Abstract
Changes in AMPA receptors have been proposed to underlie changes in synaptic efficacy in hippocampus and other brain structures. Calpain activation has also been discussed as a potential mechanism to produce lasting modifications of synaptic structure and function. Stargazin is a member of the family of transmembrane AMPA receptor associated proteins (TARPs), which participate in trafficking of AMPA receptors and regulate their kinetic properties. We report here that preincubation of thin (20 microns) frozen rat brain sections with calcium changes the immunological properties of stargazin, an effect totally blocked by a calpain inhibitor. Immunocytochemistry indicates that in situ calpain activation produces a decreased immunoreactivity for stargazin in the neuropil throughout the brain, and western blots confirmed that a similar treatment decreased stargazin levels. Interestingly, the same treatment did not modify the immunoreactivity for another TARP member, γ-8, although it increased immunoreactivity in cell bodies in hippocampus, an effect that was not blocked by calpain inhibition. These results strongly suggest the involvement of calpain in the regulation of AMPA receptor targeting and function through truncation of stargazin.
Keywords: calpain, stargazin, AMPA receptors, hippocampus, plasticity
1. Introduction
Glutamate is the major excitatory neurotransmitter in the central nervous system (CNS) and mediates its postsynaptic effects through interactions with both ionotropic and metabotropic receptors (Nakanishi and Masu, 1994). Over the last 30 years, it has been clearly demonstrated that changes in the synaptic number of one subtype of ionotropic receptors, the AMPA receptors, are responsible for the long-term changes in synaptic efficacy that underlie some forms of learning and memory (Baudry and Lynch, 2001; Kessels and Malinow, 2009). As a result, there has been considerable interest in understanding the mechanisms involved in the regulation of synaptic AMPA receptor number in various brain structures. In addition to the traditional mechanisms of receptor endocytosis and exocytosis (Caroll et al., 2001; Groc and Choquet, 2006), the relatively recent discovery of a family of transmembrane AMPA receptor associated proteins (TARPs) provided a new level of control of synaptic AMPA receptor number and function (Tomita et al., 2003; Vandenberghe et al., 2005; Nicoll et al., 2006). TARPs are auxiliary proteins for AMPA receptors and they participate in both the trafficking of AMPA receptors from the endoplasmic reticulum to the plasma membrane and postsynaptic sites, as well as in setting the kinetic properties of the receptor-channel complex. Among the TARPs, stargazin, also known as TARP-γ-2, has been the most extensively studied since its discovery in the ataxic and epipleptic stargazer mutant mouse (Chen et al., 2000). Stargazin is abundantly present in the cerebellum as well as in hippocampus and cortex. Interestingly, the absence of stargazin in cerebellar granule cells of the stargazer mutant mice results in the absence of functional synaptic AMPA receptors, clearly indicating the role of stargazin in AMPA receptor trafficking (Chen et al., 2003).
Another mechanism regulating AMPA receptor properties is through truncation of the C-terminal domain of various AMPA receptor subunits by the calcium-dependent protease, calpain (Bi et al., 1996a; Bi et al., 1997). We previously reported that calpain treatment of synaptic membranes resulted in the truncation of GluR1-3 C-terminal domains. We also showed a similar effect following calcium treatment of frozen-thawed brain sections (Bi et al., 1994), as well as in vivo following seizure activity elicited by systemic kainic acid injection in adult rats (Bi et al., 1996b). Additional experiments indicated that calpain-mediated truncation of the C-terminal domain of AMPA receptor subunits resulted in increased internalization of the receptors and further degradation (Lu et al., 2000b). We also showed that calpain could truncate several proteins involved in AMPA and NMDA receptor anchoring to postsynaptic membranes, such as PSD-95 and GRIP (Lu et al., 2000a; Lu et al., 2001). It was therefore logical to determine whether calpain activation could also regulate TARP levels in various brain regions. To answer this question, we used calcium treatment of frozen-thawed brain sections in the absence and presence of a calpain inhibitor, followed by immunohistochemistry with antibodies against stargazin and TARP-γ-8. We also performed western blots to confirm the results from immunohistochemistry. Our results indicate that calpain does modify stargazin but not γ-8 immunoreactivity in most brain regions, consistent with calpain-mediated truncation of stargazin in its C-terminal domain.
2. Experimental Procedures
Animals were treated in accordance with the principles and procedures of the National Institutes of Health Guide for the Care and Use of Laboratory Animals; all protocols were approved by the Institutional Animal Care and Use Committee of the University of Southern California. Young adult male (postnatal day 35–42) Sprague-Dawley rats were killed by decapitation following anesthesia and brains were rapidly removed, frozen in methylbutane at −40 °C and stored at −80 °C. Serial sagittal or coronal sections (20 μm thick) were cut on a cryostat, thaw-mounted onto chrome-alum gelatin-coated slides, and kept at −80 °C until used.
2.1 Tissue section treatment
Adjacent sections were thawed at room temperature (RT) and incubated for 90 min at RT in Tris-acetate buffer (100 mM, pH 7.4) containing 100 μM EGTA with or without calcium chloride (2 mM) and in the absence or presence of calpain inhibitor III (Calbiochem, 10 μM).
2.2 Immunocytochemistry
Following treatment, sections were rinsed in Tris-acetate buffer and immersed fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) containing 100 μM EGTA at 4 C for 1 h. After incubation with 10% normal goat serum for 1 h at RT, sections were incubated with primary antibodies in 5% normal goat serum overnight at 4 °C. The following antibodies were used : anti-stargazin (Millipore, cat #: 07-577; dilution 1:500) and anti-TARP-γ-8 (Abcam; dlution 1:500). Sections were then washed in PBS and incubated with biotinylated goat anti-rabbit IgG antibody (1:200, Vector Laboratories) for 2 h at RT. The sections were reacted with ABC Elite kit and DAB (Vector Laboratories) at RT for 45 min according to the manufacturer’s protocol.
2.3 Western blots
Following treatment, sections were collected in TBS containing a protease inhibitor cocktail [2.08 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, 1.6 μM aprotinin, 40 μM leupeptin, 80 μM bestatin, 30 μM pepstatin A, and 28 μM trans-3-carboxyoxirane-2-carbonyl-L-leucylagmatine] (Sigma-Aldrich, St. Louis, MO). Proteins were denatured in the presence of 0.1 M 2-mercaptoethanol. Equal amounts (20–60 μg) of protein were separated by SDS-PAGE using 8–10% gels and transferred onto polyvinylidene difluoride membranes. Membranes were blocked with 3% non-fat milk for one hour at room temperature then incubated with representative primary antibodies (stargazin and GRIP1 antibodies, from Millipore; anti-PSD95 from Cell Signaling; anti-TARP-γ-8 from Abcam) overnight at 4 °C. After wash, membranes were incubated with IRDye™ 680 goat anti-rabbit IgG (H+L) (LI-COR, Cat. # 926-32221) for an hour at RT. After wash, signals were acquired with the Odyssey infrared imaging system at 700 nm and 800 nm fluorescent channels and the bands of interest were quantified with the Odyssey software (LI-COR Bioscience, NE).
2.4 Membrane preparation and calpain treatment
Brain membranes were prepared from adult Sprague-Dawley rats (200–250 g): brains were collected in homogenization buffer (0.32 M sucrose, 10 mM HEPES pH 7.4, 2 mM EDTA, protease inhibitor cocktail (Sigma), phosphatase inhibitor cocktail (Sigma) and homogenized using a glass-Teflon homogenizer. The homogenate was centrifuged at 1,000 g and the resultant supernatant was collected and centrifuged at 100,000 g. Pellets (membrane fraction) were re-suspended in PBS containing 1% Triton-X and phosphatase inhibitors. After determining protein concentration using the BCA assay (Pierce), membranes were incubated at 37° C in the presence or absence of calpain-1 (2.4 U/ml) and 2 mM calcium for 30 min. Calpain-induced reaction was stopped by adding Laemmli's loading buffer followed by boiling at 100° C for 5 min. Samples were then processed for SDS-PAGE and western blots.
2.5 Immunohistochemistry quantification
Images were acquired using an Eclipse E600 optical Microscope (Nikon); all sections were viewed under bright-field illumination using a 4x objective and a fixed light source. Stargazin and γ8 immunolabeling was quantitatively analyzed with a computerized densitometer using the National Institutes of Health Image J 1.42q software. Density was obtained from the same regions of the granular layer and molecular layer of the cerebellum and from CA1 and CA3 areas of the hippocampus, and from the cerebral cortex. To enable comparisons between experimental and control sections, the parameters including brightness, contrast, and image size were kept constant throughout the analysis. Background was subtracted from the density values. Five sections were analyzed per animal.
2.5 Statistical analysis
Significant differences in immunodensities among the groups were determined by 1-way ANOVAs. For multiple comparisons Tukey and Fisher’s Least Square Protection Tests were used. Significance was set at P ≤ 0.05 for all tests.
3. Results
3.1 Effects of calcium treatment on stargazin immunoreactivity in different brain regions
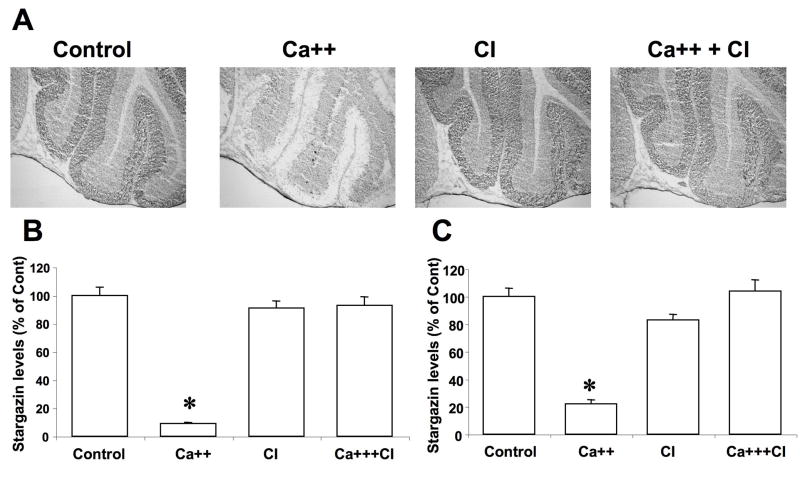
Frozen brain tissue sections were thawed at room temperature immediately before being incubated with calcium (2 mM) or incubation buffer alone in the absence or presence of calpain inhibitor III (10 μM) for 90 min. They were washed in Tris-acetate buffer, fixed in paraformaldehyde, and processed for immunohistochemistry with antibodies against stargazin. In cerebellar sections incubated with buffer alone, high levels of stargazin immunoreactivity were present in the molecular layer, and lower levels in the granule cell layer (Fig. 1A). Treatment with calcium resulted in a large decrease in stargazin immunoreactivity in both molecular layer and granule layer. These qualitative data were confirmed by image quantification using Image J software (Fig. 1B,C). Treatment with calpain inhibitor III alone did not alter stargazin immunoreactivity but completely prevented the changes elicited by calcium treatment (Fig. 1B,C).
Figure 1. Effects of calcium and calpain inhibitor on stargazin immunolabeling in cerebellum.
Thin frozen-thawed brain sections were incubated at room temperature for 90 min in the absence of calcium (Control), in the presence of 2.0 mM calcium (Ca++), and in the presence of 10 μM calpain inhibitor III (CI). They were then processed for immunohistochemistry with anti-stargazin antibodies.
A. Representative images of brain sections incubated under each condition.
B & C. Quantitative analysis of images similar to those shown in A. Staining levels were analyzed in cerebellar molecular layer (B) and granule layer (C). Results were expressed as percent of the values found under control conditions and are means ± S.E.M. of 12 sections. *p<0.001.
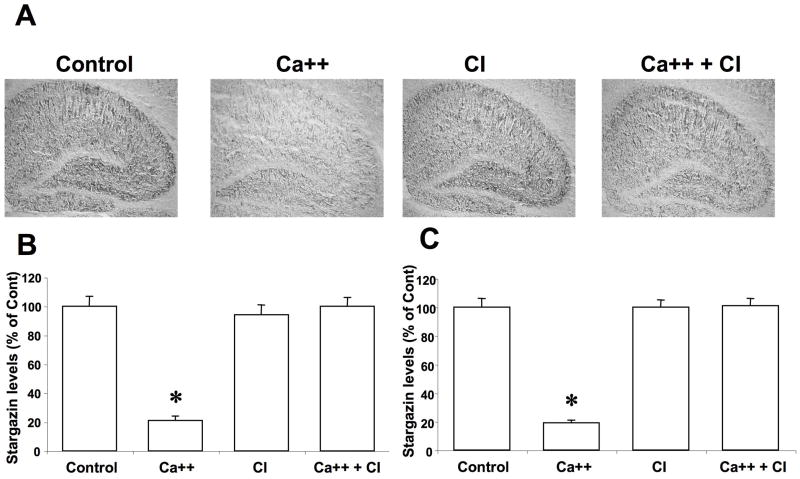
Similarly, high to moderate levels of stargazin were present throughout the hippocampus, with low levels in the cell body layer of the dentate gyrus (Fig. 2A). Incubation with calcium also resulted in a large decrease in stargazin immunoreactivity throughout the hippocampus, which was confirmed by quantitative analysis (Fig. 2B,C). Calcium treatment induced changes in stargazin immunoreactivity in hippocampus was also completely blocked by calpain inhibitor III.
Figure 2. Effects of calcium and calpain inhibitor on stargazin immunolabeling in hippocampus.
Thin frozen-thawed brain sections were incubated at room temperature for 90 min in the absence of calcium (Control), in the presence of 2.0 mM calcium (Ca++), and in the presence of 10 μM calpain inhibitor III (CI). They were then processed for immunohistochemistry with anti-stargazin antibodies.
A. Representative images of brain sections incubated under each condition.
B & C. Quantitative analysis of images similar to those shown in A. Staining levels were analyzed in CA1 (B) and CA3 (C) regions. Results were expressed as percent of the values found under control conditions and are means ± S.E.M. of 12 sections. *p<0.001.
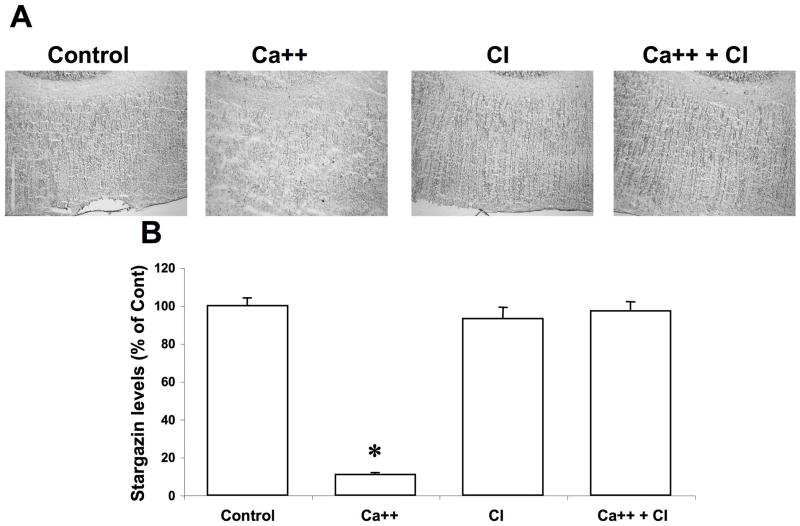
In control conditions, stargazin immunoreactivity was distributed relatively uniformly in the cerebral cortex (Fig. 3A). Following calcium treatment, immunoreactivity was also decreased throughout the cortex, an effect which was also completely prevented by calpain inhibitor III.
Figure 3. Effects of calcium and calpain inhibitor on stargazin immunolabeling in cortex.
Thin frozen-thawed brain sections were incubated at room temperature for 90 min in the absence of calcium (Control), in the presence of 2.0 mM calcium (Ca++), and in the presence of 10 μM calpain inhibitor III (CI). They were then processed for immunohistochemistry with anti-stargazin antibodies.
A. Representative images of brain sections incubated under each condition.
B. Quantitative analysis of images similar to those shown in A. Results were expressed as percent of the values found under control conditions and are means ± S.E.M. of 12 sections. *p<0.001.
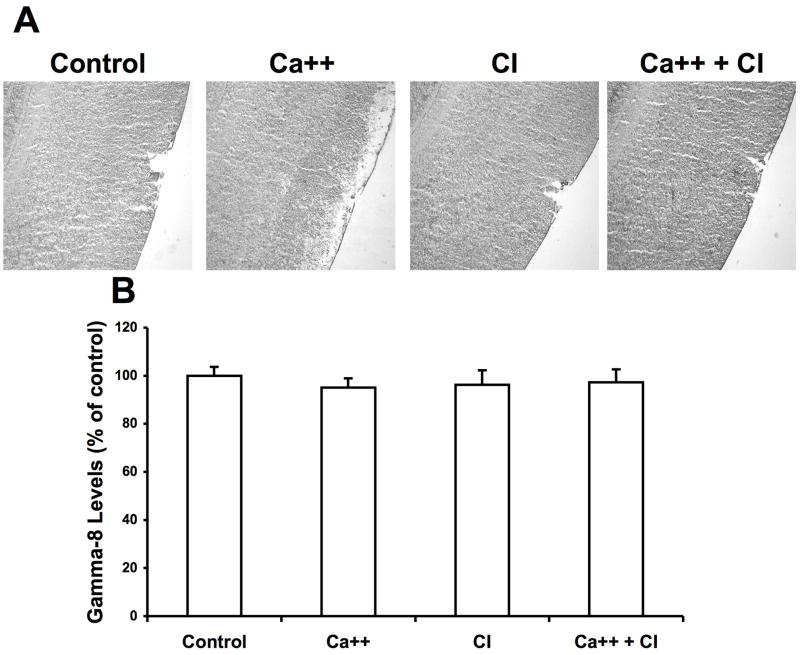
3.2 Effects of calcium treatment on TARP γ-8 immunoreactivity in hippocampus and cortex
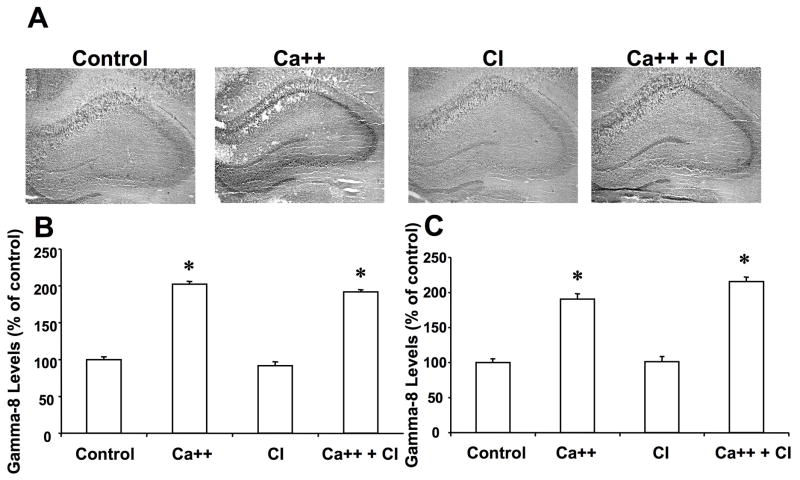
To test whether calpain also truncate other members of the TARP family proteins, we tested the effects of calcium treatment of frozen-thawed brain sections on γ-8. In hippocampus, γ-8 immunoreactivity exhibited a relatively uniform distribution, except for a higher level in the stratum pyramidale and the dentate granule cell layer (Fig. 4A). Calcium treatment did not change γ-8 immunoreactivity in the dendritic fields, but markedly increased that in the cell body layers of both hippocampus and dentate gyrus. Quantification of the images indicated that γ-8 immunoreactivity was doubled in the cell body layers of field CA1 (Fig. 4B), CA3 (Fig. 4C) and dentate gyrus (not shown). Treatment with calpain inhibitor III alone did not alter γ-8 immunoreactivity in any subfield, nor did it modify the effects of calcium in the cell body layers.
Figure 4. Effects of calcium and calpain inhibitor on γ-8 immunolabeling in hippocampus.
Thin frozen-thawed brain sections were incubated at room temperature for 90 min in the absence of calcium (Control), in the presence of 2.0 mM calcium (Ca++), and in the presence of 10 μM calpain inhibitor III (CI). They were then processed for immunohistochemistry with anti-γ-8 antibodies.
A. Representative images of brain sections incubated under each condition.
B & C. Quantitative analysis of images similar to those shown in A. Staining levels were analyzed in the cell bodies of field CA1 (B) and CA3 (C). Results were expressed as percent of the values found under control conditions and are means ± S.E.M. of 12 sections. *p<0.001.
In cerebral cortex, γ-8 immunoreactivity was distributed relatively uniformly throughout the different cortical layers (Fig. 5). Calcium treatment did not modify γ-8 immunoreactivity, nor did incubation with calpain inhibitor III.
Figure 5. Effects of calcium and calpain inhibitor on γ-8 immunolabeling in cortex.
Thin frozen-thawed brain sections were incubated at room temperature for 90 min in the absence of calcium (Control), in the presence of 2.0 mM calcium (Ca++), and in the presence of 10 μM calpain inhibitor III (CI). They were then processed for immunohistochemistry with anti-γ-8 antibodies.
A. Representative images of brain sections incubated under each condition.
B. Quantitative analysis of images similar to those shown in A. Results were expressed as percent of the values found under Control conditions and are means ± S.E.M. of 12 sections.
3.3 Effects of calcium treatment on stargazin levels in frozen-thawed brain sections
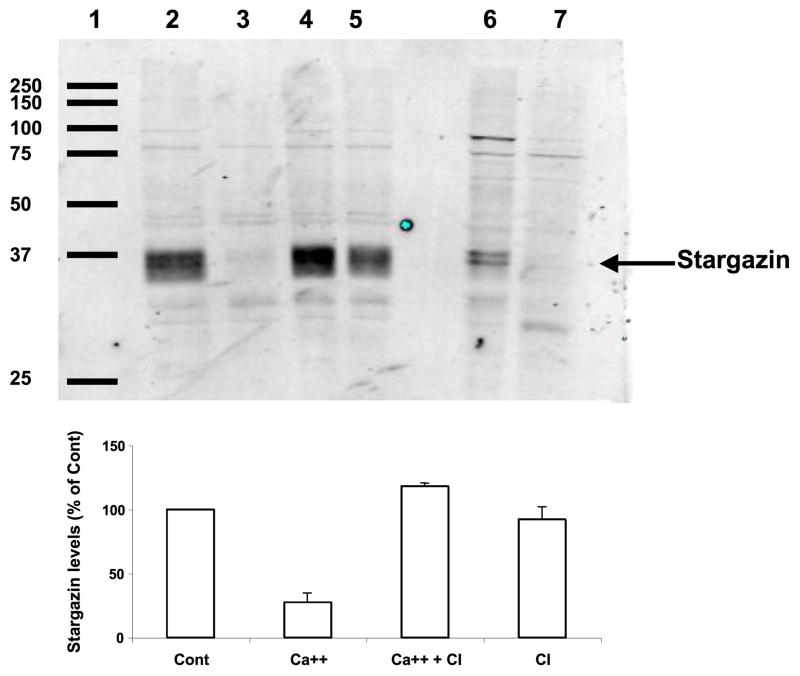
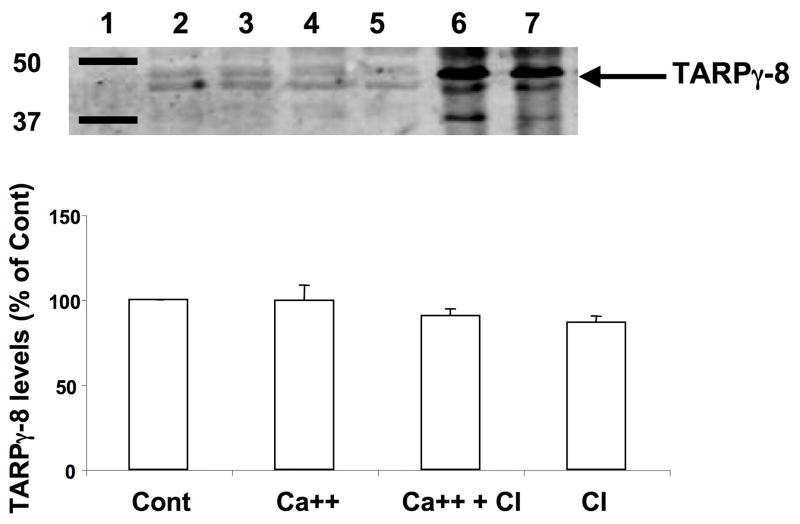
We next tested the effect of calcium treatment on levels of TARP proteins by western blot. After treatment of coronal brain sections with calcium in the absence or presence of calpain inhibitor III, sections were collected, homogenized and aliquots of the homogenates were processed for western blots with antibodies against either stargazin or γ-8. The stargazin antibodies recognized two major bands with an apparent molecular weight of 33–35 kD. This corresponds well with previously reported data from Tomita et al. (2005), who suggested that the larger molecular weight band might correspond to the phosphorylated form of stargazin (Fig. 6). Treatment of coronal brain sections with calcium resulted in a very large decrease in stargazin levels (Fig. 6). This effect was almost completely reversed by a calpain inhibitor, which by itself did not modify stargazin levels. To further confirm that the decrease in stargazin levels observed following calcium treatment was due to calpain activation, we ran on the same blots samples of membrane fractions treated without or with calpain and calcium (lanes 6–7 in Fig. 6). The results clearly indicate that the stargazin immunopositive bands in the membrane fraction are identical to those found in tissue sections and that calpain treatment results in the disappearance of these bands. Aliquots of the same homogenates were also processed for western blots with γ-8 antibody (Fig. 7). This antibody recognized a major doublet band with a molecular weight of 43–45 kD, which corresponds to the molecular weight of TARP γ-8 (Sager et al., 2009). In agreement with our immunohistochemical results, calcium treatment of brain sections did not modify γ-8 levels. To further confirm that γ-8 was not a substrate of calpain, we also ran on the same blots samples of brain membranes treated in the absence or presence of calpain and calcium (lanes 5–6 of Fig. 7). Calpain treatment did not modify γ-8 levels under these conditions.
Figure 6. Effects of calcium and calpain inhibitor on stargazin levels in brain homogenates.
Thin frozen-thawed brain sections were incubated at room temperature for 90 min in the absence of calcium (lane 2, Cont), in the presence of 2.0 mM calcium (lane 3, Ca++), and in the presence of 2.0 mM calcium and10 μM calpain inhibitor III (lane 4, Ca ++ + CI), or calpain inhibitor alone (lane 5, CI). They were then processed for western blots with anti-stargazin antibodies. Membrane fractions were prepared as described under Materials and Methods, and aliquots were treated without (lane 6) or with calpain and calcium (lane 7). Lane 1: molecular weight scale. Right arrow points to the doublet band labeled with the stargazin antibody with an apparent molecular weight of 33–35 kD.
Top: representative image of a western blot under each condition.
Bottom: quantitative analysis of western blots similar to those shown on top. Results were expressed as percent of the values found under control conditions and are means ± S.E.M. of 3 experiments. *p<0.001.
Figure 7. Effects of calcium and calpain inhibitor on TARP γ-8 levels in brain homogenates.
After calpain and calcium treatment, samples were processed for western blot analysis of TARP γ-8 as described under Figure 6 and detailed in Materials and Methods. Right arrow points to the doublet band labeled with the TARP γ-8 antibody with an apparent molecular weight of 43–45 kD.
Top: representative image of a western blot under each condition.
Bottom: quantitative analysis of western blots similar to those shown on top. Results were expressed as percent of the values found under control conditions and are means ± S.E.M. of 3 experiments.
4. Discussion
Our results indicate that in situ calpain activation modifies immunohistochemical properties of stargazin but not of TARP-γ-8 in various rat brain regions. In addition, the same treatment resulted in a large decrease in stargazin but not in TARP-γ-8 levels assessed by western blots. The decrease in stargazin immunoreactivity and levels resulting from calpain activation is consistent with calpain-mediated truncation in the C-terminal domain, which contains the antigenic domain recognized by the polyclonal antibodies we used. This was also supported by results obtained with calpain treatment of brain membranes that resulted in an identical pattern of stargazin loss in western blot. Moreover, the extent of stargazin decrease following calcium treatment of brain sections (more than 80%) in various brain regions was similar to that observed in membrane fractions following calpain treatment.
In the cerebellum, stargazin immunoreactivity was higher in the molecular layer than in the granule layer, and calcium-induced decrease in stargazin levels was relatively similar in the molecular and granule layers. Several studies have shown that calpain was present both in Purkinje cells and granule cells, although it is difficult to determine the relative distribution of calpain between these 2 neuronal populations (Simonson et al., 1985; Hamakubo et al., 1986; Li et al., 1996; Shields et al., 1998). In addition, we did not attempt to determine whether calpain-1 or calpain-2 was involved in calcium-induced changes in stargazin levels, since, under our experimental conditions, both types of calpain should be activated. However, one study reported high levels of calpain-2 in cerebellar Purkinje cells, hippocampal pyramidal neurons and neurons in the deep layers of the cerebral cortex (Li et al., 1996).
While many studies have investigated in details the functions of stargazin and TARPs in various cell types and brain structures, very few studies have investigated the cellular distribution of the various TARPs in adult brain. In hippocampus, we found that stargazin was relatively uniformly distributed except for lower levels in the granule cell and pyramidal layers. This distribution agrees well with the results from Inamura et al. (Inamura et al., 2006), although they used a different antibody. Calcium treatment resulted in a relatively uniform decrease in stargazin levels by more than 80%. Using a similar approach, we observed that the same treatment resulted in a 80% decrease in GluR1 subunits of AMPA receptors (Bi et al., 1996b), due to calpain-mediated truncation in the C-terminal domain. Stargazin has been reported to be present both in pyramidal cells and interneurons in hippocampus (Mi et al., 2004), and our data do not allow to determine whether calcium treatment results in a similar decrease in stargazin in these 2 cell populations. It would be interesting to determine whether calpain activation elicits the same truncation of stargazin in these 2 neuronal populations, as it has been suggested that stargazin has different functions for organizing excitatory synapses in these 2 types of neurons (Mi et al., 2004).
Stargazin immunoreactivity was present throughout the cerebral cortex, and calpain-mediated decrease was also relatively uniform throughout the cortex. At this level of resolution, it was not possible to determine whether the decrease was differentially distributed in certain neuronal populations. Nevertheless, the results suggest that calpain activation can produce widespread truncation of stargazin throughout the cortical layers.
In contrast to stargazin, TARP-γ-8 immunoreactivity was not modified by calpain activation in any brain region. This result was confirmed by the finding that TARP-γ-8 was not truncated by direct calpain treatment of brain membranes. Intriguingly, we found that calcium treatment of frozen-thawed brain sections resulted in increased TARP-γ-8 immunoreactivity in the cell body layers of the hippocampus. This effect was not due to calpain activation as it was not blocked by a calpain inhibitor. At present, the mechanism underlying this increase in immunoreactivity is not clear. It is conceivable that this is due to the activation of some calcium-dependent process that is more prevalent in the cell bodies than in the dendrites and that modifies the conformation of TARP-γ-8 in such a way as to increase its affinity for the antibody. This interpretation is also in agreement with the lack of effect of calcium treatment of brain sections or of calpain treatment of brain membranes on TARP-γ-8 levels.
We previously showed that calcium treatment of brain tissue sections results in calpain-mediated truncation of several proteins involved in signal transmission at glutamatergic synapses; in particular, we found that several subunits of glutamate receptors (GluR1-3), as well as GRIP and PSD95 were truncated under similar conditions (Bi et al., 1997; Lu et al., 2000, 2001). It is unlikely that these effects represent simply widespread tissue deterioration resulting from calcium treatment. Thus, we did not observe any changes in levels of actin in the same samples used for stargazin analysis. In addition, the same treatment resulted in increased 3H-AMPA binding measured with autoradiography (Tocco et al., 1992), but did not affect glutamate binding to NMDA receptors (Lapierre et al., 2000). Interestingly, the increase in 3H-AMPA binding observed following calcium treatment was at least partially blocked by calpain inhibition. It is tempting to propose that this effect could be the result of calpain-mediated stargazin truncation, as stargazin is involved in the regulation of the pharmacological properties of AMPA receptors (Nicoll et al., 2006).
In conclusion, our data indicate that calpain activation produces a widespread decrease in stargazin but not TARP-γ-8 immunoreactivity and levels throughout adult rat brain. The results are consistent with calpain-mediated truncation of the C-terminal domain of stargazin. Interestingly, Tomita et al. (2005) observed a similar decrease in stargazin levels following NMDA treatment of cultured hippocampal neurons. Although they did not comment on this effect in their manuscript, the result is consistent with the known calpain activation following NMDA receptor stimulation. Our results therefore suggest that calpain-mediated stargazin truncation represents an additional level of regulation of AMPA receptor function by calpain activation.
Acknowledgments
This research was supported by Grant NS R21 NS062167 to MB. The authors acknowledge the technical help of Ms. Erin Lee in providing us with calpain-treated brain membranes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baudry M, Lynch G. Remembrance of arguments past: how well is the glutamate receptor hypothesis of LTP holding up after 20 years? Neurobiol Learn Mem. 2001;76:284–297. doi: 10.1006/nlme.2001.4023. [DOI] [PubMed] [Google Scholar]
- Bi R, Bi X, Baudry M. Phosphorylation regulates calpain-mediated truncation of glutamate ionotropic receptors. Brain Res. 1998;797:154–158. doi: 10.1016/s0006-8993(98)00433-8. [DOI] [PubMed] [Google Scholar]
- Bi R, Rong Y, Bernard A, Khrestchatisky M, Baudry M. Src-mediated tyrosine phosphorylation of NR2 subunits of N-methyl-D-aspartate receptors protects from calpain-mediated truncation of their C-terminal domains. J Biol Chem. 2000;275:26477–26483. doi: 10.1074/jbc.M003763200. [DOI] [PubMed] [Google Scholar]
- Bi X, Chang V, Molnar E, McIlhinney RA, Baudry M. The C-terminal domain of glutamate receptor subunit 1 is a target for calpain-mediated proteolysis. Neuroscience. 1996a;73:903–906. doi: 10.1016/0306-4522(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Bi X, Chang V, Siman R, Tocco G, Baudry M. Regional distribution and time-course of calpain activation following kainate-induced seizure activity in adult rat brain. Brain Res. 1996b;726:98–108. doi: 10.1016/0006-8993(95)01360-1. [DOI] [PubMed] [Google Scholar]
- Bi X, Chen J, Dang S, Wenthold RJ, Tocco G, Baudry M. Characterization of calpain-mediated proteolysis of GluR1 subunits of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors in rat brain. J Neurochem. 1997;68:1484–1494. doi: 10.1046/j.1471-4159.1997.68041484.x. [DOI] [PubMed] [Google Scholar]
- Bi X, Tocco G, Baudry M. Calpain-mediated regulation of AMPA receptors in adult rat brain. Neuroreport. 1994;6:61–64. doi: 10.1097/00001756-199412300-00017. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001;2:315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Chen L, El-Husseini A, Tomita S, Bredt DS, Nicoll RA. Stargazin differentially controls the trafficking of alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate and kainate receptors. Mol Pharmacol. 2003;64:703–706. doi: 10.1124/mol.64.3.703. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D. AMPA and NMDA glutamate receptor trafficking: multiple roads for reaching and leaving the synapse. Cell Tissue Res. 2006;326:423–438. doi: 10.1007/s00441-006-0254-9. [DOI] [PubMed] [Google Scholar]
- Hamakubo T, Kannagi R, Murachi T, Matus A. Distribution of calpains I and II in rat brain. J Neurosci. 1986;6:3103–3111. doi: 10.1523/JNEUROSCI.06-11-03103.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura M, Itakura M, Okamoto H, Hoka S, Mizoguchi A, Fukazawa Y, Shigemoto R, Yamamori S, Takahashi M. Differential localization and regulation of stargazin-like protein, gamma-8 and stargazin in the plasma membrane of hippocampal and cortical neurons. Neurosci Res. 2006;55:45–53. doi: 10.1016/j.neures.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre L, Valastro B, Miceli D, Massicotte G. AMPA receptor modulation in previously frozen brain sections: opposite effects of calcium in cortex and hippocampus. Hippocampus. 2000;10:645–653. doi: 10.1002/1098-1063(2000)10:6<645::AID-HIPO1002>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Li J, Grynspan F, Berman S, Nixon R, Bursztajn S. Regional differences in gene expression for calcium activated neutral proteases (calpains) and their endogenous inhibitor calpastatin in mouse brain and spinal cord. J Neurobiol. 1996;30:177–191. doi: 10.1002/(SICI)1097-4695(199606)30:2<177::AID-NEU1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Lu X, Rong Y, Baudry M. Calpain-mediated degradation of PSD-95 in developing and adult rat brain. Neurosci Lett. 2000a;286:149–153. doi: 10.1016/s0304-3940(00)01101-0. [DOI] [PubMed] [Google Scholar]
- Lu X, Rong Y, Bi R, Baudry M. Calpain-mediated truncation of rat brain AMPA receptors increases their Triton X-100 solubility. Brain Res. 2000b;863:143–150. doi: 10.1016/s0006-8993(00)02112-0. [DOI] [PubMed] [Google Scholar]
- Lu X, Wyszynski M, Sheng M, Baudry M. Proteolysis of glutamate receptor-interacting protein by calpain in rat brain: implications for synaptic plasticity. J Neurochem. 2001;77:1553–1560. doi: 10.1046/j.1471-4159.2001.00359.x. [DOI] [PubMed] [Google Scholar]
- Mi R, Sia GM, Rosen K, Tang X, Moghekar A, Black JL, McEnery M, Huganir RL, O'Brien RJ. AMPA receptor-dependent clustering of synaptic NMDA receptors is mediated by Stargazin and NR2A/B in spinal neurons and hippocampal interneurons. Neuron. 2004;44:335–349. doi: 10.1016/j.neuron.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Masu M. Molecular diversity and functions of glutamate receptors. Annu Rev Biophys Biomol Struct. 1994;23:319–348. doi: 10.1146/annurev.bb.23.060194.001535. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- Sager C, Tapken D, Kott S, Hollmann M. Functional modulation of AMPA receptors by transmembrane AMPA receptor regulatory proteins. Neurosci. 2009;158:45–54. doi: 10.1016/j.neuroscience.2007.12.046. [DOI] [PubMed] [Google Scholar]
- Simonson L, Baudry M, Siman R, Lynch G. Regional distribution of soluble calcium activated proteinase activity in neonatal and adult rat brain. Brain Res. 1985;327:153–159. doi: 10.1016/0006-8993(85)91509-4. [DOI] [PubMed] [Google Scholar]
- Tocco G, Massicotte G, Standley S, Thompson RF, Baudry M. Effect of temperature and calcium on the binding propertes of the AMPA receptor in frozen rat brain sections. Eur J Neurosci. 1992;4:1093–1103. doi: 10.1111/j.1460-9568.1992.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Vandenberghe W, Nicoll RA, Bredt DS. Stargazin is an AMPA receptor auxiliary subunit. Proc Natl Acad Sci U S A. 2005;102:485–490. doi: 10.1073/pnas.0408269102. [DOI] [PMC free article] [PubMed] [Google Scholar]