Abstract
A growing number of studies have correlated higher urate levels with a lower risk of developing Parkinson’s disease (PD) and with a favorable rate of disease progression, indicating that urate could be an important biomarker of the pathophysiology underlying PD. Dietary and genetic determinants of urate have also been linked to a reduced risk or delayed onset of PD. Based on the known antioxidant and metal complexing properties of urate, together with evidence for oxidative stress as a contributor to neurodegeneration in PD, urate may serve as an endogenous neuroprotectant that helps reduce the risk and rate of the disease. In this article we review the convergent biological, epidemiological and clinical data that identify urate as a promising biomarker of the risk, diagnosis and prognosis of PD.
Keywords: blood, cerebrospinal fluid, oxidative stress, Parkinson’s disease, prognosis, substantia nigra, urate
Parkinson’s disease (PD) is a progressive neurological disorder, which classically produces motor deficits that are caused by degeneration of dopaminergic nigrostriatal neurons and is responsive to dopamine replacement therapy. However, it is increasingly appreciated that PD also afflicts other parts of the nervous system that results in substantial disability, unrelated to movement and unresponsive to dopaminergic therapy. Despite considerable progress in identifying candidate genetic and environmental influences on the development of typical PD, its pathogenic mechanisms remain uncertain and disease-modifying therapies continue to be elusive. A major hurdle in the clinical application of new insights into PD biology has been the paucity of validated biomarkers to aid in assessing the risk, prognosis and progression of the disease. The NIH Biomarkers Definition Working Group defined a biomarker as, “a characteristic objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention” [1]. The most valuable biomarkers are simple, sensitive, specific, reproducible, affordable, accessible (easily obtained and measured) and pathophysiologically relevant. In this article we describe the emergence of urate as a promising multifunctional biomarker of PD, which has many of these properties.
Urate in evolution & disease
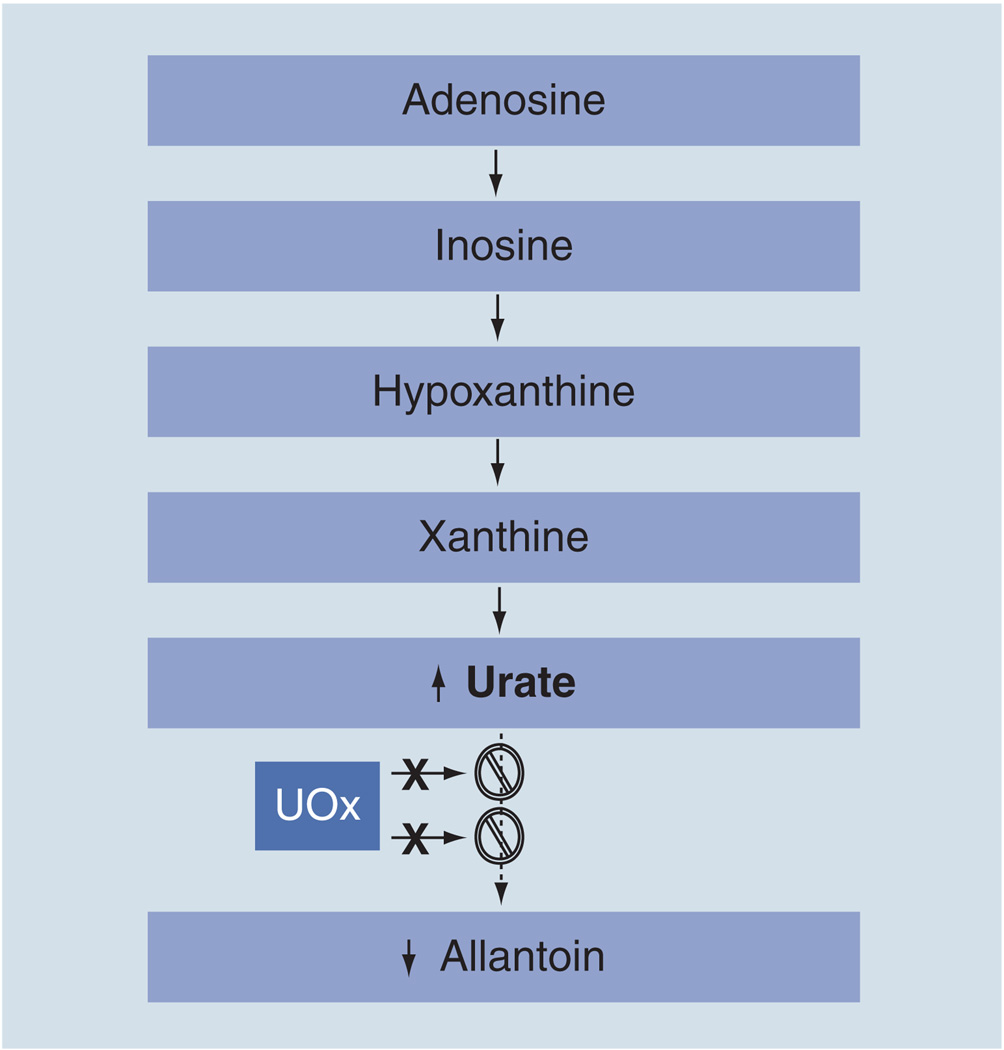
Urate, the anionic form of uric acid (2,6,8-trioxy-purine), predominates at neutral pH, and is present both intracellularly and in all body fluids. In humans, the amount of urate in the blood depends on dietary intake of purines, urate biosynthesis and urate excretion. Urate is synthesized by the xanthine oxidoreductase enzyme, which successively oxidizes hypoxanthine to xanthine and then to urate (Figure 1). In the vast majority of mammals, urate is then converted to allantoin by urate oxidase (UOx). By contrast, in humans, urate constitutes the main end product of purine metabolism and represents a major antioxidant in body fluids.
Figure 1. Urate as the end product of purine metabolism in humans.
The loss of functional UOx as a result of multiple mutations (represented by X) and resultant urate elevation distinguish humans from all other nonprimate mammals, in which urate is metabolized further by UOx to allantoin. In humans, urate concentrations approach the limit of their solubility and occasionally exceed it to cause diseases like gout. The basis for a presumed survival advantage of higher concentrations of urate remains uncertain, although its demonstrated antioxidant properties have been suggested to confer health benefits, including the possibility of neuroprotection.
UOx: Urate oxidase.
The high concentration of urate in human plasma and cerebrospinal fluid, relative to those in most other mammals, has been attributed to two independent mutational events in the UOx gene, which occured late in hominid evolution, approximately 10–15 million years ago, during the Miocene epoch, and led to incomplete transcription and, thus, the absence of functional UOx (Figure 1) [2]. Evidence for these exonic (nonsense or frameshift) mutations, which occur independently along parallel lineages of hominoids, suggests a selective advantage of the resultant urate elevation in our ancestors.
Why higher urate might be, or at least have been, of benefit to hominoid health and survival is an intriguing and still quite unanswered question. The first prominent hypothesis was proposed nearly 60 years ago, and was based on the similar purine-based structures of the cerebral stimulant, caffeine (1,3,7-trimethyl-2,6-dioxy-purine), and it is postulated that urate could have improved cognition, alertness and motivation in hominoids [3]. Alternatively, it has been proposed that primate UOx mutations may reflect a beneficial effect of urate against aging through its ability to prevent oxidative damage caused by reactive nitrogen and oxygen species (see later) [4]. Another, more recent theory suggests that urate may also have had a beneficial hypertensive effect on our primate predecessors at a time when a low-salt diet and resultant hypotension might have posed a survival threat [5].
Caused by the lack of the UOx enzyme in humans, urate circulates at high concentration near the limits of its solubility, with a physiological range between 3.6 and 6.0 mg/dl in the blood, and with higher levels in men than in women [6]. A number of diseases have been linked to both hyper- and hypo-uricemia. Elevated urate levels are known to contribute to the crystal deposition conditions of gouty arthritis (in which urate crystals accumulate and elicit inflammation in joints) and uric acid kidney stones (which require acidic urine to form). In addition, hypertension, myocardial infarction, congestive heart failure, stroke and renal disease have all been correlated with higher urate level [7–11]. On the other hand, multiple sclerosis [12] and optic neuritis [13] have been linked with reduced urate level. However, it remains unclear whether the altered urate level is the cause or a consequence in these conditions.
Oxidative stress, PD & urate
Oxidative damage has been implicated as a core contributor to the neurodegenerative process in PD [14], since PD patients have decreased levels of antioxidant enzyme activity [15] and increased oxidative stress biomarkers. Depletion of glutathione peroxidase and catalase found in PD brain [15] may render neuronal cells more susceptible to damage from reactive oxygen species (ROS) and reactive nitrogen species. Increased oxidative stress, mitochondrial dysfunction, DNA damage, lipid peroxidation and protein aggregation are common in brain tissues of PD patients [16–18]. The selective neurodegeneration in PD seems to suggest that substantia nigra pars compacta dopaminergic neurons are initially more vulnerable to ROS and reactive nitrogen species than other neurons. The reason for the susceptibility of dopaminergic neurons to oxidative stress is not fully understood. One explanation suggests that dopamine, itself, may confer neurotoxicicity, since it can generate ROS in the presence of molecular oxygen and is able to auto-oxidate to neuromelanin, which, in turn, promotes generation of oxyradicals [15]. Therefore, dopaminergic neurons may have a higher basal level of oxidative stress, which may make them more vulnerable to genetic mutation and toxicants.
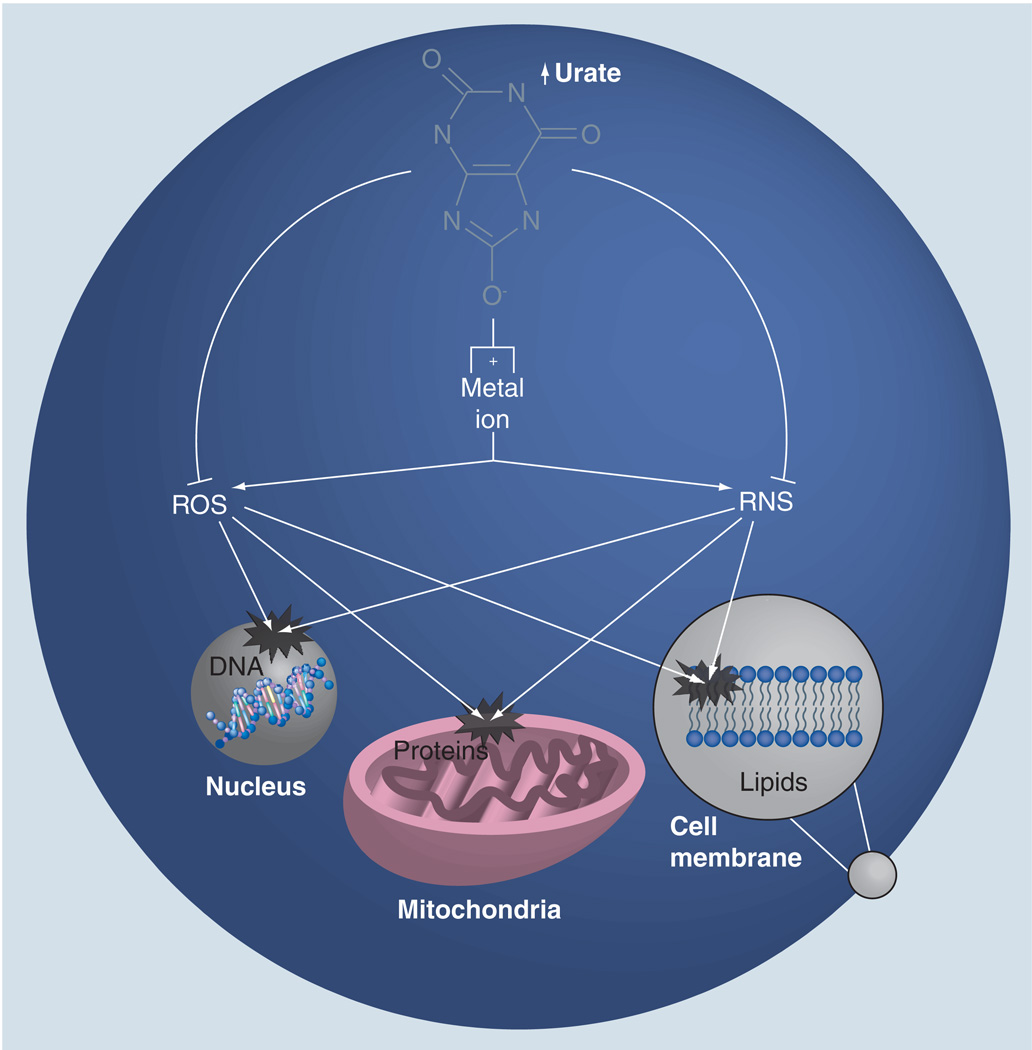
Based on its known antioxidant and metal-complexing properties (Figure 2) [4,19–21], and its relatively high levels in humans, urate could serve as an endogenous defense against the development and progression of PD. In cellular models of neurodegeneration, urate has been demonstrated to reduce oxidative stress, mitochondrial dysfunction and cell death occurring spontaneously in culture or induced by pesticides, glutamate and iron ions [22–24]. Urate has also been demonstrated to reduce oxidation of dopamine in caudate and substantia nigra of PD patients [19]. Urate’s protective effect in neuronal cell culture may be caused by its antioxidant activity. Urate is, in fact, a powerful scavenger of peroxyl radicals (ROO•) [4,20] and hydroxyl-radicals (OH•) [20], and is able to inhibit free radical-initiated DNA damage (Figure 2) [25]. Moreover, urate can be oxidized by ROS and hemoprotein/H2O2 systems, converting them to inactive forms [26]. Urate’s protective effect has also been linked to its ability to complex with metal ions (Figure 2). Urate can bind ions such as iron and copper, which promote formation of highly reactive hydroxyl free radicals [27], and, thus, reducing their oxidizing potential [28–30]. Urate can also act as a peroxynitrite scavenger, leading the production of a nitrated urate derivative [31,32], which has been demonstrated to be a vasoactive nitric oxide donor [33].
Figure 2. Hypothetical cellular mechanisms of neuroprotection by urate.
Urate can scavenge and inactivate ROS and RNS within the cell. Urate also possesses metal ion complexing properties and, thus, can reduce ROS and RNS. ROS and RNS induce destructive modification to nucleic acids (that of nuclear DNA), proteins (e.g., those required for mitochondrial energy metabolism) and lipids (e.g., those of the plasma membrane), potentially producing the dysfunctional gene expression, energy metabolism and membrane integrity that are characteristic of neurodegeneration. Therefore, through complementary direct and indirect mechanisms, urate may reduce oxidative and nitrosative damage to the metabolically stressed neurons that degenerate in Parkinson’s disease.
ROS: Reactive oxygen species; RNS: Reactive nitrogen species.
Risk biomarker
Blood urate
The first study conducted to determine plasma urate concentration in PD patients and controls demonstrated no significant difference between the two groups [34]. However this case–control study presented a small number of healthy controls and did not consider confounding factors, such as medication, BMI, gender and nutrition. The first prospective study of urate and the risk of PD demonstrated that, among some 8000 Japanese–American men (with a median age of 54 years at enrollment) in the Honolulu Heart Program, of the 92 individuals who developed PD, those who had a baseline serum urate level above the median had a 40% reduction in their rate of idiopathic PD incidence over the ensuing 30 years (rate ratio: 0.6; 95% CI: 0.4–1.0); analysis was adjusted for age and smoking [35].
Similar results were found in the Rotterdam Study, a prospective population-based study of 4695 men and women, aged 55 years and older, without PD and dementia [36]. Within 9.4 years of follow-up, 68 new cases of PD were detected. The results were normalized for sex, age, smoking, consumption of dairy products, BMI and alcohol intake. Higher serum levels of urate were associated with a significant decreased risk of PD (hazard ratio [HR]: 0.71%; 95% CI: 0.51–0.98), with evidence of a dose–effect relationship (p-value for the trend = 0.040).
In the largest prospective study to date, Weisskopf et al. analyzed blood sample data of 18,018 men who participated in the Health Professionals Follow-up Study and provided blood samples between 1993 and 1995 [37]. Between the period of blood collection and 2002, 84 subjects were diagnosed with PD. Analysis adjusted for age, smoking history and caffeine intake demonstrated that the risk of PD decreased across increasing urate quartiles. The mean plasma urate concentration was significantly lower among cases compared with controls (p < 0.01), and the rate ratio for the highest quartile compared with the lowest was 0.43 (95% CI: 0.18–1.02). This association was stronger when analysis was restricted to cases whose blood was drawn more than 4 years prior to the diagnosis, suggesting that the low level of plasma urate among individuals with PD precedes the onset of neurological symptoms, and is not a consequence of changes in diet, behavior or medical treatment early in the course of the disease.
The inverse association between blood urate level and PD risk was replicated in a fourth prospective cohort, that of the Atherosclerosis Risk in Communities (ARIC) study, comprising both women (55%) and men (45%), and African–Americans (27%) as well as Caucasians (63%) in the USA. Between 1987 and 1989, 15,792 participants (45–64 years old) were enrolled [38]. The 95 potential cases of PD included in the analysis were identified during nearly 20 years of follow-up. Plasma urate was found to be inversely associated with PD disease occurrence. Odds ratio (OR) for the occurrence of PD between the highest and lowest quartiles of baseline plasma urate was 0.4 (95% CI: 02–0.8) for the whole population after adjusting for age, sex and race. Similar inverse associations were also suggested in subgroup analyses for women and African–Americans, but were not statistically significant, possibly because of the smaller sample size.
However, the possibility that the association between urate and PD risk extends to women remains unclear. It was weakened with the recent publication of data from the large prospectively Nurses’ Health Study cohort, which demonstrated no significant change in PD risk across quartiles of baseline plasma urate (p-value for the trend = 0.4) [39]. On the other hand, a recent community-based prevalence study of probable PD cases found a strong association with lower serum urate in women as well as in men [40] (see the ‘Diagnostic biomarker’ section).
Determinants & correlates of blood urate
Dietary factors influencing urate
Blood urate level can be affected by diet, with a 1.0 mg/dl reduction achieved by strict avoidance of dietary purines, of which, meats are a major source [41]. In addition to meat, alcohol and fructose have been demonstrated to increase blood urate levels, whereas dairy products have been found to reduce them. Gao et al. reported that a composite index of such dietary factors predictive of higher plasma urate levels also predict a reduced risk of developing PD [42]. Using data from a subpopulation of the Health Professionals Follow-up Study, Gao et al. derived an optimized dietary urate index, comprising lower dairy protein, higher alcohol and other factors that correlate with higher plasma urate levels. Over 14 years of follow-up, 248 incident cases of PD were documented among 47,406 men in this cohort. A higher dietary urate index at baseline was associated with a lower risk of PD (top quintile vs bottom: relative risk = 0.47, p-value of the trend = 0.0008), after adjustment for age, smoking, caffeine intake and other potential confounders.
Some of the dietary correlates of higher urate levels have also been individually linked to a reduced risk of PD in other epidemiological studies. Amongst these individual dietary components, dairy product consumption has been most consistently linked to PD. Lower intake of dairy products and, specifically, milk was first linked to a reduced risk of PD among 7504 men (45–68 years of age) enrolled in the Honolulu Heart Program [43]. During 30 years of follow-up, 128 subjects developed PD. After adjustment for age, dietary and other factors, there was a 2.3-fold lower risk of PD (95% CI: 1.3–4.1) among those who consumed no milk compared with those in the highest intake group. The positive association between intake of dairy products and risk of PD was confirmed in another prospectively followed cohort of 57,689 men and 73,175 women from the American Cancer Society’s Cancer Prevention Study II Nutrition Cohort. A total of 250 men and 138 women with PD were identified during the study [44]. The authors found a higher risk among those who consumed higher amounts of dairy products in both men and women. A meta-analysis of all prospective studies confirmed a moderately elevated risk of PD among persons with higher dairy product consumption [44].
Individual dietary components that are known to elevate blood urate, such as meat and alcohol, may also be linked to a reduced PD risk. In a nested–case control study of a prospectively followed population in rural China, individuals consuming more meat at baseline were significantly less likely to develop PD [45]. Similarly, significant inverse associations between the consumption of meat (broiled or smoked) and PD were reported in a Swedish case–control study [46]. Alcohol, which can elevate blood urate by increasing its generation via purine loading and by reducing its renal clearance [47], has also been linked, in some studies, to a reduced risk of PD [48–50]. However, this association has not been consistently observed [50,51].
Genetic factors influencing urate
In addition to the universal ancestral UOx mutations that preclude urate degradation in humans, rare mutations in other purine metabolism genes, such as that for hypoxanthine phosphoribosyl-transferase that causes Lesch–Nyhan syndrome, can profoundly alter urate levels [52]. Recently, common allelic variants of a urate transporter gene glut9 have been identified as important contributors to the normal variation in blood urate levels in humans [53], as well as to the risk of gout, but not coronary artery disease or hypertension [54–56]. To data, no data have been published on polymorphisms of glut9 (or other genetic determinants of serum urate) and PD risk.
However, a recent study of glut9 polymorphisms in three PD cohorts demonstrated a significantly earlier age of onset amongst PD subjects, with the rs1014290 (intron 3) allelic variant of glut9 [57], which is known to be associated with a lower serum urate level [55]. Limitations of this study included the lack of corroboration from blood urate levels (not measured) or from corresponding haplotype results (conducted but not clear). Nevertheless, the result supports the hypothesis that higher urate levels may delay the development of PD. Whether this allelic variant or other genetic determinants of lower serum urate levels also reduce the risk of PD is now an important, answerable question.
Gout
The identification of higher urate as an inverse risk factor for PD, as well as a known cause of gout, led to the hypothesis that a history of gout should be relatively uncommon amongst PD patients. Two independent epidemiological studies have tested the hypothesis and, indeed, their results support this. A study of the General Practice Research Database – a computerized dataset comprising information on more than 3 million Britons who were followed up by their general practitioners – identified 1052 PD cases and 6634 controls [58]. Individuals with a previous history of gout were found to have a lower risk of developing PD (OR: 0.69; 95% CI: 0.48–0.99), with a significant association among men (OR: 0.60; 95% CI: 0.40–0.91), but not among women (OR: 1.26; 95% CI: 0.57–2.81; p-value for interaction: 0.11). Moreover, initiation of antigout medication was associated with a lower risk of PD (OR: 0.57; 95% CI: 0.19–1.70). Together these findings indicate that a diagnosis of gout is associated with a reduced risk of developing PD.
In a separate population cohort in western Canada, the incidence rates for PD were compared between 11,258 gout patients and approximately five-times as many matched control subjects [59]. Over an average 8 years of follow-up, nearly 1180 new cases of PD were identified. In a multivariate analysis, the adjusted relative risk of PD among those with gout was 0.70 (95% CI: 0.59–0.83) compared with those without a history of gout, which confirms a history of gout as an inverse risk factor for PD.
Prognostic biomarker
Blood urate
The growing evidence for serum urate as an inverse risk factor for PD prompted the hypothesis that, amongst those already diagnosed with the disease, serum urate may also be a predictor of favorable outcomes. Two rigorously characterized PD cohorts were investigated to test the hypothesis. First was the Parkinson Research Examination of CEP-1347 Trial (PRECEPT) cohort, comprising 806 subjects with early PD who were not on dopaminergic therapy at enrollment [60]. They were followed for approximately 2 years on average until 2005, by which time 493 patients reached the original trial’s primary end point of developing disability that required dopamine treatment. After adjustment for potential confounders, the HR of reaching the primary disability end point declined with increasing baseline concentration of serum urate. Subjects in the top quintile of urate reached the end point at only half the rate of subjects in the bottom quintile (HR: 0.51; 95% CI: 0.37–0.72; p-value for the trend <0.001). This association was stronger in men (HR: 0.39; 95% CI: 0.26–0.60; p-value for the trend <0.001) than in woman (HR: 0.77: 95% CI: 0.39–1.50), for whom the trend was not statistically significant (p = 0.3).
Baseline urate also predicted favorable outcomes using the Secondary Clinical Unified PD Rating Scale (UPDRS) and radiographic (dopamine transporter brain scan) outcomes in the PRECEPT study [60]. For the latter, loss of striatal dopamine-transporter binding sites was estimated as the change in striatal uptake of [123I] β-CIT – a cocaine analog that binds the transporter – over the course of the trial. Increasing serum urate concentration was predictive of slower rates of losing [123I]β-CIT binding sites in striatum (p-value for the trend = 0.002). Again, the relationship was robust in men (p = 0.001) but not significant in women (p = 0.43), despite a similar trend [60].
Ascherio et al. were able to test the hypothesis in a second, independent cohort of early PD, by using the clinical and biochemical datasets of the Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism (DATATOP) trial [61]. Of the 800 US and Canadian subjects with early, untreated PD, who enrolled in DATATOP over 20 years ago, 774 had available values of baseline serum urate (originally measured as a routine safety laboratory). Of these, 50% progressed to disability sufficient to require levodopa therapy (primary end point) during the 2 years of follow-up. As in the PRECEPT study, higher serum urate concentrations at baseline were predictive of slower rates of clinical decline. The HR of progressing to the primary end point decreased with increasing serum urate concentrations (adjusted HR for highest vs lowest quintile: 0.64; 95% CI: 0.44–0.94; HR for a one standard deviation increase: 0.82; CI: 0.73–0.93) and declined with increasing concentrations of serum urate (p = 0.002).
Once again, the inverse association between baseline serum urate and clinical progression value was significant for men but not for women (p-value for the trend: 0.005 and 0.3, respectively). The basis for the recurrent sex difference is not clear. Although it may be biological, there was no statistically significant interaction between serum urate and sex for either the DATATOP or PRECEPT cohorts [60,61]. Moreover, the weaker relationship in women may reflect the fact that there are fewer women than men with PD (1:2 ratio of women to men in both cohorts) and that women, on average, have considerably lower blood urate levels. As a consequence, in both studies, women accounted for approximately 75% of the first (lowest) quintile of serum urate, but only approximately 15% of the fourth and fifth (uppermost) quintiles. The paucity of women in the uppermost quintiles may contribute to the lack of a clear link between urate and progression in women because the urate–PD relationship (for PD risk as well as rate of progression) relies primarily on changes above the population’s median serum urate concentration.
Cerebrospinal fluid urate
Cerebrospinal fluid (CSF) can reflect metabolic processes in the brain in a direct manner owing to the relatively free exchange of molecules between the brain and the CSF. There exists a steep, approximately tenfold, gradient of urate concentration from blood down to CSF (in which control values typically range from 0.2 to 0.5 mg/dl), suggesting that urate generated in the periphery does not readily cross the blood–brain barrier (BBB). Evidence that xanthine oxidoreductase is detectable and functional locally in brain [62] has challenged the old hypothesis that urate is only formed on the blood side of the BBB – mainly in the liver and the small intestine [63,64]. Nevertheless, limited passive diffusion or transport from blood may still contribute substantially to the CSF urate pool, which is consistent with a tight correlation between serum and CSF urate despite the gradient [61,65]. The extent to which CSF urate comprises peripherally generated urate that trickles across the BBB versus production within the CNS remains to be clarified.
Similarly, it remains unclear to what extent variation or frank disruption in BBB integrity for urate may contribute to the pathophysiology of PD or to the utility of CSF urate as a biomarker for the disease. In order to maintain this serum:CSF urate concentration gradient, normal BBB function is required and, for this reason, the ratio of serum urate:CSF urate has been used as a marker for BBB integrity [65,66]. In conditions where BBB function breaks down, serum urate molecules would be expected to leak through the damaged BBB. Although it is unclear whether BBB breakdown is important in the pathogenesis of PD, abnormal BBB function has been demonstrated in PD in vivo [67–69]. Available data on CSF urate concentrations in PD patients are scarce. Although one previous study did not demonstrate a difference in CSF urate between PD and control patients, the sample size was limited (with only 11 PD subjects) and the sex of the subjects was not noted [70].
A recent study by Ascherio and colleagues identified an inverse relationship between CSF urate concentrations and subsequent rates of clinical decline in PD patients [61]. The study utilized the clinical database of the DATATOP trial of 800 subjects with early PD (Hoehn and Yahr stages I and II). At baseline (pretreatment), serum urate concentrations were measured as part of a routine safety laboratory panel, and CSF was collected and stored for specified studies and future research. The primary end point of the study was the appearance of disability sufficient to start dopaminergic treatment, and secondary variables included the responses to the UPDRS score (sum of the cognitive, motor and activity of daily living subscale scores). For the 713 subjects with available CSF urate levels, a total of 48% reached the primary end point during the 2-year follow-up period. Similar to the concentration of serum urate (see previously), the concentration of CSF urate was inversely related to the primary end point (HR for highest vs lowest quintile: 0.65; 95% CI: 0.44–0.96) and the rate of change in the UPDRS.
Interestingly, the inverse association between urate and disability progression was much stronger among those not randomized to α-tocopherol treatment upon enrollment into DATATOP, and was absent among those assigned to treatment with this antioxidant at 2000 IU per day [61]. Conversely, amongst those individuals with the lowest levels of CSF (or serum) urate, α-tocopherol treatment, which, overall, was demonstrated to be ineffective at slowing disease progression [70], did seem to slow progression. For example, randomized assignment to α-tocopherol appeared to slow the rate of UPDRS worsening by 40% (p < 0.05) among those in the lowest quintile of CSF urate at baseline. By contrast, α-tocopherol demonstrated no effect on UPDRS change among those with higher CSF urate. If replicated, these findings would indicate that urate could also serve as a so-called predictive biomarker [71], useful for determining which patients are likely to be ‘responders’ to a given treatment – in this case α-tocopherol. Mechanistically, the study also raises the possibility of a competitive interaction between antioxidant protectants with the lipophilic antioxidant α-tocopherol, which is capable of improving disease progression only in those who have low endogenous antioxidant levels, such as those of the prominent hydrophilic antioxidant urate. Although a similar interaction between urate and α-tocopherol was suggested in a study of mild, cognitive impairment progression in Alzheimer’s disease [72], it remains to be determined whether the interaction between urate and α-tocopherol observed in DATATOP is reproducible in PD.
As urate concentrations are an order of magnitude lower in CSF than in serum (e.g., 14-fold in DATATOP), the integrity of the BBB is likely to be a particularly important factor that may have contributed to or confounded the relationship between CSF urate and PD progression. Although BBB function was not addressed in this study, estimates of BBB integrity may be available by the classical measurement of the serum:CSF ratio of albumin, or even of urate itself. The complex interactions between the BBB, CNS generation of urate and disease progression will need to be clarified for CSF urate to be developed as a reliable, independent biomarker of PD progression. Despite the accumulating evidence indicating distinct antioxidant, metal ion-complexing and ascorbate-stabilizing effects of urate [73,74], it remains unknown whether the link between higher CSF urate and slower clinical decline is based on a direct neuroprotective action of urate or whether urate is simply a marker of another protective factor. Regardless, this study provides the first evidence that CSF urate, similar to serum urate, may serve as a prognostic biomarker of PD (Figure 3) [60].
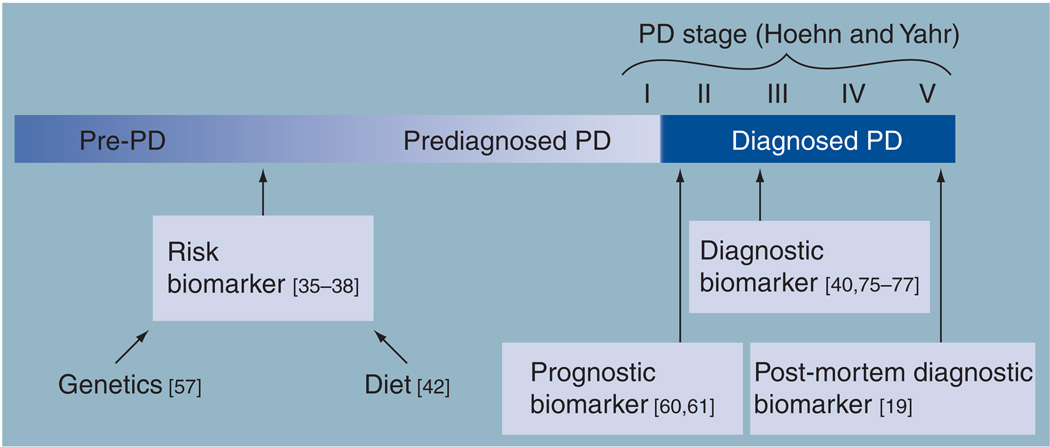
Figure 3. Biomarker roles of urate along the timeline of Parkinson’s disease.
Urate levels in blood, cerebrospinal fluid or brain have been consistently linked to PD across its preclinical phases (pre-PD, prediagnosed PD) and its clinical stages (Hoehn and Yahr I–V). Multiple prospective epidemiological studies of blood urate and its genetic and dietary determinants have identified them as inverse risk factors or risk biomarkers for the disease. Urate measured in the serum or cerebrospinal fluid shortly after diagnosis is predictive of favorable clinical and/or radiographic features of disease progression, and, as such, urate has been identified as the first prognostic biomarker of typical idiopathic PD. Blood urate can also help differentiate between PD subjects and non-PD/healthy controls and, thus, also can serve as a diagnostic biomarker for the disease. Similarly, brain urate concentrations were found to be lower in post-mortem specimens from PD versus control subjects.
PD: Parkinson’s disease.
Diagnostic biomarker
Blood urate
Lower plasma or serum urate levels have been reported in PD patients and compared with healthy individuals in several case–control studies (Figure 3), although the overlap is considerable, suggesting that the diagnostic biomarker utility of urate, alone, would be limited. A Spanish study that investigated markers of oxidative stress enrolled 79 patients with idiopathic PD and 107 control subjects and controlled for age, sex and place or residence. The study demonstrated that plasma urate levels were significantly lower in PD patients than in controls [75]. Lower urate levels were also found in 40 idiopathic PD patients with a mean disease duration of 4 years compared with 29 spousal controls. The difference was statistically significant, despite the fact that the analysis did not control for the overrepresentation of men and hypertension among the PD cases, which may have led to an underestimation of the association, since men and hypertensive individuals typically have higher serum urate than women and normotensive individuals, respectively [76].
A recent study investigated serum urate levels in PD patients compared with age- and sex-matched controls, and their possible relationship with clinical parameters and pharmaceutical treatment. In 43 patients with clinically definite PD, the plasma urate level was found to be reduced in comparison to that of 47 healthy control individuals (p < 0.01). Serum urate concentration was also inversely correlated with disease duration (Spearman’s rank correlation coefficient [Rs] = −0.397, p = 0.009); an association that was significant for men (Rs = −0.44, p = 0.004) but not for women (Rs = −0.22, p = 0.34) [77]. The data from this cross-sectional analysis raise the possibility that serum urate might also be a biomarker of disease severity, which could be further investigated with serial urate measurement in a prospectively followed PD cohort.
Both prospective longitudinal and cohort studies of women have reported absent or weaker urate associations with PD, possibly because women are less likely to have PD, but more likely because women have substantially lower urate concentrations than men. The importance of the far smaller number of women with higher (>6 mg/dl) serum urate, compared with men, is amplified by the apparent ‘steepness’ of the inverse urate–PD association at greater concentration than 6 mg/dl, as observed in several studies (e.g., [37]). If the difference was, in fact, caused by a small number of women with relatively high serum urate, then the relationship should still exist, even if weaker and more variable across studies compared with that for men. The results of a recent community-based case–control study, in which women as well as men demonstrated a clear inverse association between serum urate and probable PD are consistent with this interpretation [40]. Out of 69,000 residents living near a chemical plant, 59 participants self-reported a history of PD and were taking specific PD medication. Analyses controlling for potential confounders demonstrated that urate at or above the median had a lower probability of reporting treated PD compared with those participants with urate levels below the median (OR: 0.33; 95% CI: 0.19–0.60, p = 0.0002), with a similar reduction in women (OR: 0.31; CI: 0.11–0.87, p = 0.025) despite their smaller numbers, as well as men (OR: 0.34; CI: 0.17–0.69, p = 0.0027).
Finally, the relationship between plasma urate level and cognitive impairment in PD has been explored by two recent studies. Annanmaki et al. found a positive association between plasma or urine urate/uric acid levels and preserved neuropsychological performance [78]. These results were corroborated in a Chinese study by Wang and colleagues, which demonstrated that patients with cognitive impairment had significantly lower urate levels compared with PD patients without cognitive impairment [79]. Therefore, serum urate may possess additive properties as a diagnostic biomarker for cognitive dysfunction superimposed on the classic motor deficits of PD – consistent with a broader diagnostic biomarker potential for degenerative disease (see later).
Post-mortem brain urate
Cerebrospinal fluid biomarkers of neurodegenerative disease are generally considered more informative than their blood homologs owing to their closer proximity to the degenerating neurons that define the disease. Accordingly, CNS tissue-based biomarkers (e.g., α-synuclein immunoreactive cytoplasmic inclusions within nigral neurons) are ideally located for pathophysiological relevance, despite their obvious lack of practical utility as a diagnostic biomarker (Figure 3). Furthermore, insights from the characterization of diagnostic biomarkers in post-mortem brain specimens can be of high value in validating the importance of more peripherally sampled version of the same biomarker. Therefore, the demonstration that nigrostriatal tissue contains lower concentrations of urate than corresponding control brain tissue has had important implications for the therapeutic, diagnostic and prognostic potential of urate in PD. Arguably, it was the results of post-mortem neurochemical study by Church and Ward [19] – conducted some 20 years ago – that led to the wave of epidemiological interest in urate as potential risk biomarker and, in turn, its current clinical application. The authors examined postmortem caudate and substantia nigra samples from PD patients and controls. Although the study was small (n = 4 for PD and normal control groups), dopamine urate was present at significantly (p < 0.05) and markedly (54%) lower concentrations in the substantia nigra of PD patients compared with controls, with a similar trend found in the caudate (p < 0.1 for urate). Church and Ward also reported that the dopamine oxidation rate constant in PD nigra was much higher than in controls, supporting the concept of a local ‘pro-oxidative stress’ in the disease state. Furthermore, they found that the dopamine oxidation rate could be reduced in PD nigral homogenates by adding urate, and increased in the corresponding control homogenates by adding urate oxidase. These data support the hypothesis that in vivo urate acts an endogenous inhibitor of dopamine oxidation reactions and, potentially, oxidative damage. More recently, Fitzmaurice et al. reported that, in autopsy specimens from ten PD patients, nigral urate levels appeared to be 26% lower than that in the specimens from 16 control patients, although the association did not reach statistical significance [80].
Conclusion & future perspective
Based on a remarkable convergence of evolutionary, laboratory, epidemiological and clinical findings, urate has emerged as a promising biomarker for the risk, diagnosis and prognosis of PD. The biochemical plausibility of endogenous urate also having a pivotal protective influence has added therapeutic significance as well. Indeed, these biomarker and mechanistic data have facilitated rapid translation to an initial clinical trial of urate elevation as a candidate disease-modifying strategy for PD [101].
The putative antioxidant mechanism suggests that any true neuroprotective effect of urate could be expected to extend beyond dopaminergic neuron degeneration in PD. Indeed, the evidence that urate may serve as diagnostic biomarker for cognitive function in PD, as described previously, is consistent with increasingly appreciated cortical involvement in the disease. Furthermore, a few studies suggest that urate as a biomarker for favorable CNS outcomes in neurodegeneration is not specific for PD. For example, preliminary findings have identified blood urate as a potential biomarker of favorable prognosis in Huntington’s disease [81] and, possibly, the conversion from mild cognitive impairment to Alzheimer’s disease [71]. Whether urate is, in fact, as strongly linked to outcomes of neurodegenerative diseases other than PD, will be addressed in the coming years.
Despite the association between serum or CSF urate and multiple favorable PD outcomes, several caveats should be noted. Although it is tempting to make clinical use of the emerging biomarker properties of urate for PD – particularly for its prognosis – it remains an experimental link. It is unlikely to be sufficiently predictive, alone, to warrant its routine measurement in clinical practice; at least not without integrating it into a composite index of risk, diagnosis or prognosis – indices that have not yet been developed and validated. Over the next 10 years, it is likely that the field of PD biomarkers will advance in this direction, in a manner analogous to that of the cardiovascular field, in which composite indices of cardiac risk are routinely employed in both research and clinical practice. Therefore, it can be envisioned that a ‘Parkinson’s Risk Index’ will soon be quantifiable and useful, and might be based on genetic test results, family and exposure histories, olfaction testing, and simple blood tests for urate, specific α-synuclein species and other factors. A similar ‘Parkinson’s Progression Index’ may comprise information from functional neuroimaging of dopaminergic deficits, blood and CSF determinations of urate, gene tests and other molecular factors that are yet to be validated, as well as clinical phenotype (e.g., tremor predominance).
Although urate elevation has compelling neuroprotective potential therapeutically, and can be achieved in humans through the administration of widely available nutritional supplements, this candidate treatment strategy is unproven and carries substantial health risks (e.g., gout, urolithiasis and possible cardiovascular disease), and should not be attempted outside of a carefully conducted clinical study in which the potential risks and benefits can be balanced. Over the next 10 years, the prospects for targeting urate, or related purines and antioxidants, as disease-modifying therapy are likely to be clarified.
Similarly, although urate testing as a PD biomarker cannot be recommended in routine clinical practice yet, its link to PD outcomes is sufficiently robust to warrant immediate application in research studies of PD. For example, measurement of baseline serum urate in a randomized clinical trial of a candidate neuroprotectant should now be standard in any new ‘disease modification’ trial for PD. Its inclusion in the analysis as a covariate linked to progression will increase the power and/or reduce the sample size/cost of the trial. Testing for an interaction between endogenous urate levels and the study drug in a neuroprotection trial is also important, as urate may have a predictive biomarker role, as was suggested in our post-hoc analysis of its interaction with α-tocopherol in the DATATOP trial, described previously [60]. Therefore, under some circumstances, serum urate measurement might be conducted as a screening test prior to enrollment, in order to select PD subjects with relatively low urate as potential ‘responders’ based on low endogenous antioxidant capacity.
Finally, in addition to further developing the risk, diagnostic, prognostic and possibly other applications of urate as a biomarker of PD, it is critical to enhance our insufficient understanding of why urate is linked to the disease. Carefully designed laboratory and clinical studies are required to elucidate the exact role of urate and related purines in the development and progression of the disease.
Acknowledgements
The authors wish to thank CA Desjardins for his assistance with the manuscript.
This work was supported by grants from the NIH (R01NS054978, K24NS60991 and R21NS058324), Michael J Fox Foundation, American Parkinson’s Disease Association and RJG Foundation.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 2.Wu XW, Muzny DM, Lee CC, et al. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J. Mol. Evol. 1992;34(1):78–84. doi: 10.1007/BF00163854. [DOI] [PubMed] [Google Scholar]
- 3.Orowan E. The origin of man. Nature. 1955;175(4459):683–684. doi: 10.1038/175683a0. [DOI] [PubMed] [Google Scholar]
- 4. Ames BN, Cathcart R, Schwiers E, et al. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc. Natl Acad. Sci. USA. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. ▪▪ Describes the discovery that urate possesses antioxidant properties comparable to those of ascorbate.
- 5.Watanabe S, Kang DH, Feng L, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40(3):355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- 6.Hediger MA, Johnson RJ, Miyazaki H, et al. Molecular physiology of urate transport. Physiology (Bethesda) 2005;20:125–133. doi: 10.1152/physiol.00039.2004. [DOI] [PubMed] [Google Scholar]
- 7.Jossa F, Farinaro E, Panico S, et al. Serum uric acid and hypertension: the Olivetti heart study. J. Hum. Hypertens. 1994;8(9):677–681. [PubMed] [Google Scholar]
- 8.Freedman DS, Williamson DF, Gunter EW, et al. Relation of serum uric acid to mortality and ischemic heart disease. The NHANES I Epidemiologic Follow-up Study. Am. J. Epidemiol. 1995;141(7):637–644. doi: 10.1093/oxfordjournals.aje.a117479. [DOI] [PubMed] [Google Scholar]
- 9.Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J. Am. Soc. Nephrol. 2002;13(12):2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 10.Choi HK, Mount DB, Reginato AM, et al. Pathogenesis of gout. Ann. Intern. Med. 2005;143(7):499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- 11.Bos MJ, Koudstaal PJ, Hofman A, et al. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37(6):1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- 12.Toncev G, Milicic B, Toncev S, et al. Serum uric acid levels in multiple sclerosis patients correlate with activity of disease and blood-brain barrier dysfunction. Eur. J. Neurol. 2002;9(3):221–226. doi: 10.1046/j.1468-1331.2002.00384.x. [DOI] [PubMed] [Google Scholar]
- 13.Knapp CM, Constantinescu CS, Tan JH, et al. Serum uric acid levels in optic neuritis. Mult. Scler. 2004;10(3):278–280. doi: 10.1191/1352458504ms1042oa. [DOI] [PubMed] [Google Scholar]
- 14.Burkhardt CR, Weber HK. Parkinson’s disease: a chronic, low-grade antioxidant deficiency? Med. Hypotheses. 1994;43(2):111–114. doi: 10.1016/0306-9877(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 15.Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson’s disease: evidence supporting it. Ann. Neurol. 1992;32(6):804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- 16.Yoritaka A, Hattori N, Uchida K, et al. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl Acad. Sci. USA. 1996;93(7):2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danielson SR, Andersen JK. Oxidative and nitrative protein modifications in Parkinson’s disease. Free Radic. Biol. Med. 2008;44(10):1787–1794. doi: 10.1016/j.freeradbiomed.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swerdlow RH, Parks JK, Miller SW, et al. Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann. Neurol. 1996;40(4):663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 19. Church WH, Ward VL. Uric acid is reduced in the substantia nigra in Parkinson’s disease: effect on dopamine oxidation. Brain Res. Bull. 1994;33(4):419–425. doi: 10.1016/0361-9230(94)90285-2. ▪▪ First study to report a significant association between urate and Parkinson’s disease (PD); it encouraged initial epidemiological investigations into blood urate and PD risk.
- 20.Regoli F, Winston GW. Quantification of total oxidant scavenging capacity of antioxidants for peroxynitrite, peroxyl radicals, and hydroxyl radicals. Toxicol. Appl. Pharmacol. 1999;156(2):96–105. doi: 10.1006/taap.1999.8637. [DOI] [PubMed] [Google Scholar]
- 21.Kaur H, Halliwell B. Action of biologically-relevant oxidizing species upon uric acid. Identification of uric acid oxidation products. Chem. Biol. Interact. 1990;73(2–3):235–247. doi: 10.1016/0009-2797(90)90006-9. [DOI] [PubMed] [Google Scholar]
- 22.Duan W, Ladenheim B, Cutler RG, et al. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J. Neurochem. 2002;80(1):101–110. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 23.Haberman F, Tang SC, Arumugam TV, et al. Soluble neuroprotective antioxidant uric acid analogs ameliorate ischemic brain injury in mice. Neuromolecular Med. 2007;9(4):315–323. doi: 10.1007/s12017-007-8010-1. [DOI] [PubMed] [Google Scholar]
- 24.Guerreiro S, Ponceau A, Toulorge D, et al. Protection of midbrain dopaminergic neurons by the end-product of purine metabolism uric acid: potentiation by low-level depolarization. J. Neurochem. 2009;109(4):1118–1128. doi: 10.1111/j.1471-4159.2009.06040.x. [DOI] [PubMed] [Google Scholar]
- 25.Cohen AM, Aberdroth RE, Hochstein P. Inhibition of free radical-induced DNA damage by uric acid. FEBS Lett. 1984;174(1):147–150. doi: 10.1016/0014-5793(84)81094-7. [DOI] [PubMed] [Google Scholar]
- 26.Kaur H, Halliwell B. Action of biologically-relevant oxidizing species upon uric acid. Identification of uric acid oxidation products. Chem. Biol. Interact. 1990;73(2–3):235–247. doi: 10.1016/0009-2797(90)90006-9. [DOI] [PubMed] [Google Scholar]
- 27.Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149(1):43–50. doi: 10.1016/s0300-483x(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 28.Davies KJ, Sevanian A, Muakkassah-Kelly SF, et al. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem. J. 1986;235(3):747–754. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Einsele H, Clemens MR, Wegner U, et al. Effect of free radical scavengers and metal ion chelators on hydrogen peroxide and phenylhydrazine induced red blood cell lipid peroxidation. Free Radic. Res. Commun. 1987;3(1–5):257–263. doi: 10.3109/10715768709069791. [DOI] [PubMed] [Google Scholar]
- 30.Miura T, Muraoka S, Ogiso T. Inhibitory effect of urate on oxidative damage induced by adriamycin-Fe3+ in the presence of H2O2. Res. Commun. Chem. Pathol. Pharmacol. 1993;79(1):75–85. [PubMed] [Google Scholar]
- 31.Aruoma OI, Halliwell B. Inactivation of α1-antiproteinase by hydroxyl radicals. The effect of uric acid. FEBS Lett. 1989;244(1):76–80. doi: 10.1016/0014-5793(89)81166-4. [DOI] [PubMed] [Google Scholar]
- 32.Whiteman M, Ketsawatsakul U, Halliwell B. A reassessment of the peroxynitrite scavenging activity of uric acid. Ann. NY Acad. Sci. 2002;962:242–259. doi: 10.1111/j.1749-6632.2002.tb04072.x. [DOI] [PubMed] [Google Scholar]
- 33.Skinner KA, White CR, Patel R, et al. Nitrosation of uric acid by peroxynitrite. Formation of a vasoactive nitric oxide donor. J. Biol. Chem. 1998;273(38):24491–24497. doi: 10.1074/jbc.273.38.24491. [DOI] [PubMed] [Google Scholar]
- 34.Ahlskog JE, Uitti RJ, Low PA, et al. No evidence for systemic oxidant stress in Parkinson’s or Alzheimer’s disease. Mov. Disord. 1995;10(5):566–573. doi: 10.1002/mds.870100507. [DOI] [PubMed] [Google Scholar]
- 35.Davis JW, Grandinetti A, Waslien CI, et al. Observations on serum uric acid levels and the risk of idiopathic Parkinson’s disease. Am. J. Epidemiol. 1996;144(5):480–484. doi: 10.1093/oxfordjournals.aje.a008954. [DOI] [PubMed] [Google Scholar]
- 36.de Lau LM, Koudstaal PJ, Hofman A, et al. Serum uric acid levels and the risk of Parkinson disease. Ann. Neurol. 2005;58(5):797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 37. Weisskopf MG, O’Reilly E, Chen H, et al. Plasma urate and risk of Parkinson’s disease. Am. J. Epidemiol. 2007;166(5):561–567. doi: 10.1093/aje/kwm127. ▪▪The largest prospective epidemiological study of urate and PD risk; it confirmed the inverse association and prompted investigation of the urate-PD rate hypothesis.
- 38.Chen H, Mosley TH, Alonso A, et al. Plasma urate and Parkinson’s disease in the Atherosclerosis Risk in Communities (ARIC) study. Am. J. Epidemiol. 2009;169(9):1064–1069. doi: 10.1093/aje/kwp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Reilly EJ, Gao X, Weisskopf MG, et al. Plasma urate and Parkinson’s disease in women. Am. J. Epidemiol. 2010;172(6):666–670. doi: 10.1093/aje/kwq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winquist A, Steenland K, Shankar A. Higher serum uric acid associated with decreased Parkinson’s disease prevalence in a large community-based survey. Mov. Disord. 2010;25(7):932–936. doi: 10.1002/mds.23070. [DOI] [PubMed] [Google Scholar]
- 41.Wortmann RL. Disorders of purine and pyrimidine metabolism. In: Fauci AS, Braunwald E, Kasper DL, et al., editors. Harrison’s Principles of Internal Medicine. NY, USA: McGraw-Hill Medical Publishing Division; 2008. [Google Scholar]
- 42. Gao X, Chen H, Choi HK, et al. Diet, urate, and Parkinson’s disease risk in men. Am. J. Epidemiol. 2008;167(7):831–838. doi: 10.1093/aje/kwm385. ▪Demonstrated that dietary influences on plasma urate may contribute to a lower risk of PD.
- 43.Park M, Ross GW, Petrovitch H, et al. Consumption of milk and calcium in midlife and the future risk of Parkinson disease. Neurology. 2005;64(6):1047–1051. doi: 10.1212/01.WNL.0000154532.98495.BF. [DOI] [PubMed] [Google Scholar]
- 44.Chen H, O’Reilly E, McCullough ML, et al. Consumption of dairy products and risk of Parkinson’s disease. Am. J. Epidemiol. 2007;165(9):998–1006. doi: 10.1093/aje/kwk089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma L, Zhang L, Gao XH, et al. Dietary factors and smoking as risk factors for PD in a rural population in China: a nested case-control study. Acta Neurol. Scand. 2006;113(4):278–281. doi: 10.1111/j.1600-0404.2005.00571.x. [DOI] [PubMed] [Google Scholar]
- 46.Fall PA, Fredrikson M, Axelson O, et al. Nutritional and occupational factors influencing the risk of Parkinson’s disease: a case-control study in southeastern Sweden. Mov. Disord. 1999;14(1):28–37. doi: 10.1002/1531-8257(199901)14:1<28::aid-mds1007>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 47.Choi HK, Atkinson K, Karlson EW, et al. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363(9417):1277–1281. doi: 10.1016/S0140-6736(04)16000-5. [DOI] [PubMed] [Google Scholar]
- 48.Grandinetti A, Morens DM, Reed D, et al. Prospective study of cigarette smoking and the risk of developing idiopathic Parkinson’s disease. Am. J. Epidemiol. 1994;139(12):1129–1138. doi: 10.1093/oxfordjournals.aje.a116960. [DOI] [PubMed] [Google Scholar]
- 49.Paganini-Hill A. Risk factors for Parkinson’s disease: the leisure world cohort study. Neuroepidemiology. 2001;20(2):118–124. doi: 10.1159/000054770. [DOI] [PubMed] [Google Scholar]
- 50.Hernan MA, Chen H, Schwarzschild MA, et al. Alcohol consumption and the incidence of Parkinson’s disease. Ann. Neurol. 2003;54(2):170–175. doi: 10.1002/ana.10611. [DOI] [PubMed] [Google Scholar]
- 51.Palacios N, Gao X, O’Reilly E, et al. Intake of alcohol and risk of Parkinson disease in a large prospective study of men and women. Am. J. Epidemiol. 2010;171 Suppl.:S149. (Abstract 599) [Google Scholar]
- 52.Lesch M, Nyhan WL. A familial disorder of uric acid metabolism and central nervous system function. Am. J. Med. 1964;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- 53.So A, Thorens B. Uric acid transport and disease. J. Clin. Invest. 2010;120(6):1791–1799. doi: 10.1172/JCI42344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stark K, Reinhard W, Neureuther K, et al. Association of common polymorphisms in GLUT9 gene with gout but not with coronary artery disease in a large case-control study. PLoS ONE. 2008;3(4):e1948. doi: 10.1371/journal.pone.0001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vitart V, Rudan I, Hayward C, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 2008;40(4):437–442. doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]
- 56.Caulfield MJ, Munroe PB, O’Neill D, et al. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med. 2008;5(10):e197. doi: 10.1371/journal.pmed.0050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Facheris MF, Hicks AA, Minelli C, et al. Variation in the uric acid transporter gene SLC2A9 and its association with AAO of Parkinson’s disease. J. Mol. Neurosci. 2010 doi: 10.1007/s12031-010-9409-y. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 58.Alonso A, Rodriguez LA, Logroscino G, et al. Gout and risk of Parkinson disease: a prospective study. Neurology. 2007;69(17):1696–1700. doi: 10.1212/01.wnl.0000279518.10072.df. [DOI] [PubMed] [Google Scholar]
- 59.De Vera M, Rahman MM, Rankin J, et al. Gout and the risk of Parkinson’s disease: a cohort study. Arthritis Rheum. 2008;59(11):1549–1554. doi: 10.1002/art.24193. [DOI] [PubMed] [Google Scholar]
- 60. Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch. Neurol. 2008;65(6):716–723. doi: 10.1001/archneur.2008.65.6.nct70003. ▪Identified serum urate as the first blood biomarker to be predictive of progression in typical idiopathic PD. The first study to correlate serum urate with changes in striatal functionality.
- 61. Ascherio A, LeWitt PA, Xu K, et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch. Neurol. 2009;66(12):1460–1468. doi: 10.1001/archneurol.2009.247. ▪▪Established serum urate and identified cerebrospinal fluid urate as prognostic biomarkers of clinical progression in idiopathic PD, prompting clinical development of urate elevation as a therapeutic strategy for disease modification.
- 62.O’Neill RD, Lowry JP. On the significance of brain extracellular uric acid detected with in-vivo monitoring techniques: a review. Behav. Brain Res. 1995;71(1–2):33–49. doi: 10.1016/0166-4328(95)00035-6. [DOI] [PubMed] [Google Scholar]
- 63.Higgins P, Dawson J, Walters M. The potential for xanthine oxidase inhibition in the prevention and treatment of cardiovascular and cerebrovascular disease. Cardiovasc. Psychiatry Neurol. 2009;2009:282059. doi: 10.1155/2009/282059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niklasson F, Hetta J, Degrell I. Hypoxanthine, xanthine, urate and creatinine concentration gradients in cerebrospinal fluid. Ups. J. Med. Sci. 1988;93(3):225–232. doi: 10.3109/03009738809178548. [DOI] [PubMed] [Google Scholar]
- 65.Niklasson F, Agren H. Brain energy metabolism and blood-brain barrier permeability in depressive patients: analyses of creatine, creatinine, urate, and albumin in CSF and blood. Biol. Psychiatry. 1984;19(8):1183–1206. [PubMed] [Google Scholar]
- 66.Kastenbauer S, Koedel U, Becker BF, et al. Oxidative stress in bacterial meningitis in humans. Neurology. 2002;58(2):186–191. doi: 10.1212/wnl.58.2.186. [DOI] [PubMed] [Google Scholar]
- 67.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 68. Kortekaas R, Leenders KL, van Oostrom JC, et al. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 2005;57(2):176–179. doi: 10.1002/ana.20369. ▪Not only proposed a possible role of blood-brain barrier dysfunction in PD pathogenesis, but also raised the question of how urate homeostasis in cerebrospinal fluid might contribute to or confound the observed relationship between cerebrospinal fluid urate and PD progression.
- 69.Tohgi H, Abe T, Takahashi S, et al. The urate and xanthine concentrations in the cerebrospinal fluid in patients with vascular dementia of the Binswanger type, Alzheimer type dementia, and Parkinson’s disease. J. Neural Transm. Park. Dis. Dement. Sect. 1993;6(2):119–126. doi: 10.1007/BF02261005. [DOI] [PubMed] [Google Scholar]
- 70.DATATOP: a multicenter controlled clinical trial in early Parkinson’s disease. Parkinson Study Group. Arch. Neurol. 1989;46(10):1052–1060. doi: 10.1001/archneur.1989.00520460028009. [DOI] [PubMed] [Google Scholar]
- 71.Simon R. Development and validation of biomarker classifiers for treatment selection. J. Stat. Plan. Inference. 2008;138(2):308–320. doi: 10.1016/j.jspi.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Irizarry MC, Raman R, Schwarzschild MA, et al. Plasma urate and progression of mild cognitive impairment. Neurodegener. Dis. 2009;6(1–2):23–28. doi: 10.1159/000170883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson RF, Harris TA. Dopamine and uric acid act as antioxidants in the repair of DNA radicals: implications in Parkinson’s disease. Free Radic. Res. 2003;37(10):1131–1136. doi: 10.1080/10715760310001604134. [DOI] [PubMed] [Google Scholar]
- 74.Bowman GL, Shannon J, Frei B, et al. Uric acid as a CNS antioxidant. J. Alzheimers Dis. 2010;19(4):1331–1336. doi: 10.3233/JAD-2010-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Larumbe Ilundain R, Ferrer Valls JV, Vines Rueda, et al. Case-control study of markers of oxidative stress and metabolism of blood iron in Parkinson’s disease. Rev. Esp. Salud. Publica. 2001;75(1):43–53. [PubMed] [Google Scholar]
- 76.Annanmaki T, Muuronen A, Murros K. Low plasma uric acid level in Parkinson’s disease. Mov. Disord. 2007;22(8):1133–1137. doi: 10.1002/mds.21502. [DOI] [PubMed] [Google Scholar]
- 77.Andreadou E, Nikolaou C, Gournaras F, et al. Serum uric acid levels in patients with Parkinson’s disease: their relationship to treatment and disease duration. Clin. Neurol. Neurosurg. 2009;111(9):724–728. doi: 10.1016/j.clineuro.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 78.Annanmaki T, Pessala-Driver A, Hokkanen L, et al. Uric acid associates with cognition in Parkinson’s disease. Parkinsonism Relat. Disord. 2008;14(7):576–578. doi: 10.1016/j.parkreldis.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 79.Wang XJ, Luo WF, Wang LJ, et al. Study on uric acid and the related factors associated with cognition in the patients with Parkinson’s disease. Zhonghua Yi Xue Za Zhi. 2009;89(23):1633–1635. [PubMed] [Google Scholar]
- 80.Fitzmaurice PS, Ang L, Guttman M, et al. Nigral glutathione deficiency is not specific for idiopathic Parkinson’s disease. Mov. Disord. 2003;18(9):969–976. doi: 10.1002/mds.10486. [DOI] [PubMed] [Google Scholar]
- 81.Auinger P, Kieburtz K, McDermott MP. The relationship between uric acid levels and Huntington’s disease progression. Mov. Disord. 2010;25(2):224–228. doi: 10.1002/mds.22907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 101.The Parkinson Study Group. Safety of Urate Elevation in Parkinson’s Disease (SURE-PD) [Accessed 1 September 2010]; http://clinicaltrials.gov/ct2/show/ NCT00833690.