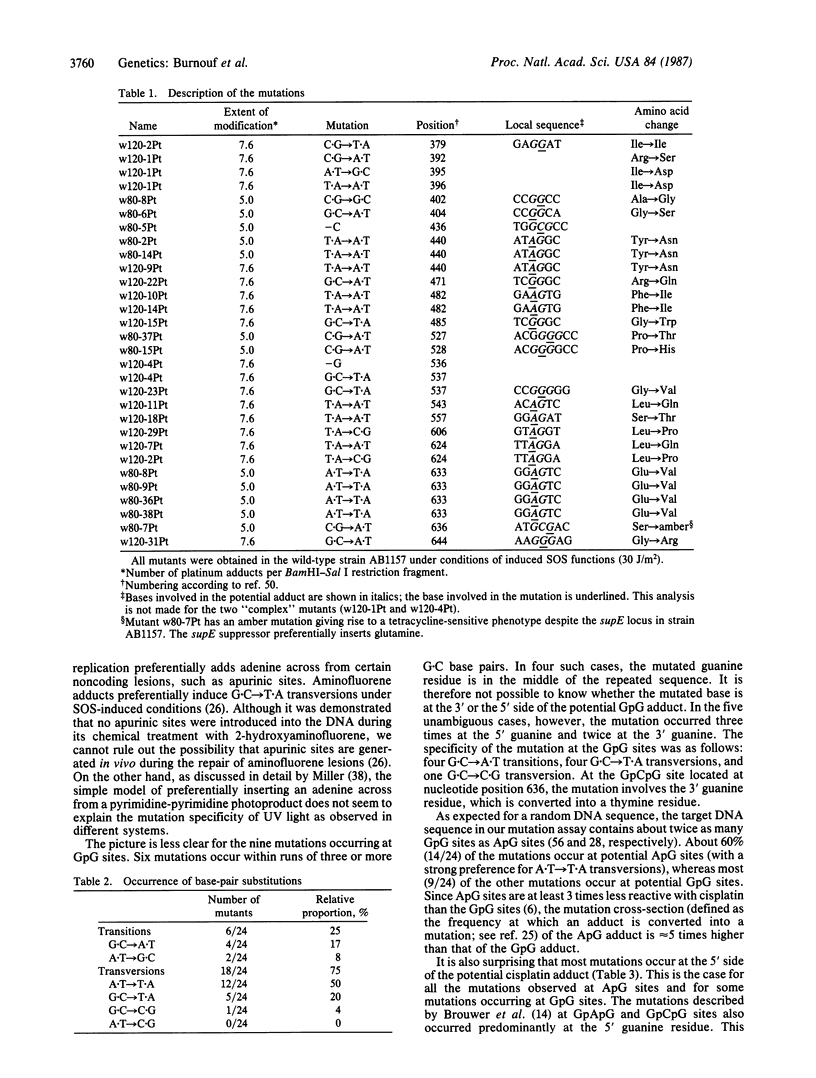
Using a forward-mutation assay based on the inactivation of the tetracycline-resistance gene located on plasmid pBR322, we have determined the mutation spectrum induced in Escherichia coli by cisplatin [cis-diamminedichloroplatinum(II)], a widely used antitumor drug. Cisplatin is known to form mainly intrastrand diadducts at ApG and GpG sites. We found that cisplatin efficiently induces mutations in an SOS-dependent way (i.e., dependent upon UV irradiation of the host bacteria). More than 90% of the mutations are single-base-pair substitutions occurring at the potential sites of cisplatin adducts (ApG and GpG). Taking into account the relative proportions of ApG and GpG adducts, we found that the ApG adducts are at least 5 times more mutagenic than the GpG adducts. Moreover, a strong mutation specificity was seen at the 5' side of the ApG adducts (A X T----T X A transversions). The observation that most mutations occur at the 5' end of the adduct at both ApG and GpG sites is discussed in relation to recent structural data.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. S. Platinum(II) complexes generate frame-shift mutations in test strains of Salmonella typhimurium. Mutat Res. 1979 Jul;67(3):209–214. doi: 10.1016/0165-1218(79)90014-4. [DOI] [PubMed] [Google Scholar]
- Beck D. J., Brubaker R. R. Effect of cis-platinum(II)diamminodichloride on wild type and deoxyribonucleic acid repair deficient mutants of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1247–1252. doi: 10.1128/jb.116.3.1247-1252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D. J., Brubaker R. R. Mutagenic properties of cis-plantinum(II)diammino-dichloride in Escherichia coli. Mutat Res. 1975 Feb;27(2):181–189. doi: 10.1016/0027-5107(75)90077-9. [DOI] [PubMed] [Google Scholar]
- Beck D. J., Fisch J. E. Mutagenicity of platinum coordination complexes in Salmonella typhimurium. Mutat Res. 1980 Jan;77(1):45–54. doi: 10.1016/0165-1218(80)90119-6. [DOI] [PubMed] [Google Scholar]
- Bichara M., Fuchs R. P. DNA binding and mutation spectra of the carcinogen N-2-aminofluorene in Escherichia coli. A correlation between the conformation of the premutagenic lesion and the mutation specificity. J Mol Biol. 1985 Jun 5;183(3):341–351. doi: 10.1016/0022-2836(85)90005-1. [DOI] [PubMed] [Google Scholar]
- Brouwer J., Adhin M. R., van de Putte P. Effect of pKM101 on cell killing and specificity of mutation induction by cis-diaminedichloroplatinum(II) in Escherichia coli K-12. J Bacteriol. 1983 Dec;156(3):1275–1281. doi: 10.1128/jb.156.3.1275-1281.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer J., van de Putte P., Fichtinger-Schepman A. M., Reedijk J. Base-pair substitution hotspots in GAG and GCG nucleotide sequences in Escherichia coli K-12 induced by cis-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1981 Nov;78(11):7010–7014. doi: 10.1073/pnas.78.11.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. III. Additional correlation of mutational sites with specific amino acid residues. J Mol Biol. 1977 Dec 15;117(3):525–567. doi: 10.1016/0022-2836(77)90056-0. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum(II) and cis-dichloro(ethylenediamine)platinum(II). Biochemistry. 1983 Aug 2;22(16):3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- Eastman A. Interstrand cross-links and sequence specificity in the reaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1985 Sep 10;24(19):5027–5032. doi: 10.1021/bi00340a011. [DOI] [PubMed] [Google Scholar]
- Eastman A. Reevaluation of interaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1986 Jul 1;25(13):3912–3915. doi: 10.1021/bi00361a026. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Proteins required for ultraviolet light and chemical mutagenesis. Identification of the products of the umuC locus of Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):175–192. doi: 10.1016/0022-2836(83)90074-8. [DOI] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., Lohman P. H., Reedijk J. Detection and quantification of adducts formed upon interaction of diamminedichloroplatinum (II) with DNA, by anion-exchange chromatography after enzymatic degradation. Nucleic Acids Res. 1982 Sep 11;10(17):5345–5356. doi: 10.1093/nar/10.17.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van der Veer J. L., den Hartog J. H., Lohman P. H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985 Jan 29;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Foster P. L., Eisenstadt E., Miller J. H. Base substitution mutations induced by metabolically activated aflatoxin B1. Proc Natl Acad Sci U S A. 1983 May;80(9):2695–2698. doi: 10.1073/pnas.80.9.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R. P. DNA binding spectrum of the carcinogen N-acetoxy-N-2-acetylaminofluorene significantly differs from the mutation spectrum. J Mol Biol. 1984 Jul 25;177(1):173–180. doi: 10.1016/0022-2836(84)90063-9. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Schwartz N., Daune M. P. Hot spots of frameshift mutations induced by the ultimate carcinogen N-acetoxy-N-2-acetylaminofluorene. Nature. 1981 Dec 17;294(5842):657–659. doi: 10.1038/294657a0. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I., Chaney S. G., Sancar A. Repair of cis-platinum-DNA adducts by ABC excinuclease in vivo and in vitro. J Bacteriol. 1985 Sep;163(3):817–823. doi: 10.1128/jb.163.3.817-823.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Maenhaut-Michel G., Fuchs R. P. Specific strand loss in N-2-acetylaminofluorene-modified DNA. J Mol Biol. 1987 Feb 20;193(4):651–659. doi: 10.1016/0022-2836(87)90348-2. [DOI] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Verdier J. M., Bichara M., Freund A. M., Daune M. P., Fuchs R. P. Carcinogen-induced mutation spectrum in wild-type, uvrA and umuC strains of Escherichia coli. Strain specificity and mutation-prone sequences. J Mol Biol. 1984 Jul 25;177(1):33–51. doi: 10.1016/0022-2836(84)90056-1. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Mutational specificity of depurination. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1494–1498. doi: 10.1073/pnas.81.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClerc J. E., Istock N. L. Specificity of UV mutagenesis in the lac promoter of M13lac hybrid phage DNA. Nature. 1982 Jun 17;297(5867):596–598. doi: 10.1038/297596a0. [DOI] [PubMed] [Google Scholar]
- Leopold W. R., Batzinger R. P., Miller E. C., Miller J. A., Earhart R. H. Mutagenicity, tumorigenicity, and electrophilic reactivity of the stereoisomeric platinum(II) complexes of 1,2-diaminocyclohexane. Cancer Res. 1981 Nov;41(11 Pt 1):4368–4377. [PubMed] [Google Scholar]
- Leopold W. R., Miller E. C., Miller J. A. Carcinogenicity of antitumor cis-platinum(II) coordination complexes in the mouse and rat. Cancer Res. 1979 Mar;39(3):913–918. [PubMed] [Google Scholar]
- Malinge J. M., Schwartz A., Leng M. Characterization of the ternary complexes formed in the reaction of cis-diamminedichloroplatinum (II), ethidium bromide and nucleic acids. Nucleic Acids Res. 1987 Feb 25;15(4):1779–1797. doi: 10.1093/nar/15.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Ganem D., Lu P., Schmitz A. Genetic studies of the lac repressor. I. Correlation of mutational sites with specific amino acid residues: construction of a colinear gene-protein map. J Mol Biol. 1977 Jan 15;109(2):275–298. doi: 10.1016/s0022-2836(77)80034-x. [DOI] [PubMed] [Google Scholar]
- Miller J. H. Mutagenic specificity of ultraviolet light. J Mol Biol. 1985 Mar 5;182(1):45–65. doi: 10.1016/0022-2836(85)90026-9. [DOI] [PubMed] [Google Scholar]
- Pinto A. L., Lippard S. J. Binding of the antitumor drug cis-diamminedichloroplatinum(II) (cisplatin) to DNA. Biochim Biophys Acta. 1985;780(3):167–180. doi: 10.1016/0304-419x(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Plooy A. C., Fichtinger-Schepman A. M., Schutte H. H., van Dijk M., Lohman P. H. The quantitative detection of various Pt-DNA-adducts in Chinese hamster ovary cells treated with cisplatin: application of immunochemical techniques. Carcinogenesis. 1985 Apr;6(4):561–566. doi: 10.1093/carcin/6.4.561. [DOI] [PubMed] [Google Scholar]
- ROSENBERG B., VANCAMP L., KRIGAS T. INHIBITION OF CELL DIVISION IN ESCHERICHIA COLI BY ELECTROLYSIS PRODUCTS FROM A PLATINUM ELECTRODE. Nature. 1965 Feb 13;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Gordon L. K., Haseltine W. A. Use of exonuclease III to determine the site of stable lesions in defined sequences of DNA: the cyclobutane pyrimidine dimer and cis and trans dichlorodiammine platinum II examples. Nucleic Acids Res. 1981 Sep 25;9(18):4595–4609. doi: 10.1093/nar/9.18.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles B., Lesca C. Induction of recA protein in Escherichia coli by three platinum (II) compounds. Biochem Biophys Res Commun. 1982 Mar 15;105(1):202–208. doi: 10.1016/s0006-291x(82)80031-4. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Kunkel T. A., Loeb L. A. Infidelity of DNA synthesis associated with bypass of apurinic sites. Proc Natl Acad Sci U S A. 1983 Jan;80(2):487–491. doi: 10.1073/pnas.80.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss B., Rabkin S., Sagher D., Moore P. The role of DNA polymerase in base substitution mutagenesis on non-instructional templates. Biochimie. 1982 Aug-Sep;64(8-9):829–838. doi: 10.1016/s0300-9084(82)80138-7. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Skopek T. R., Hutchinson F. Changes in DNA base sequence induced by targeted mutagenesis of lambda phage by ultraviolet light. J Mol Biol. 1984 Mar 5;173(3):273–291. doi: 10.1016/0022-2836(84)90121-9. [DOI] [PubMed] [Google Scholar]
- den Hartog J. H., Altona C., van Boom J. H., van der Marel G. A., Haasnoot C. A., Reedijk J. cis-diamminedichloroplatinum(II) induced distortion of a single and double stranded deoxydecanucleosidenonaphosphate studied by nuclear magnetic resonance. J Biomol Struct Dyn. 1985 Jun;2(6):1137–1155. doi: 10.1080/07391102.1985.10507629. [DOI] [PubMed] [Google Scholar]
- van Hemelryck B., Guittet E., Chottard G., Girault J. P., Herman F., Huynh-Dinh T., Lallemand J. Y., Igolen J., Chottard J. C. A high melting cis-[Pt(NH3)2[d(GpG)]]adduct of a decanucleotide duplex. Biochem Biophys Res Commun. 1986 Jul 31;138(2):758–763. doi: 10.1016/s0006-291x(86)80561-7. [DOI] [PubMed] [Google Scholar]


