Abstract
Rationale
The birth of neurons, their migration to appropriate positions in the brain, and their establishment of the proper synaptic contacts happen predominately during the prenatal period. Environmental stressors during gestation can exert a major impact on brain development and thereby contribute to the pathogenesis of neuropsychiatric illnesses, such as depression and psychotic disorders including schizophrenia.
Objective
The objectives here are to present recent preclinical studies of the impact of prenatal exposure to gestational stressors on the developing fetal brain and discuss their relevance to the neurobiological basis of mental illness. The focus is on maternal immune activation, psychological stresses, and malnutrition, due to the abundant clinical literature supporting their role in the etiology of neuropsychiatric illnesses.
Results
Prenatal maternal immune activation, viral infection, unpredictable psychological stress, and malnutrition all appear to foster the development of behavioral abnormalities in exposed offspring that may be relevant to the symptom domains of schizophrenia and psychosis, including sensorimotor gating, information processing, cognition, social function, and subcortical hyperdopaminergia. Depression-related phenotypes, such as learned helplessness or anxiety, are also observed in some model systems. These changes appear to be mediated by the presence of proinflammatory cytokines and/or corticosteroids in the fetal compartment that alter the development the neuroanatomical substrates involved in these behaviors.
Conclusion
Prenatal exposure to environmental stressors alters the trajectory of brain development and can be used to generate animal preparations that may be informative in understanding the pathophysiological processes involved in several human neuropsychiatric disorders.
Keywords: Prenatal stress, Prenatal immune activation, Prenatal protein malnutrition, Prenatal viral infection, Schizophrenia, Psychosis, Depression, Anxiety, Brain
Introduction
Fetal development takes place in a privileged environment in the mother’s body, behind the placental barrier. The placenta is both a structural and a chemical barrier, which insulates the developing fetus from any adversity that might arise in the maternal environment. In situations where the maternal environment is experiencing extreme challenge, however, the placental barrier can become compromised, thereby exposing the developing fetal brain to maternally derived substances, such as cytokines or stress hormones (Mueller and Bale 2008; Seckl 2004; Welberg et al. 2000). Brain development is a delicately choreographed sequence of overlapping events in which timing is crucial, and any deviation from this pattern has the potential to lead to inappropriately timed neuronal birth, migration, and/or synaptogenesis, and ultimately, to miswiring and significant compromises in the function of the brain. Evidence for each of these putative developmental impairments has been identified upon inspection of postmortem human brain samples obtained from individuals afflicted with neuropsychiatric disorders, such as schizophrenia, depression, and bipolar disorder, which supports the neurodevelopmental origin of these common neuropsychiatric disorders (Jaaro-Peled et al. 2009; Lewis and Levitt 2002; Rapoport et al. 2005; Talge et al. 2007). In this review, we will summarize data from experimental animal studies that reveal the powerful effects that several prominent prenatal stressors have on fetal brain development, their behavioral and neural consequences, and the relevance of these findings to modeling psychotic and depressive illnesses in rodent preparations. Due to space limitations, an exhaustive review of this literature is not possible; but, we hope to provide readers with an overview of several important and relevant research fields.
Maternal immune activation during gestation
Maternal infection during pregnancy has been consistently associated with an increased risk of schizophrenia in the offspring (reviewed by Brown and Susser 2002). This association has been found for numerous infectious agents, including rubella, influenza, herpes simplex, toxoplasma gondii, measles, polio, and genital and/or reproductive infections (see Meyer and Feldon 2009). A mechanism common to the immune response associated with these and other infections is the stimulated release of inflammatory cytokines. In fact, elevation of maternal cytokine levels during the second trimester has been found for mothers whose children later developed schizophrenia (Brown et al. 2004). Immunological abnormalities, including, most notably, cytokine imbalances, have also been characterized for individuals with schizophrenia themselves (Muller et al. 2000).
It is less clear whether immune activation during pregnancy increases offspring's risk for developing mood disorders. Several studies have reported a link between maternal immune activation and immune development of offspring; for instance, an elevation of circulating cytokines occurs in adult women whose mothers experienced psychosocial stress during pregnancy (Entringer et al. 2008), and maternal depressive symptoms during the second trimester of pregnancy can impact neonatal immune function (Mattes et al. 2009). Because it is well established that elevated cytokines are associated with both psychosocial stress and depressive symptoms in adults (Christian et al. 2009), such epidemiological studies hint at a potential relationship between maternal immune function and the development of depression in offspring; but, to date, they have fallen short of establishing one. Recently, a large study in the UK that included more than 6,000 subjects failed to find any association between prenatal viral infection and nonpsychotic depression later in life (Pang et al. 2009).
Animal models of immune challenge use different immunogenic agents, but are all designed to induce a cytokine-associated inflammatory response in the mothers, which, in turn, results in increased levels of inflammatory cytokines in the fetal environment—sometimes including the fetal brain, though not always (Ashdown et al. 2006; Gayle et al. 2004; Liverman et al. 2006; Urakubo et al. 2001). Cytokines such as interleukin-1, interleukin-6, and tumor necrosis factor-alpha, in addition to their roles in the immune system, have been shown to impact numerous aspects of brain development including neuronal apoptosis, differentiation, and morphology (e.g., Gilmore et al. 2004; Jarskog et al. 1997; Yang et al. 2002). Maternal immune activation models of psychiatric illness have generally been conducted in rats and mice, with some differences in findings between the two species. Bacterial endotoxin [LPS (lipopolysaccharides)] as well as viral (human influenza) and viral mimic (poly I:C) agents have all been employed to stimulate the maternal immune system and will be discussed separately here. A major hypothesis common to these models is that elevated in utero exposure to cytokines adversely impacts fetal brain development, resulting in the development of neurobehavioral phenotypes characteristic of mental illness.
Although a wealth of promising data has been published in support of the prenatal immune activation hypothesis of psychiatric illness, several caveats must be kept in mind when interpreting the results generated with these models. In addition to inducing cytokine production, immune activation in rodents (as in humans) is associated with fever and anorexia-induced weight loss (Hopwood et al. 2009), leaving open the possibility that the in utero metabolic needs of offspring may be significantly (although transiently) compromised in these models. This is not a trivial point, as both prenatal malnutrition and prenatal hyperthermia adversely affect fetal brain development and have themselves been advanced as preclinical models of schizophrenia (Hopwood et al. 2009; Palmer et al. 2004, 2008) (see below). Additionally, immune activation during pregnancy can lead to reduced placental blood flow and impaired oxygen delivery to the fetal brain (Dalitz et al. 2003). Hypoxia is known to significantly and negatively impact fetal brain development, and while cytokine release can result from oxygen deprivation, injury to the brain is also caused through non-cytokine mediated mechanisms, including creation of reactive oxygen species (ROS) and excitotoxic cell death (Forder and Tymianski 2009; Rees et al. 2008). Finally, a crucial component of the immune response is activation of the hypothalamic-pituitary-adrenal (HPA) axis, which results in increased glucocorticoid release. As discussed below, glucocorticoids can also readily cross the placenta to directly impact neural development. At least one study has shown that maternal immune activation can result in fetal stress axis activation that is not accompanied by increases in cytokines within the fetal brain (Gayle et al. 2004). Although this study did not examine the behavioral profile of animals exposed to the particular dose/timing of immune activation it used (studies vary widely in this regard), it does suggest that studies that fail to confirm cytokine activation in fetal brain tissue following the specific dose/timing regimen used could be misattributing the neurobehavioral effects of immunestimulated fetal stress activation to direct effects of cytokines. With few exceptions to date, studies of immune challenge models of psychiatric illness have focused on the impact of inflammatory cytokines, without disentangling it from the impact of these other factors.
Lipopolysaccharides
LPS are large molecules consisting of a lipid joined to a polysaccharide by a covalent bond, which are found in the outer membrane of Gram-negative bacteria. LPS act as endotoxins (i.e., structural rather than soluble components of bacteria that are released primarily when the bacteria are lysed) to evoke a powerful immune response. LPS challenge causes inflammation due to secretion of inflammatory cytokines by immune cells, particularly macrophages, and results in the development of a fever as well as weight loss in the exposed animal. Systemic LPS administration stimulates both peripheral cytokine release (Wright et al. 1990) and central cytokine expression (Quan et al. 1999). The available evidence suggests that, when administered systemically to pregnant animals, LPS can be detected in both maternal tissues and the placenta, but not in the fetus itself (Ashdown et al. 2006), indicating that effects of maternal LPS exposure on the fetal brain are not the result of direct action of LPS, but by proxy factors such as elevated cytokines and/or glucocorticoids.
Rats born to mothers who were administered systemic LPS during gestation show a number of phenotypes relevant to schizophrenia as adults. Most, though not all, studies indicate that these animals show impaired sensory gating as measured by prepulse inhibition (PPI) of the acoustic startle reflex (Borrell et al. 2002; Fortier et al. 2004, 2007; Romero et al. 2007). They also show increases in serum cytokines (Borrell et al. 2002; Romero et al. 2007) and corticosterone (Romero et al. 2008). The PPI deficit is more pronounced and develops earlier in males than females (Borrell et al. 2002; Romero et al. 2008). This behavioral abnormality, along with the increase in serum cytokines, can be ameliorated by adult treatment with the typical (first generation) antipsychotic haloperidol (Borrell et al. 2002; Romero et al. 2007). Animals exposed to prenatal LPS also show increased psychostimulant-induced locomotion (Fortier et al. 2004). Behavioral hyperresponsivity to amphetamine, which increases brain dopamine levels, is relevant to psychosis and schizophrenia in part because antipsychotic drugs work by blocking dopamine D2 receptors. Adult males (but not females) exposed to prenatal LPS also have a higher dopamine concentration in the nucleus accumbens, a brain area integral to the brain's reward system (Borrell et al. 2002; Romero et al. 2008), which appears to be dysfunctional in schizophrenia (Gold et al. 2008). All of these studies employed systemic administration of LPS during the prenatal time period, but in a recent study, O’Donnell and colleagues examined the effects of LPS when delivered directly into the developing ventral hippocampus. They delivered LPS during the neonatal period, since, in rodents, this time period roughly corresponds to the third trimester of human pregnancy, and found that neonatal intrahippocampal LPS resulted in a persistent elevation of cytokines in several brain regions as well as impaired PPI, and prevented the peri-adolescent maturation of the response of prefrontal cortical fast-spiking interneurons to dopamine (Feleder et al. 2010). This study makes it clear that immune challenge during early life, even when delivered just to the hippocampus, can impact periadolescent development of the prefrontal cortex and result in schizophrenia-like behavioral and neural phenotypes during adulthood.
Animals whose mothers were exposed to LPS during gestation also exhibit some behaviors relevant to major affective disorders such as depression and anxiety. For instance, adult mice exposed to prenatal LPS show signs of heightened anxiety on standard rodent tests of anxiety such as the elevated plus maze (Hava et al. 2006). In the same study, LPS exposure was associated with reduced locomotor activity during social interaction, perhaps due to increased anxiety or fearfulness (Hava et al. 2006). Additionally, there is evidence that these animals show an enhanced acoustic startle response, which can be indicative of heightened anxiety (Fortier et al. 2004), although not all studies replicate this finding (Borrell et al. 2002).
In addition to behavioral abnormalities, the course of brain development is also altered as a consequence of prenatal exposure to LPS. For instance, there are age-dependent alterations in expression of synaptophysin (a synaptic vesicle membrane protein) and GSK-3 β (part of the Wnt signaling pathway) in the frontal cortex (Romero et al. 2008), a primary location of dysfunction in both schizophrenia and depression (Koenigs and Grafman 2009; Lewis and Sweet 2009). Similar to what is observed in schizophrenia (Broadbelt et al. 2002), animals exposed to prenatal LPS show disrupted dendritic development in the medial prefrontal cortex (Baharnoori et al. 2009). Both dendritic development and synaptic transmission in CA1 of hippocampus are also impacted by prenatal LPS exposure; functional alterations include heightened excitability of pyramidal neurons and impaired short-term (but not long-term) plasticity (Baharnoori et al. 2009; Lowe et al. 2008). Additionally, prenatal LPS exposure results in the reduced survival of newly born hippocampal cells during postnatal development (Cui et al. 2009). Interestingly, this last phenotype is not prevented by concurrent prenatal administration of ibuprofen (although it did prevent fever in the pregnant dam), suggesting that LPS-induced deficits in neurogenesis are not mediated by LPS-induced fever (Cui et al. 2009). These findings are important because pathology in the prefrontal cortex and hippocampus is considered critical for the clinical abnormalities observed in schizophrenia (Lewis and Sweet 2009).
Influenza virus
The impact of prenatal human influenza virus exposure on the neonatal brain has been studied extensively. In these studies, a sublethal dose of the virus was delivered via intranasal inoculation to pregnant mice (typically on day 9 of gestation, but sometimes later). Unlike other maternal immune activation paradigms, this one does not result in fever, though inoculated animals do exhibit common sickness behaviors including lethargy, sleepiness, ruffled fur, and lack of grooming (Sidwell et al. 1986). Although complete loss of pregnancy is uncommon in this paradigm, loss of some pups is inferred from the fact that the average litter size of influenza-infected dams is about 50% that of sham-infected dams (Shi et al. 2003).
Prenatal influenza infection results in a variety of behavioral phenotypes in mice that may be relevant to human psychiatric illness. Adult mice exposed to prenatal influenza infection show impaired sensory gating asmeasured by PPI (Shi et al. 2003). As in schizophrenia, the PPI deficit can be worsened by the N-methyl-D-aspartic acid (NMDA) receptor antagonist ketamine, and ameliorated with antipsychotic drug treatment (Shi et al. 2003; Swerdlow et al. 2006). Prenatal influenza exposure also affects social behavior in adult mice; exposed animals show a nearly threefold reduction in the number of contacts made with a novel conspecific (Shi et al. 2003). This effect may be due to reduced interest in social contact, as in schizophrenia, or a state of anxiety, as in affective disorders—indeed, adult mice exposed to prenatal influenza infection fail to display normal novelty-induced exploration in an open field and take almost twice as long as control mice to initiate investigation of a novel object; both measures likely reflect a state of heightened anxiety (Shi et al. 2003).
Prenatal influenza infection alters the development of the cerebral cortex and hippocampus, which are dysfunctional in both schizophrenia and depression. In both these areas, prenatal exposure to influenza causes a dramatic reduction in Reelin expression (Fatemi et al. 1999), similar to what is observed in postmortem tissue from schizophrenic patients (Guidotti et al. 2000). Early in brain development, during the embryonic stage, Reelin is first expressed by the transient GABAergic Cajal–Retzius neurons and is crucial for establishing neocortical and allocortical laminar structure (Ogawa et al. 1995). Beyond this stage and continuing into adulthood, Reelin appears to play a role in synaptic plasticity, as it is released by gamma-amino-butyric acid (GABA) containing interneurons and binds to integrin receptors located in postsynaptic densities on dendritic spines (Pesold et al. 1998). So, reduced Reelin may be involved in the reduction in synapses and dendrites that have been observed in schizophrenia, which are reflected in increased packing density of pyramidal neurons and reduced cortical thickness and hippocampal volume (Broadbelt et al. 2002; Glantz and Lewis 2000; Selemon et al. 1998; Steen et al. 2006). Interestingly, mice exposed to prenatal influenza also show increased pyramidal neuron density into adulthood and reduced cortical and hippocampal thickness, at least at birth (Fatemi et al. 1999, 2002a).
Numerous genes show altered expression—some upregulated, some downregulated—in the brains of animals exposed to prenatal influenza virus, especially when the infection occurs at a point in gestation that corresponds to mid-second trimester in humans; some show continued disruption into adulthood (Fatemi et al. 1998, 2000, 2005, 2008a, b, 2009). Expression of neuronal nitric oxide is greatly increased in the hippocampus, especially in the dentate gyrus, of neonatal mice prenatally exposed to influenza, perhaps indicative of enhanced vulnerability to excitotoxic cell death (Fatemi et al. 1998). In tissue homogenates from the rostral brain (which would presumably include the frontal cortex but not the hippocampus), prenatal influenza infection induces an increase in neuronal nitric oxide synthase only prior to adolescence, but a decrease is actually observed at adulthood (Fatemi et al. 2000). Also notably affected are some major components of myelin, including myelin basic protein (MBP), proteolipid protein (PLP), myelin-associated glycoprotein (MAG), and myelin oligodendrocyte protein (MOG; Fatemi et al. 2005, 2009). Not surprisingly, given these findings, abnormal measures of fractional anisotropy (a diffusion tensor imaging measure indicating degree of myelination) are also found in these animals, with the direction of change being fiber tract-specific and age-dependent (Fatemi et al. 2008a, 2009). Of significant interest is that reductions in brain MBP, PLP, MAG, and MOG have each also been reported to occur in schizophrenia, bipolar disorder, and major depression, in addition to reduced fractional anisotropy (reviewed by Sokolov 2007). Axonal loss and/or reduced myelination (both of which would be reflected by reduced fractional anisotropy) could significantly contribute to the cognitive deficits observed in each of these illnesses (Dwork et al. 2007; Fields 2008).
Examination of monoamine neurotransmitters has revealed some effects of prenatal influenza infection. One limitation of this literature is that, to date, neurotransmitter levels have only been evaluated in the cerebellum. Serotonin levels are reduced in the cerebellum at adolescence, although this effect does not persist to adulthood, whereas levels of serotonin's primary metabolite 5-hydroxyindoleacetic acid (5-HIAA) are reduced from birth until postnatal day 14 (Winter et al. 2008). In contrast, cerebellar levels of dopamine and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), as well as GABA, glutamate, and glutamine all appear indistinguishable from controls at multiple postnatal ages from birth through adulthood (Winter et al. 2008). Because the mechanism of action for most antidepressant medications is to increase the levels of serotonin in the brain, the neurochemical profile of animals prenatally exposed to the influenza virus suggests these animals may model aspects of depression. It remains to be seen whether these patterns hold for areas known to show alterations in these neurotransmitters in psychiatric illness, such as the cerebral cortex.
With the exception of the neurochemical findings, which appear more relevant to depression, the evidence discussed above appears encouraging for a link between prenatal influenza exposure and neurodevelopmental alterations that could be relevant to schizophrenia. There are some discrepancies, however, between what is found in animals following prenatal infection with the influenza virus and what is observed in schizophrenia. One major difference is that prenatal influenza exposure causes reactive gliosis, a common response of the brain to injury, which is evident at birth and continues at least until adolescence; increased density of cells expressing the astrocyte-specific marker glial fibrillary acidic protein (GFAP) is observed, as well as hypertrophy of individual GFAP immunoreactive cells (Fatemi et al. 2002b). These findings are problematic because there is no evidence of reactive gliosis in schizophrenia (Falkai et al. 1999). Additionally, expression of GAD65 and GAD67 is apparently increased in the brain as a consequence of prenatal influenza infection (Fatemi et al. 2004). In contrast, a widespread reduction in GAD67 is among one of the most replicable findings in schizophrenia (e.g., Akbarian et al. 1995; Guidotti et al. 2000; Hashimoto et al. 2008).
Finally, mice exposed to prenatal influenza fail to consistently show either the reduction in whole brain volume or the increase in ventricular volume that is observed in individuals with schizophrenia, even first-episode patients (Steen et al. 2006). Inoculation with the virus at murine embryonic day 9 (E9)—the paradigm that has generated the majority of the findings—actually results in macrocephaly and decreased ventricular volume at adulthood (Fatemi et al. 2002a). Inoculation with the virus later in embryonic development can result in increased juvenile brain volume (Fatemi et al. 2008b, 2009), but even after inoculation at E18, ventricular volume is not reduced (Fatemi et al. 2008b). In terms of brain volume changes following prenatal influenza exposure, the monkey may more closely model schizophrenia—a recent study reported that rhesus monkeys exposed to maternal influenza infection during pregnancy show significant loss of cortical gray matter, particularly in medial prefrontal areas, as well as reduced white matter volume and slightly (though not significantly) enlarged lateral ventricles (Short et al. 2010). Cognitive functioning was not measured in these animals, but monkeys exposed to prenatal influenza engaged in behaviors that may indicate a heightened state of anxiety—they spent less time in contact with their mothers, developed autonomy earlier than control monkeys, and showed an increased likelihood to vocalize. Importantly, this study confirmed the absence of virus-specific antibodies in the fetal compartment, precluding a primary response to the virus in the offspring. Additionally, maternal infection did not affect gestation length, birth weight, and major motor abilities. Basal and stressed cortisol levels were also normal, as was dexamethasone suppression of cortisol, at an age (1.5 years) that is roughly equivalent to late childhood in humans. These findings indicated that the stress axis, including glucocorticoid negative feedback, was not disrupted by prenatal influenza infection in monkeys and are therefore unlikely to account for the observed brain atrophy.
Polyriboinosinic–polyribocytidilic acid
Polyriboinosinic–polyribocytidilic acid (poly I:C) is a viral mimic agent that is structurally similar to double-stranded RNA. It interacts with toll-like receptor3, which is expressed by lymphocytes and other immune system cells, to induce a strong immune response. Similar to other immunogenic agents discussed in this review, prenatal administration of poly I:C to pregnant rodents can result in an elevation of cytokines in fetal brain tissue (Meyer et al. 2005, 2006b). One advantage of using poly I:C in a prenatal paradigm is that the immune response it generates is non-specific—that is, it stimulates production of inflammatory cytokines but not specific anti-viral antibodies. Another advantage is that its effects are limited to approximately 48 h, so administration can be designed to target a specific window of fetal brain development. The profile of maternal and fetal cytokines that are activated in response to prenatal poly I:C exposure depends on the gestational age at which it is delivered, as does the resulting phenotype of the offspring (Meyer et al. 2006b). Although it is beyond the scope of this review to treat exhaustively the differences in the neurobehavioral profile resulting from early versus late gestational administration of poly I:C, this topic has been discussed in detail elsewhere, and we refer the reader to those reviews (Meyer and Feldon 2009; Meyer et al. 2009). Prenatal poly I:C administration has been employed in both rats and mice where it results in significant maternal weight loss and sickness behavior; in mice, fully half the litters are aborted, and the remaining litters are approximately 50% the size of control litters (Ozawa et al. 2006). Many of the disrupted behavioral and neurochemical phenotypes observed in offspring born to mothers exposed to poly I:C during pregnancy (described below) are caused by prenatal factors and not exclusively by changes postnatal maternal care—adoption of pups exposed to poly I:C in utero by control mothers does not rescue the phenotype of these animals, although, interestingly, adoption of a control pup by an immune-challenged surrogate mother is sufficient to induce the phenotype (Meyer et al. 2006c, 2008c). Finally, aberrant phenotypes do not emerge when the anti-inflammatory cytokine IL-10 is co-administered with prenatal poly I:C, providing further support for the idea that neurobehavioral alteration in offspring of immune-challenged mothers is due to the stimulated release of pro-inflammatory cytokines rather than some other mechanism (Meyer et al. 2008b). Interestingly, other studies have revealed that poly I:C administration may increase maternal production of the pro-inflammatory cytokine, IL-6, which can cross the placental barrier and disrupt cortical development (Dahlgren et al. 2006; Gilmore et al. 2004). Since blockade of IL-6 mechanisms, either by administration of a monoclonal antibody or using IL-6 knockout mice, prevents poly I:C-induced behavioral changes (Samuelsson et al. 2006; Smith et al. 2007), IL-6 maybe a regulator of viral infection-mediated changes in brain development underlying some facets of the psychotic illness phenotype.
Poly I:C administration to pregnant mice and rats generates offspring that show impaired PPI and enhanced psychostimulant (amphetamine and MK-801)-induced hyperactivity; these phenotypes are evident only in adult animals; most studies find there is no difference between exposed and unexposed groups prior to puberty (Li et al. 2009; Meyer et al. 2005, 2008c, d, e; Ozawa et al. 2006; Shi et al. 2003; Vuillermot et al. 2010; Wolff and Bilkey 2008; Zuckerman et al. 2003). Latent inhibition (LI) appears to be completely abolished in adult poly I:C-treated rats and mice, but is intact prior to puberty (Meyer et al. 2005, 2006a, c, 2008e; Zuckerman et al. 2003; Zuckerman and Weiner 2005). Consistent with the interpretation of these behavioral abnormalities as reflective of a hyperdopaminergic state, prenatal poly I:C results in increased dopamine turnover and decreased binding of D2-like (but not D1-like) receptors in the striatum of adult, but not prepubertal mice (Ozawa et al. 2006). Additional evidence of enhanced dopamine metabolism following prenatal poly I:C exposure has been found by Meyer's group in the nucleus accumbens, striatum, and prefrontal cortex (Meyer et al. 2008d; Vuillermot et al. 2010; Winter et al. 2009), including a postpubertal onset of increased dopamine receptor expression in both dorsal and ventral striatal regions (Vuillermot et al. 2010). Perhaps as a compensatory measure, expression of D1 and D2 receptors is reduced in the medial prefrontal cortex following prenatal poly I:C treatment; interestingly, this effect is only evident in male offspring (Meyer et al. 2008c, d). It is clear that the altered neurochemical profile of adult animals exposed to prenatal poly I:C has its origins in fetal development—the increased number of midbrain dopaminergic neurons found in adult animals is already present by late gestation in these animals (Meyer et al. 2008a; Vuillermot et al. 2010). A causal relationship between a subcortical hyperdopaminergic state and impaired PPI in this paradigm is suggested by the finding that pre-testing administration of either a selective D1 or D2 receptor antagonist normalizes PPI of adult animals prenatally exposed to poly I:C (Vuillermot et al. 2010). Further support is lent by the finding that treatment with antipsychotic drugs during the periadolescent time period prevents the development of abnormal LI, PPI, and psychostimulant-induced hyperlocomotion phenotypes in animals prenatally exposed to poly I:C (Meyer et al. 2008e).
The behavioral deficits in PPI and LI, combined with the evidence of subcortical hyperdopaminergia, strongly suggest that prenatal poly I:C exposure provides a good model for psychosis. One caveat regarding the interpretation of these studies comes from a recent study, which found that only offspring of mothers who lost weight as a consequence of poly I:C treatment showed hyperlocomotion in response to amphetamine and MK-801; offspring of mothers who did not experience weight loss did not show enhanced sensitivity to psychostimulants (Bronson et al. 2009). This could indicate that cytokine production was stimulated in the former group of animals and not in the latter; but, since all pregnant mothers were administered the same dose of poly I:C, it could instead suggest that malnutrition rather than cytokine production is the mechanism underlying these phenotypes. Further research is required to distinguish between these possibilities.
Cognitive deficits have also been reported following prenatal exposure to poly I:C in rodents. Impaired novel object recognition is evident in adult (but not prepubertal) exposed mice; this deficit can be mitigated by treatment with clozapine but not haloperidol (Ozawa et al. 2006). Spatial working memory and learning of a two-way active avoidance paradigm are also impaired, whereas spatial discrimination reversal learning in an operant task is enhanced, in mice exposed to prenatal poly I:C (Bitanihirwe et al. 2010; Meyer et al. 2005, 2008d; Vuillermot et al. 2010). Spatial reversal learning is not universally enhanced by prenatal exposure to poly I:C in mice, however; in a subsequent study, this group found impaired reversal learning (i.e., enhanced perseverative behavior) using left–right discrimination in a water version of the T maze (Meyer et al. 2006b). The former study administered poly I:C early in gestation, whereas the latter administered it late, which could account for the difference; in both cases, initial learning of the task was not disrupted. Interestingly, a recent study reported that expression of the cytoplasmic serine–threonine protein kinase AKT1, polymorphisms in which have been associated with schizophrenia (e.g., Karege et al. 2010), is both reduced in the prefrontal cortex of mice prenatally exposed to poly I: C and correlated with their cognitive impairments on a spatial working memory task (Bitanihirwe et al. 2010). In rats, prenatal exposure to poly I:C disrupts some aspects of cognition including rapid reversal learning, which like the deficit in LI, may be alleviated by treatment with the antipsychotic clozapine (Zuckerman and Weiner 2005). However, prenatal poly I:C does not result in a generalized learning deficit in rats, as this same study found no effect of exposure on learning in classical fear conditioning, active avoidance, discrimination learning, and spatial cognition (Morris water maze) paradigms (Zuckerman and Weiner 2005). Combined, the deficits in LI and reversal learning suggest that the cognitive profile resulting from prenatal poly I:C exposure in rats is one of excessive cognitive switching.
There has been considerably less exploration of the link between prenatal poly I:C exposure and behavioral phenotypes relevant to major affective disorders. Although not all studies report this effect (Ozawa et al. 2006), mice exposed to poly I:C in utero have been shown to spend less time in the center of an open field box compared with unexposed mice; in the absence of any difference in overall locomotor activity, this suggests increased anxiety (Meyer et al. 2005, 2006b, 2008e). Animals exposed to poly I:C during gestation have not been evaluated using classic rodent tests of depressive-like behavior (i.e., the forced swim test and sucrose preference test); however, it is interesting to note that treatment with the antidepressant fluoxetine during the periadolescent time period is capable of preventing the development of PPI deficits and psychostimulant-induced hyperlocomotion in these animals (Meyer et al. 2008e).
Neuroanatomical and neurochemical development are both also significantly altered by prenatal exposure to poly I:C. Similar to what is observed in schizophrenia, the lateral ventricles are enlarged in adult animals prenatally exposed to poly I:C, although unlike in schizophrenia, there do not appear to be changes in overall white or gray matter volumes (Li et al. 2009). Alterations within the white matter have, however, been reported; there is evidence for reduced MBP expression and reduced myelination in the hippocampus of juvenile mice exposed to poly I:C in utero, although somewhat paradoxically, these abnormalities appear to resolve by adulthood (Makinodan et al. 2008). Consistent with a schizophrenia-like phenotype, there is no evidence for reactive gliosis resulting from prenatal poly I: C treatment; at least, in the hippocampus, the number of astrocytes is unchanged following prenatal poly I:C exposure (Meyer et al. 2006b). In rats, there is evidence of increased pyknotic cells in the medial temporal lobe, particularly CA1 (Zuckerman et al. 2003); but, in mice, this was not observed. The total number of glial and pyknotic cells in the hippocampal subfields and dentate gyrus, as well as hippocampal volume, have all been found to be normal in adult mice exposed to poly I:C in utero (Nyffeler et al. 2006). Prenatal administration of poly I:C, like prenatal LPS administration, does result in a reduction in the postnatal survival of newly born neurons in the dentate gyrus of the hippocampus, which appears to be due to increased apoptosis (Meyer et al. 2006b). Interestingly, adult treatment with the antipsychotic clozapine rescues the deficit in hippocampal-dependent spatial working memory without bringing hippocampal neurogenesis to normal levels (Meyer et al. 2010), indicating that the behavioral deficit may be due to other pathological features of the hippocampus in poly I:C-exposed animals. Similar to what is observed following prenatal influenza exposure, prenatal exposure to poly I:C leads to a significant reduction in Reelin-expressing cells in both the hippocampus and prefrontal cortex (Meyer et al. 2008d); interestingly, this abnormality is observed in pre-pubertal animals and thus emerges prior to behavioral deficits (Meyer et al. 2006b). Additionally, although overall levels of both glutamate and GABA are normal in multiple brain regions following prenatal exposure to poly I:C (Winter et al. 2009), parvalbumin-expressing inhibitory interneurons are significantly reduced in both the hippocampus and prefrontal cortex (Meyer et al. 2008d); this latter finding has been repeatedly observed in schizophrenia (e.g., Akbarian et al. 1995; Guidotti et al. 2000; Hashimoto et al. 2008). Perhaps in order to compensate for the loss of inhibitory input provided by these cells, there is increased expression of the alpha2 subunit of GABAA receptors in the hippocampus and basolateral amygdala of adult offspring prenatally exposed to poly I:C compared with control mice (Nyffeler et al. 2006). Finally, expression of the NR1 subunit of the NMDA-type glutamate receptor is reduced in the hippocampus of animals prenatally exposed to poly I:C, although expression is normal in the prefrontal cortex of these animals (Meyer et al. 2008d), a region that does experience loss of NR1 expression in schizophrenia (Beneyto and Meador-Woodruff 2008). Collectively, these findings indicate that maternal immune activation in the absence of a specific viral pathogen is capable of altering numerous neuronal phenotypes across the postnatal developmental timeframe and continuing into adulthood, providing further support for the cytokine hypothesis of mental illness.
Maternal psychological stress exposure
Recent epidemiological studies have revealed contributions of the prenatal environment to later life psychopathology, including psychotic disorders, depression, and anxiety (Bresnahan et al. 2005; Brown 2002; Field and Diego 2008; Rice et al. 2007, 2010). With regard to schizophrenia, these investigations related maternal psychological distress, including bereavement, unwantedness of a pregnancy, natural disaster, or war to an increased risk that vulnerable offspring will develop schizophrenia later in life (Brown 2002; Spauwen et al. 2004; Sullivan 2005). These studies, like those looking at prenatal viral infections, indicate a period of vulnerability in the first and second trimesters of pregnancy. Unfortunately, the literature to support a strong link between prenatal stress and depression or anxiety in humans is not as well developed as in the schizophrenia field. Nonetheless, several studies do suggest that exposure to gestational stress can indeed increase the risk of later life depression and anxiety-related disorders (Brown et al. 2000; Torrey et al. 1996; Watson et al. 1999). Several outstanding recent reviews have been written about many of the effects of prenatal stress on activity of the HPA axis and behavioral phenotypes associated with this early-life manipulation, and readers are directed to these reviews for a more historical perspective on this field (Koenig 2006; Owen et al. 2005; Weinstock 2005, 2008). In this section, emphasis will be given to recent preclinical work that has been done using prenatal stress to elicit phenotypes related to psychotic and depressive illnesses.
In contrast to the limited support for the role of prenatal stress in the etiology of depression and anxiety in humans, there is a robust preclinical literature that reveals the importance of the prenatal period in the development of anxiety- and depression-related behaviors in experimental animal preparations. Numerous studies show that prenatal stress reprograms the HPA axis and induces either increased basal secretion or enhanced stress-related secretion of glucocorticoid hormones (for review, see Owen et al. 2005; Weinstock 2005, 2008), and this reprogramming of the HPA axis appears to be due, in part, to a reduction in the activity of the protective 11-beta-hydroxysteroid dehydrogenase resident in the placenta, which protects the fetus from maternal glucocorticoids (Welberg et al. 2000, 2005). This enhancement of HPA axis activity appears to involve inefficient glucocorticoid negative feedback due to the diminished expression of receptors for the glucocorticoids in the hippocampus. However, augmentation of corticotrophin-releasing hormone (CRH) expression may also be involved, as increased expression of CRH in the amygdala, which is one of the primary initiators of anxiogenic behaviors in the brain, has also been reported (Brunton and Russell 2010; Cratty et al. 1995; Mueller and Bale 2008). Thus, many preclinical studies of prenatal stress focus on anxiogenic phenotypes. The most commonly used behavioral endpoint for anxiety in animal studies is reduced time spent in the open arms of the elevated plus maze, which has predictive validity for anxiolytic compounds. The ability of prenatal stress to decrease time spent in the open arms of the elevated plus maze was among the first behavioral deficits to be associated with prenatal stress exposure (Fride and Weinstock 1988; Poltyrev et al. 1996). In this assay, the majority of studies find a diminution in time spent in the open arms of the elevated plus maze, especially in male rats (Brunton and Russell 2010; Chung et al. 2005; Szymanska et al. 2009; Walf and Frye 2007; Weinstock 2008; Zuena et al. 2008) as well as mice (Oliver and Davies 2009), but other studies have failed to find a similar enhancement of anxiety-related behavior (Bogoch et al. 2007; Estanislau and Morato 2005; Gotz and Stefanski 2007; Richardson et al. 2006; Zagron and Weinstock 2006). In female rats, the stage of the estrus cycle may influence behavioral responses to anxiogenic stimuli, and prenatally stressed female rats in behavioral estrus actually spend more time in the open arms of the EPM suggesting lower levels of anxiety (Walf and Frye 2007; Zuena et al. 2008), although others have reported that female rats develop anxious behaviors in response to prenatal stressors like males (Brunton and Russell 2010). In other tests of anxiogenic behaviors such as the light–dark emergence test or the defensive burying test, minimal anxiogenic behaviors were observed in male rats (Lee et al. 2007; Richardson et al. 2006), but stronger effects were observed in females (Richardson et al. 2006), which may be related to decreased hippocampal neurogenesis (Mandyam et al. 2008). Interestingly, one of the most widely used depression-related endpoints in rodents is the forced swim test, and this test has only been used in a limited number of studies of prenatal stress. Prenatal stress exposure interestingly appears to have little effect on immobility in the forced swim test of male rats and mice in most cases (Alonso et al. 1991; Oliver and Davies 2009; Van den Hove et al. 2005, 2006), while increased immobility time has been reported in females (Alonso et al. 1991). In several recent reports, Szymanska and colleagues found an increase in immobility time in male rats, while Mueller and Bale reported enhanced immobility in both the forced swim test and the tail suspension test in prenatally stressed male mice that did not occur in female mice (Mueller and Bale 2008; Szymanska et al. 2009). Obviously additional studies are needed to clarify the effect of prenatal stress on depressive behaviors, in general and across genders more specifically. One potential explanation for the array of anxiety- and depression-related findings following prenatal stress, aside from the usual strain and paradigm differences, may not only be within-group individual animal variability of anxious phenotypes, as suggested in the recent findings (Bosch et al. 2006; Clinton et al. 2008), but also changes in epigenetic mechanisms that control expression of genes associated with HPA axis activity and the serotonergic system in the brain (Mueller and Bale 2008).
Cognitive impairments are important features of both mood disorders and psychotic illnesses and there has been much interest in exploring these phenotypes in animals following exposure to gestational stress. Lemaire and colleagues reported in 2000 that rats exposed to a prenatal restraint stress regimen during the final week of pregnancy, showed reduced spatial memory in the Morris water maze as adults (Lemaire et al. 2000). Subsequent studies have confirmed the impaired spatial memory in the Morris water maze although the effects appear to be modest (Meunier et al. 2004; Son et al. 2006; Yaka al. 2007; Yang et al. 2006). Spatial memory has also been examined using the radial arm maze but prenatal restraint stress did not appear to have significant effects in this test or in the object recognition task (Bowman et al. 2004), although studies performed in our laboratory indicate that spatial working memory deficits as well as a compromised ability to perform the novel object recognition task can be induced by exposure of pregnant rats to a variable stress procedure (Markham et al. 2010). Furthermore, Mueller and Bale (2007) examined learning and memory using the Barnes maze in mice following a variable prenatal stress exposure (Mueller and Bale 2007). Male mice exposed to the prenatal manipulation early in gestation but not later, had reduced performance on this task while female mice showed improved memory. Prenatal stress also appears to disrupt performance in delayed and spontaneous alternation tasks (Gue et al. 2004; Meunier et al. 2004). However, the deficits reported were typically assessed in rats prior to puberty when the animals are more active, and this may be an important facet of the behavioral testing procedure to consider in planning future cognition-related experiments. Many of the memory tasks used interrogate hippocampal-dependent memory and electrophysiological studies have reported that prenatal stress induces a diminution in hippocampal long-term potentiation (LTP) and an enhancement of long-term depression (Son et al. 2006; Yaka et al. 2007; Yang et al. 2006). LTP is dependent on NMDA receptor activity, and expression and activation of NMDA receptors are altered by prenatal stress (Fumagalli et al. 2009; Jia et al. 2010; Kinnunen et al. 2003; Son et al. 2006; Yaka et al. 2007). Hypofunctionality of the glutamate system appears to be an underlying feature of schizophrenia (Javitt 2007), and the diminished expression and activation of NMDA receptors may be a reflection of a similar molecular pathology in prenatally stress rat preparations. Additionally, NMDA receptors are known to reside on dendritic spines of hippocampal pyramidal neurons, and several studies have reported that prenatal exposure to stress decreases dendritic spine density in the hippocampus and cortex of male and female rat pups (Fujioka et al. 2006; Jia et al. 2010; Murmu et al. 2006), although similar effects have not been found in the prefrontal cortex (Michelsen et al. 2007). Compromised cognitive function could also be related to the replenishment of new neurons in the hippocampus, and, indeed, reduced hippocampal neurogenesis following prenatal stress was originally reported by Lemaire and colleagues in 2000, and this finding has been confirmed by a number of other investigators (Van den Hove et al. 2005, 2006; Yaka et al. 2007; Yang et al. 2006; Zuena et al. 2008). One factor that can contribute to the birth and survival of neurons is brain-derived neurotrophic factor (BDNF). In the hippocampus taken from prenatally stressed rats, levels of BDNF have been reported to be decreased (Neeley et al. 2010; Van den Hove et al. 2005, 2006) or increased (Zuena et al. 2008). Together, these studies suggest that hippocampal-dependent memory is compromised by exposure to stress during gestation and that change in either hippocampal cell morphology or number may contribute to the deficits that have been reported. An extensive interrogation of prefrontal cortex-dependent memory function following prenatal stress exposure has not been undertaken, but data from our laboratory suggest that prefrontal cortex function may also be compromised by prenatal stress exposure (Markham et al. 2010). Given the importance of changes in executive functioning in neuropsychiatric disorder, additional studies to address the function of additional higher-order brain regions following prenatal stress are needed.
In contrast to the abundant preclinical literature on the effects of prenatal stress on anxiety- and depression-related behavioral assays, there is only a limited number of studies attempting to address endpoints related to psychotic features of schizophrenia. This may be due to the difficulty in establishing animal models for psychotic disorders. However, it is clear that psychosis involves over-activation of the subcortical dopamine system (Abi-Dargham et al. 2000, 2009; Laruelle et al. 1999). Studies have demonstrated that prenatal stress exposure in rats does, indeed, lead to enhancement of the subcortical dopamine system that can be observed by directly measuring dopamine turnover or output (Alonso et al. 1994; Fride and Weinstock 1988; Silvagni et al. 2008). The increase in dopaminergic activity may be the consequence of complex changes in the ventral tegmental area dopamine-producing cells following prenatal stress (Katunar et al. 2010). Behavioral assays of locomotor activity under either baseline conditions or following psychostimulant treatment can be used to infer subcortical dopamine activity, and there appears to be good agreement about enhanced responsiveness of the subcortical dopamine system involved in regulating locomotor activity (Henry et al. 1995; Koenig et al. 2005). However, this subcortical enhancement of dopamine release is not generalizable to the prefrontal cortex, where prenatal stress induces a diminished dopamine response to amphetamine challenge suggestive of a hypodopaminergic state in the cortex (Carboni et al. 2010). This pattern of dopamine activity (increased subcortical and decreased prefrontal cortex) following prenatal stress is consistent with current theories of the dopamine system in schizophrenia. Another behavioral endpoint associated closely with schizophrenia is PPI. PPI deficits are commonly seen in patients with schizophrenia and reflect fundamental changes in the information processing system of the brain (Green et al. 2009). Experimental animals exposed to a variable stress paradigm during the last week of pregnancy show prominent deficits in PPI (Koenig et al. 2005) without changes in acoustic startle responses (Hougaard et al. 2005; Koenig et al. 2005). Interestingly, employing prenatal stress paradigms where the pregnant rats can habituate to the stress, i.e., repeated exposures to the same stress, failed to produce deficits in PPI (Burton et al. 2006; Lehmann et al. 2000) suggesting that not all prenatal stress paradigms produce the same behavioral phenotypes—a situation that is probably further complicated by the use of different strains and species of experimental animals. In addition to sensorimotor gating deficits revealed by PPI testing, electrophysiological techniques designed to mimic P50 event-related potential testing in humans (Potter et al. 2006) have shown that hippocampal gating responses to auditory stimuli are compromised in animals following prenatal stress exposure (Koenig et al. 2005). Interestingly, the importance of gene by environment interactions has been recognized in schizophrenia research as underlying a sizable portion of the risk for developing the illness (European Network of Schizophrenia Networks for the Study of Gene-Environment Interactions 2008; van Os et al. 2008). Oliver and Davies (2009) provide the first experimental evidence for a gene by environment interaction related to PPI. Exposure of SNAP-25 knockout mice to a variable prenatal stress procedure leads to PPI deficits that are not observed with either manipulation alone. This seminal observation confirms the importance of neurodevelopmental interactions between genetic factors and environmental stimuli in generating phenotypes related to schizophrenia.
The negative symptom domain of the schizophrenia, including the social withdrawal manifested by many patients, is not treated well by antipsychotic medications. Rodents are highly social and recent work has devised a procedure to study social interactions in experimental animals under non-anxiogenic conditions (Lee et al. 2005; Moy et al. 2004). Under these conditions, prenatally stressed rats exhibit a compromised interest in engaging in normal social activities (Lee et al. 2007), which is related to a deficit in the brain oxytocin system. Moreover, Oliver and Davies (2009) detected a social deficit in the SNAP-25 knockout mice only after the animals were exposed to the prenatal stress procedure, further indicating that the prenatal stress procedure can unmask genetic vulnerability for psychiatric illness-related phenotypes. Further studies will be required to determine whether these changes in social behaviors are more relevant to anxiety-related or schizophrenia-related outcomes, but they suggest that prenatal psychological stress paradigms have a broad use in modeling aspects of both types of neuropsychiatric disorders.
Prenatal malnutrition studies
Prospective studies about the importance of adequate prenatal nutrition on psychosis and depression have not been conducted, and the studies that have been done relied on retrospective experimental study designs. These studies have used information generated following several major famines that occurred in The Netherlands during 1944–1945 and in China during the late 1950s. In each of these instances, there has been a reported approximate twofold increase in the risk of developing schizophrenia or depressive disorders in the affected offspring of women who were pregnant during these famines (Brown and Susser 2008; Brown et al. 1995, 2000; St Clair et al. 2005; Stein et al. 2009; Xu et al. 2009). Although the data are not abundant, they point to the importance of prenatal nutrition in normal fetal brain development. However, it is not clear from human studies if specific components of the diet can be tied to the adverse outcomes of the offspring, but animal studies have identified several specific dietary components that contribute to the development of normal behaviors, including protein, choline, and vitamin D. Only a small number of studies have been performed in this important area, however, which makes interpreting the findings challenging. An additional caveat, of course, is that reduced maternal caloric intake during gestation not only results in potential fetal nutritional deficiencies, but also stimulates maternal glucocorticoid release and reprograms the HPA axis of the offspring (Langley-Evans et al. 1996; Lesage et al. 2001; Nunez et al. 2008). Thus, alterations in brain biochemistry and behavior could be related to either malnutrition, exposure of the developing brain to glucocorticoids, or the combination of these mechanisms. Studies have not been done to disentangle these possibilities.
Choline is an important contributor to phospholipids and other cellular membrane components. A gestational deficiency in choline reportedly disrupts development of the hippocampus and other brain regions in rats and may be related to an overproduction of GABAergic interneurons (Glenn et al. 2007; Jones et al. 1999; Stevens et al. 2008). This disruption in normal development may contribute to the diminished sensorimotor gating and impaired cognition that follows prenatal choline deficiency (Glenn et al. 2008; Stevens et al. 2008; Wong-Goodrich et al. 2008). Interestingly, however, there are no changes in hippocampal alpha-7 nicotinic receptor binding following maternal dietary choline restriction (McCann et al. 2006; Stevens et al. 2008; Wong-Goodrich et al. 2008). However, the more recent study demonstrates the complexity of the interaction between a gestational deficit in choline and the availability of choline in the adult diet, suggesting that prenatal choline availability creates susceptibilities that can be manifested in later life and may play out differently depending on the adult dietary composition. While there is a clear link between prenatal choline and adult cognitive function, the relationship between developmental choline deficiencies and other aspects of depression or psychotic illnesses have not been explored.
Maternal malnutrition is commonly induced by either restricting protein in the diet or by limiting the daily intake of calories (for example, Almeida et al. 1996a, b; Mokler et al. 2007; Palmer et al. 2004). The majority of studies are designed to investigate the effect of limited maternal protein ingestion. In this case, juvenile offspring appear to show a decrease in anxiogenic behaviors in the elevated plus maze and also manifest a decrease in social interactions, as juveniles (Almeida et al. 1996a, b). In the one study that has looked at elevated plus maze behavior after prenatal calorie restriction, no change in open arm time was reported, but time in the center of an open field was increased, suggesting a diminution in anxiety in response to prenatal calorie restriction (Levay et al. 2008). In addition, prenatal protein restriction generated sex-dependent deficits in prepulse inhibition and locomotor activity that were present predominantly in female rats (Palmer et al. 2004, 2008). These changes in behavior appear to be related to alterations in the dopamine system, where prenatal protein restriction has been reported to disrupt normal dopaminergic tone, although the mixed directionality of the findings precludes a clear interpretation of them (Palmer et al. 2008; Vucetic et al. 2010). Cognitive function is disrupted by prenatal calorie and protein restriction in mice with the major effect being an increase in the number of incorrect working memory errors in the radial arm maze task. Interestingly, prenatally malnourished mice consistently take longer to perform this task than the normal control animals (Ranade et al. 2008). In the limited studies that have been performed, it would appear that prenatal malnutrition can disrupt behaviors associated with psychotic illnesses, but the effects appear to be more prominent in female than in males which do not match our current understanding of the gender bias in schizophrenia.
Vitamin D has recently become popularized in the media as an essential factor for energy and vitality. Recent studies have begun to explore the importance of this vitamin in prenatal brain development. Depletion of vitamin D from the maternal diet during gestation leads to marked potentiation of the locomotor enhancing effects of MK-801 (Kesby et al. 2006; O'Loan et al. 2007) and enhanced activity in the elevated plus maze but without an increase in anxiety or depression-related behaviors (Burne et al. 2004; Harms et al. 2008). In addition, prenatal vitamin D deficiency (VDD) is apparently not associated with alterations in HPA axis function (Eyles et al. 2006). Prenatal VDD rats also show a deficit in latent inhibition without a change in prepulse inhibition (Becker et al. 2005). While prenatal VDD exhibit enlarged lateral ventricles they also had enhanced hippocampal LTP without impairments in spatial learning and memory (Becker et al. 2005; Harms et al. 2008). Clearly, additional studies are needed using this interesting model to examine other behavioral features that may be related to depression or schizophrenia, but it is clear that disrupting prenatal vitamin D intake can have significant effects on the developing fetal brain with adverse consequences later in life.
Conclusions
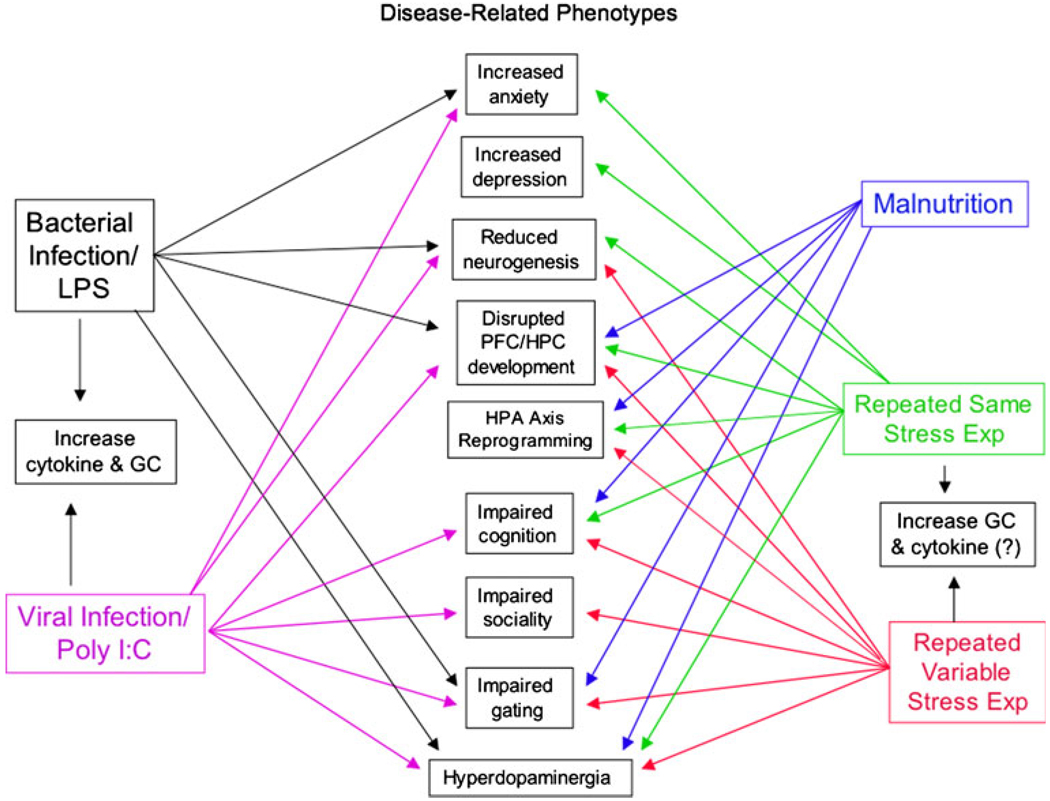
The prenatal period is a time when the developing brain is highly susceptible to the adverse effects of stressors of diverse modalities, including stressors related to immune activation, psychological adversity, or malnutrition, as shown in Fig. 1. In each of these cases, the experimental evidence supports the notion that pathological changes in adult behaviors related to psychosis, cognition, and schizophrenia may arise as a result of stressful perturbations during the prenatal period. These observations complement the epidemiological literature on schizophrenia and psychosis nicely and indicate that animal preparations may be useful in the investigation of some of the fundamental origins of these illnesses, particularly in regard to the role of environmental factors in the etiopathophysiology of the illness. In contrast to psychotic disorders, there is only cursory clinical evidence pointing to a prenatal origin for anxiety or depressive illnesses, despite the preclinical animal findings that support the hypothesis that at least some features of anxiety and depressive disorders might arise following exposure to prenatal psychological insults. Schizophrenia and major depression are characterized by multiple domains of psychopathology, some of which are shared across illnesses. For example, impaired cognition and hippocampal dysfunction are prominent features of both diseases, but it is not clear if the pathology and epidemiological origins of these features are shared or distinct. This pattern of overlapping clinical phenotypes presents a challenge for modeling psychotic illnesses and major depression in animals. Figure 1 highlights some of the phenotypic similarities and differences in the prenatal developmental animal models of psychiatric illnesses. It is probable that differences in animal species and strains, as well as the timing of exposure, create some of the differences shown in this figure. A prominent question requiring further investigation is the identification of the causal agent(s) leading to each of the phenotypic domains. Some findings support a role of pro-inflammatory cytokines in the immune/infection-based models, but there are also abundant data to show that immune agents activate glucocorticoid release, at least in adult animals. On the other hand, in the prenatal models of psychiatric illness not involving maternal immune activation, evidence strongly supports a role for maternal glucocorticoids, but there are scant data to support involvement of cytokines in mediating offspring phenotypes. Thus, it is clear that the occurrence of maternal stress during fetal development creates changes in the brain and ultimately, behavior of the offspring. These changes reflect many features of psychiatric illnesses, especially those with a clear environmental component in their etiology. Understanding the pathologies that could be related to developmental alterations offers a mechanism to develop novel intervention strategies that might aid patients afflicted with psychotic and mood disorders.
Fig. 1.
This figure shows a general summary of the major disease-related phenotypes that can be induced by prenatal exposure to immune-related stresses, psychological stresses, or nutritional stress in rodent preparations. The diagram also indicates that, in the immune models, both cytokines and glucocorticoid hormones should be considered as the major factors that impact the developing fetal nervous system to elicit phenotypic changes in the offspring, while in the case of prenatal psychological stress models, the evidence more strongly supports an action of the glucocorticoids on the developing brain as involvement of cytokine factors is less clear in these models. In the case of nutritional models, it has yet to be determined whether either or both of these mechanisms are involved in the genesis of the developmental changes that occur following dietary manipulations
Acknowledgements
This work is supported by grants MH073826 (JIK), MH082999 (JIK), and K12HD043489 (JAM).
Footnotes
Conflict of interest The authors have no conflicts of interest to declare.
Contributor Information
Julie A. Markham, Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland School of Medicine, PO Box 21247, Baltimore, MD 21228, USA
James I. Koenig, Email: jkoenig@mprc.umaryland.edu, Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland School of Medicine, PO Box 21247, Baltimore, MD 21228, USA.
References
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol Psychiatry. 2009;65:1091–1093. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Almeida SS, Tonkiss J, Galler JR. Prenatal protein malnutrition affects exploratory behavior of female rats in the elevated plus-maze test. Physiol Behav. 1996a;60:675–680. doi: 10.1016/s0031-9384(96)80047-3. [DOI] [PubMed] [Google Scholar]
- Almeida SS, Tonkiss J, Galler JR. Prenatal protein malnutrition affects the social interactions of juvenile rats. Physiol Behav. 1996b;60:197–201. doi: 10.1016/0031-9384(95)02236-8. [DOI] [PubMed] [Google Scholar]
- Alonso SJ, Arevalo R, Afonso D, Rodriquez M. Effects of maternal stress during pregnancy on forced swimming test behavior of the offspring. Physiol Behav. 1991;50:511–517. doi: 10.1016/0031-9384(91)90538-y. [DOI] [PubMed] [Google Scholar]
- Alonso SJ, Navarro E, Rodriquez M. Permanent dopaminergic alterations in the n. accumbens after prenatal stress. Pharmacol Biochem Behav. 1994;49:353–358. doi: 10.1016/0091-3057(94)90433-2. [DOI] [PubMed] [Google Scholar]
- Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11:47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- Baharnoori M, Brake WG, Srivastava LK. Prenatal immune challenge induces developmental changes in the morphology of pyramidal neurons of the prefrontal cortex and hippocampus in rats. Schizophr Res. 2009;107:99–109. doi: 10.1016/j.schres.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Becker A, Eyles DW, McGrath JJ, Grecksch G. Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behav Brain Res. 2005;161:306–312. doi: 10.1016/j.bbr.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology. 2008;33:2175–2178. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Weber L, Feldon J, Meyer U. Cognitive impairment following prenatal immune challenge in mice correlates with prefrontal cortical AKT1 deficiency. Int J Neuropsychopharmacol. 2010;13:981–996. doi: 10.1017/S1461145710000192. [DOI] [PubMed] [Google Scholar]
- Bogoch Y, Biala YN, Linial M, Weinstock M. Anxiety induced by prenatal stress is associated with suppression of hippocampal genes involved in synaptic function. J Neurochem. 2007;101:1018–1030. doi: 10.1111/j.1471-4159.2006.04402.x. [DOI] [PubMed] [Google Scholar]
- Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–215. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Kromer SA, Neumann ID. Prenatal stress: opposite effects on anxiety and hypothalamic expression of vasopressin and corticotropin-releasing hormone in rats selectively bred for high and low anxiety. Eur J Neurosci. 2006;23:541–551. doi: 10.1111/j.1460-9568.2005.04576.x. [DOI] [PubMed] [Google Scholar]
- Bowman RE, MacLusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 2004;145:3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- Bresnahan M, Schaefer CA, Brown AS, Susser ES. Prenatal determinants of schizophrenia: what we have learned thus far? Epidemiol Psichiatr Soc. 2005;14:194–197. doi: 10.1017/s1121189x00007946. [DOI] [PubMed] [Google Scholar]
- Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res. 2002;58:75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- Bronson SL, Ahlbrand R, Horn PS, Kern JR, Roenker NL, Richtand NM. Environmental influence on maternal response in a prenatal immune activation model of schizophrenia. Biol Psychiatry. 2009:45S. [Google Scholar]
- Brown AS. Prenatal risk factors and schizophrenia. Expert Rev Neurother. 2002;2:53–60. doi: 10.1586/14737175.2.1.53. [DOI] [PubMed] [Google Scholar]
- Brown AS, Susser ES. In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev. 2002;8:51–57. doi: 10.1002/mrdd.10004. [DOI] [PubMed] [Google Scholar]
- Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull. 2008;34:1054–1063. doi: 10.1093/schbul/sbn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Susser ES, Lin SP, Neugebauer R, Gorman JM. Increased risk of affective disorders in males after second trimester prenatal exposure to the Dutch hunger winter of 1944–45. Br J Psychiatry. 1995;166:601–606. doi: 10.1192/bjp.166.5.601. [DOI] [PubMed] [Google Scholar]
- Brown AS, van Os J, Driessens C, Hoek HW, Susser ES. Further evidence of relation between prenatal famine and major affective disorder. Am J Psychiatry. 2000;157:190–195. doi: 10.1176/appi.ajp.157.2.190. [DOI] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Perrin M, Gorman JM, Susser ES. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J Neuroendocrinol. 2010;22:258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- Burne TH, Becker A, Brown J, Eyles DW, Mackay-Sim A, McGrath JJ. Transient prenatal vitamin D deficiency is associated with hyperlocomotion in adult rats. Behav Brain Res. 2004;154:549–555. doi: 10.1016/j.bbr.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Burton C, Lovic V, Fleming AS. Early adversity alters attention and locomotion in adult Sprague–Dawley rats. Behav Neurosci. 2006;120:665–675. doi: 10.1037/0735-7044.120.3.665. [DOI] [PubMed] [Google Scholar]
- Carboni E, Barros VG, Ibba M, Silvagni A, Mura C, Antonelli MC. Prenatal restraint stress: an in vivo microdialysis study on catecholamine release in the rat prefrontal cortex. Neuroscience. 2010;168:156–166. doi: 10.1016/j.neuroscience.2010.03.046. [DOI] [PubMed] [Google Scholar]
- Christian LM, Franco A, Glaser R, Iams JD. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav Immun. 2009;23:750–754. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Son GH, Park SH, Park E, Lee KH, Geum D, Kim K. Differential adaptive responses to chronic stress of maternally stressed male mice offspring. Endocrinology. 2005;146:3202–3210. doi: 10.1210/en.2004-1458. [DOI] [PubMed] [Google Scholar]
- Clinton S, Miller S, Watson SJ, Akil H. Prenatal stress does not alter innate novelty-seeking behavioral traits, but differentially affects individual differences in neuroendocrine stress responsivity. Psychoneuroendocrinology. 2008;33:162–177. doi: 10.1016/j.psyneuen.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cratty MS, Ward HE, Johnson EA, Azzaro AJ, Birkle DL. Prenatal stress increases corticotropin-releasing factor content and release in rat amygdala minces. Brain Res. 1995;675:297–302. doi: 10.1016/0006-8993(95)00087-7. [DOI] [PubMed] [Google Scholar]
- Cui K, Ashdown H, Luheshi GN, Boksa P. Effects of prenatal immune activation on hippocampal neurogenesis in the rat. Schizophr Res. 2009;113:288–297. doi: 10.1016/j.schres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Dahlgren J, Samuelsson AM, Jansson T, Holmang A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res. 2006;60:147–151. doi: 10.1203/01.pdr.0000230026.74139.18. [DOI] [PubMed] [Google Scholar]
- Dalitz P, Harding R, Rees SM, Cock ML. Prolonged reductions in placental blood flow and cerebral oxygen delivery in preterm fetal sheep exposed to endotoxin: possible factors in white matter injury after acute infection. J Soc Gynecol Investig. 2003;10:283–290. doi: 10.1016/s1071-5576(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Dwork AJ, Mancevski B, Rosoklija G. White matter and cognitive function in schizophrenia. Int J Neuropsychopharmacol. 2007;10:513–536. doi: 10.1017/S1461145707007638. [DOI] [PubMed] [Google Scholar]
- Entringer S, Kumsta R, Nelson EL, Hellhammer DH, Wadhwa PD, Wust S. Influence of prenatal psychosocial stress on cytokine production in adult women. Dev Psychobiol. 2008;50:579–587. doi: 10.1002/dev.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estanislau C, Morato S. Prenatal stress produces more behavioral alterations than maternal separation in the elevated plus-maze and in the elevated T-maze. Behav Brain Res. 2005;163:70–77. doi: 10.1016/j.bbr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- European Network of Schizophrenia Networks for the Study of Gene-Environment Interactions. Schizophrenia aetiology: do gene-environment interactions hold the key? Schizophr Res. 2008;102:21–26. doi: 10.1016/j.schres.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Eyles DW, Rogers F, Buller K, McGrath JJ, Ko P, French K, Burne TH. Developmental vitamin D (DVD) deficiency in the rat alters adult behaviour independently of HPA function. Psychoneuroendocrinology. 2006;31:958–964. doi: 10.1016/j.psyneuen.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Falkai P, Honer WG, David S, Bogerts B, Majtenyi C, Bayer TA. No evidence for astrogliosis in brains of schizophrenic patients. A post-mortem study. Neuropathol Appl Neurobiol. 1999;25:48–53. doi: 10.1046/j.1365-2990.1999.00162.x. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Sidwell R, Akhter P, Sedgewick J, Thuras P, Bailey K, Kist D. Human influenza viral infection in utero increases nNOS expression in hippocampi of neonatal mice. Synapse. 1998;29:84–88. doi: 10.1002/(SICI)1098-2396(199805)29:1<84::AID-SYN8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, Shier A, Sheikh S, Bailey K. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry. 1999;4:145–154. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Cuadra AE, El-Fakahany EE, Sidwell RW, Thuras P. Prenatal viral infection causes alterations in nNOS expression in developing mouse brains. NeuroReport. 2000;11:1493–1496. [PubMed] [Google Scholar]
- Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L, Sidwell R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002a;22:25–33. doi: 10.1023/a:1015337611258. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Sidwell RW, Kist DA, Stary JM, Earle JA, Thuras P. Human influenza viral infection in utero alters glial fibrillary acidic protein immunoreactivity in the developing brains of neonatal mice. Mol Psychiatry. 2002b;7:633–640. doi: 10.1038/sj.mp.4001046. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Araghi-Niknam M, Laurence JA, Stary JM, Sidwell RW, Lee S. Glial fibrillary acidic protein and glutamic acid decarboxylase 65 and 67 kDa proteins are increased in brains of neonatal BALB/c mice following viral infection in utero. Schizophr Res. 2004;69:121–123. doi: 10.1016/S0920-9964(03)00175-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Pearce DA, Brooks AI, Sidwell RW. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: a potential animal model for schizophrenia and autism. Synapse. 2005;57:91–99. doi: 10.1002/syn.20162. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Sidwell RW. Viral regulation of aquaporin 4, connexin 43, microcephalin and nucleolin. Schizophr Res. 2008a;98:163–177. doi: 10.1016/j.schres.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, Smee DF, Pearce DA, Winter C, Sohr R, Juckel G. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr Res. 2008b;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Abu-Odeh D, Mori S, Huang H, Oishi K. Abnormal expression of myelination genes and alterations in white matter fractional anisotropy following prenatal viral influenza infection at E16 in mice. Schizophr Res. 2009;112:46–53. doi: 10.1016/j.schres.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feleder C, Tseng KY, Calhoon GG, O'Donnell P. Neonatal intrahippocampal immune challenge alters dopamine modulation of prefrontal cortical interneurons in adult rats. Biol Psychiatry. 2010;67:386–392. doi: 10.1016/j.biopsych.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M. Cortisol: the culprit prenatal stress variable. Int J Neurosci. 2008;1:1181–1205. doi: 10.1080/00207450701820944. [DOI] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forder JP, Tymianski M. Postsynaptic mechanisms of excitotoxicity: Involvement of postsynaptic density proteins, radicals, and oxidant molecules. Neuroscience. 2009;158:293–300. doi: 10.1016/j.neuroscience.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Joober R, Luheshi GN, Boksa P. Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. J Psychiatr Res. 2004;38:335–345. doi: 10.1016/j.jpsychires.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Fride E, Weinstock M. Prenatal stress increases anxiety related behavior and alters cerebral lateralization of dopamine activity. Life Sci. 1988;42:1059–1065. doi: 10.1016/0024-3205(88)90561-9. [DOI] [PubMed] [Google Scholar]
- Fujioka A, Fujioka T, Ishida Y, Maekawa T, Nakamura S. Differential effects of prenatal stress on the morphological maturation of hippocampal neurons. Neuroscience. 2006;141:907–915. doi: 10.1016/j.neuroscience.2006.04.046. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Pasini M, Frasca A, Drago F, Racagni G, Riva MA. Prenatal stress alters glutamatergic system responsiveness in adult rat prefrontal cortex. J Neurochem. 2009;109:1733–1744. doi: 10.1111/j.1471-4159.2009.06088.x. [DOI] [PubMed] [Google Scholar]
- Gayle DA, Beloosesky R, Desai M, Amidi F, Nunez SE, Ross MG. Maternal LPS induces cytokines in the amniotic fluid and corticotropin releasing hormone in the fetal rat brain. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1024–R1029. doi: 10.1152/ajpregu.00664.2003. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Fredrik Jarskog L, Vadlamudi S, Lauder JM. Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology. 2004;29:1221–1229. doi: 10.1038/sj.npp.1300446. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur J Neurosci. 2007;25:2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn MJ, Kirby ED, Gibson EM, Wong-Goodrich SJ, Mellott TJ, Blusztajn JK, Williams CL. Age-related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats. Brain Res. 2008;1237:110–123. doi: 10.1016/j.brainres.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34:835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz AA, Stefanski V. Psychosocial maternal stress during pregnancy affects serum corticosterone, blood immune parameters and anxiety behaviour in adult male rat offspring. Physiol Behav. 2007;90:108–115. doi: 10.1016/j.physbeh.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Green MF, Butler PD, Chen Y, Geyer MA, Silverstein S, Wynn JK, Yoon JH, Zemon V. Perception measurement in clinical trials of schizophrenia: promising paradigms from CNTRICS. Schizophr Bull. 2009;35:163–181. doi: 10.1093/schbul/sbn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gue M, Bravard A, Meunier J, Veyrier R, Gaillet S, Recasens M, Maurice T. Sex differences in learning deficits induced by prenatal stress in juvenile rats. Behav Brain Res. 2004;150:149–157. doi: 10.1016/S0166-4328(03)00250-X. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Harms LR, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6 J mice. Behav Brain Res. 2008;187:343–350. doi: 10.1016/j.bbr.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hava G, Vered L, Yael M, Mordechai H, Mahoud H. Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Dev Psychobiol. 2006;48:162–168. doi: 10.1002/dev.20116. [DOI] [PubMed] [Google Scholar]
- Henry C, Guegant G, Cador M, Arnauld E, Arsaut J, LeMoal M, Demotes-Mainard J. Prenatal stress in rats facilitates amphetamine-induced sensitization and induces long-lasting changes in dopamine receptors in the nucleus accumbens. Brain Res Dev Brain Res. 1995;685:179–186. doi: 10.1016/0006-8993(95)00430-x. [DOI] [PubMed] [Google Scholar]
- Hopwood N, Maswanganyi T, Harden LM. Comparison of anorexia, lethargy, and fever induced by bacterial and viral mimetics in rats. Can J Physiol Pharmacol. 2009;87:211–220. doi: 10.1139/y09-003. [DOI] [PubMed] [Google Scholar]
- Hougaard KS, Andersen MB, Kjaer SL, Hansen AM, Werge T, Lund SP. Prenatal stress may increase vulnerability to life events: comparison with the effects of prenatal dexamethasone. Brain Res Dev Brain Res. 2005;159:55–63. doi: 10.1016/j.devbrainres.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarskog LF, Xiao H, Wilkie MB, Lauder JM, Gilmore JH. Cytokine regulation of embryonic rat dopamine and serotonin neuronal survival in vitro. Int J Dev Neurosci. 1997;15:711–716. doi: 10.1016/s0736-5748(97)00029-4. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-d-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Jia N, Yang K, Sun Q, Cai Q, Li H, Cheng D, Fan X, Zhu Z. Prenatal stress causes dendritic atrophy of pyramidal neurons in hippocampal CA3 region by glutamate in offspring rats. Dev Neurobiol. 2010;70:114–125. doi: 10.1002/dneu.20766. [DOI] [PubMed] [Google Scholar]
- Jones JP, Meck WH, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Brain Res Dev Brain Res. 1999;118:159–167. doi: 10.1016/s0165-3806(99)00103-0. [DOI] [PubMed] [Google Scholar]
- Karege F, Perroud N, Schurhoff F, Meary A, Marillier G, Burkhardt S, Ballmann E, Fernandez R, Jamain S, Leboyer M, La Harpe R, Malafosse A. Association of Akt1 gene variants and protein expression in both schizophrenia and bipolar disorder. Genes Brain Behav. 2010;9:503–511. doi: 10.1111/j.1601-183X.2010.00578.x. [DOI] [PubMed] [Google Scholar]
- Katunar MR, Saez T, Brusco A, Antonelli MC. Ontogenetic expression of dopamine-related transcription factors and tyrosine hydroxylase in prenatally stressed rats. Neurotox Res. 2010;18:69–81. doi: 10.1007/s12640-009-9132-z. [DOI] [PubMed] [Google Scholar]
- Kesby JP, Burne TH, McGrath JJ, Eyles DW. Developmental vitamin D deficiency alters MK 801-induced hyperlocomotion in the adult rat: An animal model of schizophrenia. Biol Psychiatry. 2006;60:591–596. doi: 10.1016/j.biopsych.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Kinnunen AK, Koenig JI, Bilbe G. Repeated variable prenatal stress alters pre- and postsynaptic gene expression in the rat frontal pole. J Neurochem. 2003;86:736–748. doi: 10.1046/j.1471-4159.2003.01873.x. [DOI] [PubMed] [Google Scholar]
- Koenig JI. Schizophrenia: a unique translational opportunity in behavioral neuroendocrinology. Horm Behav. 2006;50:602–611. doi: 10.1016/j.yhbeh.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res. 2005;156:251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley-Evans SC, Gardner DS, Jackson AA. Maternal protein restriction influences the programming of the rat hypothalamic–pituitary–adrenal axis. J Nutr. 1996;126:1578–1585. doi: 10.1093/jn/126.6.1578. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psych. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady D, Shapiro RA, Dorsa DM, Koenig JI. Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology. 2005;30:1883–1894. doi: 10.1038/sj.npp.1300722. (published online 2/16/05) [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Feldon J. Lack of effect of an early stressful life event on sensorimotor gating in adult rats. Schizophr Res. 2000;41:365–371. doi: 10.1016/s0920-9964(99)00080-8. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage J, Blondeau B, Grino M, Breant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology. 2001;142:1692–1702. doi: 10.1210/endo.142.5.8139. [DOI] [PubMed] [Google Scholar]
- Levay EA, Paolini AG, Govic A, Hazi A, Penman J, Kent S. Anxiety-like behaviour in adult rats perinatally exposed to maternal calorie restriction. Behav Brain Res. 2008;191:164–172. doi: 10.1016/j.bbr.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119:706–716. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Cheung C, Wei R, Hui ES, Feldon J, Meyer U, Chung S, Chua SE, Sham PC, Wu EX, McAlonan GM. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PLoS ONE. 2009;4:e6354. doi: 10.1371/journal.pone.0006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liverman CS, Kaftan HA, Cui L, Hersperger SG, Taboada E, Klein RM, Berman NE. Altered expression of pro-inflammatory and developmental genes in the fetal brain in a mouse model of maternal infection. Neurosci Lett. 2006;399:220–225. doi: 10.1016/j.neulet.2006.01.064. [DOI] [PubMed] [Google Scholar]
- Lowe GC, Luheshi GN, Williams S. Maternal infection and fever during late gestation are associated with altered synaptic transmission in the hippocampus of juvenile offspring rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1563–R1571. doi: 10.1152/ajpregu.90350.2008. [DOI] [PubMed] [Google Scholar]
- Makinodan M, Tatsumi K, Manabe T, Yamauchi T, Makinodan E, Matsuyoshi H, Shimoda S, Noriyama Y, Kishimoto T, Wanaka A. Maternal immune activation in mice delays myelination and axonal development in the hippocampus of the offspring. J Neurosci Res. 2008;86:2190–2200. doi: 10.1002/jnr.21673. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Crawford EF, Eisch AJ, Rivier CL, Richardson HN. Stress experienced in utero reduces sexual dichotomies in neurogenesis, microenvironment, and cell death in the adult rat hippocampus. Dev Neurobiol. 2008;68:575–589. doi: 10.1002/dneu.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Taylor AR, Taylor SB, Brady-Bell D, Koenig JI. Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. 2010 doi: 10.3389/fnbeh.2010.00173. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes E, McCarthy S, Gong G, van Eekelen JA, Dunstan J, Foster J, Prescott SL. Maternal mood scores in mid-pregnancy are related to aspects of neonatal immune function. Brain Behav Immun. 2009;23:380–388. doi: 10.1016/j.bbi.2008.12.004. [DOI] [PubMed] [Google Scholar]
- McCann JC, Hudes M, Ames BN. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci Biobehav Rev. 2006;30:696–712. doi: 10.1016/j.neubiorev.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Meunier J, Gue M, Recasens M, Maurice T. Attenuation by a sigma1 (sigma1) receptor agonist of the learning and memory deficits induced by a prenatal restraint stress in juvenile rats. Br J Pharmacol. 2004;142:689–700. doi: 10.1038/sj.bjp.0705835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav Brain Res. 2009;204:322–334. doi: 10.1016/j.bbr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun. 2006a;20:378–388. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006b;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Schwendener S, Feldon J, Yee BK. Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Exp Brain Res. 2006c;173:243–257. doi: 10.1007/s00221-006-0419-5. [DOI] [PubMed] [Google Scholar]
- Meyer U, Engler A, Weber L, Schedlowski M, Feldon J. Preliminary evidence for a modulation of fetal dopaminergic development by maternal immune activation during pregnancy. Neuroscience. 2008a;154:701–709. doi: 10.1016/j.neuroscience.2008.04.031. [DOI] [PubMed] [Google Scholar]
- Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry. 2008b;13:208–221. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology. 2008c;33:441–456. doi: 10.1038/sj.npp.1301413. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008d;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Spoerri E, Yee BK, Schwarz MJ, Feldon J. Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia. Schizophr Bull. 2008e;34:1083–1094. doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35:959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Knuesel I, Nyffeler M, Feldon J. Chronic clozapine treatment improves prenatal infection-induced working memory deficits without influencing adult hippocampal neurogenesis. Psychopharmacol Berl. 2010;208:531–543. doi: 10.1007/s00213-009-1754-6. [DOI] [PubMed] [Google Scholar]
- Michelsen KA, van den Hove DL, Schmitz C, Segers O, Prickaerts J, Steinbusch HW. Prenatal stress and subsequent exposure to chronic mild stress influence dendritic spine density and morphology in the rat medial prefrontal cortex. BMC Neurosci. 2007;8:107. doi: 10.1186/1471-2202-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokler DJ, Torres OI, Galler JR, Morgane PJ. Stress-induced changes in extracellular dopamine and serotonin in the medial prefrontal cortex and dorsal hippocampus of prenatally malnourished rats. Brain Res. 2007;1148:226–233. doi: 10.1016/j.brainres.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav. 2007;91:51–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Riedel M, Gruber R, Ackenheil M, Schwarz MJ. The immune system and schizophrenia. An integrative view. Ann NY Acad Sci. 2000;917:456–467. doi: 10.1111/j.1749-6632.2000.tb05410.x. [DOI] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- Neeley EW, Berger R, Koenig JI, Leonard S. Prenatal stress differentially alters BDNF expression and signaling across rat strains. 2010 doi: 10.1016/j.neuroscience.2011.03.065. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez H, Ruiz S, Soto-Moyano R, Navarrete M, Valladares L, White A, Perez H. Fetal undernutrition induces overexpression of CRH mRNA and CRH protein in hypothalamus and increases CRH and corticosterone in plasma during postnatal life in the rat. Neurosci Lett. 2008;448:115–119. doi: 10.1016/j.neulet.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Nyffeler M, Meyer U, Yee BK, Feldon J, Knuesel I. Maternal immune activation during pregnancy increases limbic GABAA receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience. 2006;143:51–62. doi: 10.1016/j.neuroscience.2006.07.029. [DOI] [PubMed] [Google Scholar]
- O'Loan J, Eyles DW, Kesby J, Ko P, McGrath JJ, Burne TH. Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology. 2007;32:227–234. doi: 10.1016/j.psyneuen.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Oliver PL, Davies KE. Interaction between environmental and genetic factors modulates schizophrenic endophenotypes in the Snap-25 mouse mutant blind-drunk. Hum Mol Genet. 2009;18:4576–4589. doi: 10.1093/hmg/ddp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Printz DJ, Butler PD, Dulawa SC, Printz MP. Prenatal protein deprivation in rats induces changes in prepulse inhibition and NMDA receptor binding. Brain Res. 2004;996:193–201. doi: 10.1016/j.brainres.2003.09.077. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Brown AS, Keegan D, Siska LD, Susser E, Rotrosen J, Butler PD. Prenatal protein deprivation alters dopamine-mediated behaviors and dopaminergic and glutamatergic receptor binding. Brain Res. 2008;1237:62–74. doi: 10.1016/j.brainres.2008.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang D, Syed S, Fine P, Jones PB. No association between prenatal viral infection and depression in later life–a long-term cohort study of 6152 subjects. Can J Psychiatry. 2009;54:565–570. doi: 10.1177/070674370905400809. [DOI] [PubMed] [Google Scholar]
- Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, Caruncho HJ. Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci USA. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltyrev T, Keshet GI, Kay G, Weinstock M. Role of experimental conditions in determining differences in exploratory behavior of prenatally stressed rats. Dev Psychobiol. 1996;29:453–462. doi: 10.1002/(SICI)1098-2302(199607)29:5<453::AID-DEV4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Potter D, Summerfelt A, Gold J, Buchanan RW. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr Bull. 2006;32:692–700. doi: 10.1093/schbul/sbj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Stern EL, Whiteside MB, Herkenham M. Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol. 1999;93:72–80. doi: 10.1016/s0165-5728(98)00193-3. [DOI] [PubMed] [Google Scholar]
- Ranade SC, Rose A, Rao M, Gallego J, Gressens P, Mani S. Different types of nutritional deficiencies affect different domains Psychopharmacology of spatial memory function checked in a radial arm maze. Neuroscience. 2008;152:859–866. doi: 10.1016/j.neuroscience.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Rees S, Harding R, Walker D. An adverse intrauterine environment: implications for injury and altered development of the brain. Int J Dev Neurosci. 2008;26:3–11. doi: 10.1016/j.ijdevneu.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Rice F, Jones I, Thapar A. The impact of gestational stress and prenatal growth on emotional problems in offspring: a review. Acta Psychiatr Scand. 2007;115:171–183. doi: 10.1111/j.1600-0447.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Boivin J, van den Bree M, Hay DF, Thapar A. The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychol Med. 2010;40:335–345. doi: 10.1017/S0033291709005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- Romero E, Ali C, Molina-Holgado E, Castellano B, Guaza C, Borrell J. Neurobehavioral and immunological consequences of prenatal immune activation in rats. Neuropsychopharmacology. 2007;32:1791–1804. doi: 10.1038/sj.npp.1301292. [DOI] [PubMed] [Google Scholar]
- Romero E, Guaza C, Castellano B, Borrell J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol Psychiatry. 2008;15:372–383. doi: 10.1038/mp.2008.44. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1345–R1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151 Suppl 3:U49–U62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short SJ, Lubach GR, Karasin AI, Olsen CW, Styner M, Knickmeyer RC, Gilmore JH, Coe CL. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry. 2010;67:965–973. doi: 10.1016/j.biopsych.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell RW, Huffman JH, Call EW, Alaghamandan H, Cook PD, Robins RK. Effect of selenazofurin on influenza A and B virus infections of mice. Antivir Res. 1986;6:343–353. doi: 10.1016/0166-3542(86)90016-1. [DOI] [PubMed] [Google Scholar]
- Silvagni A, Barros VG, Mura C, Antonelli MC, Carboni E. Prenatal restraint stress differentially modifies basal and stimulated dopamine and noradrenaline release in the nucleus accumbens shell: an ‘in vivo’ microdialysis study in adolescent and young adult rats. Eur J Neurosci. 2008;28:744–758. doi: 10.1111/j.1460-9568.2008.06364.x. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov BP. Oligodendroglial abnormalities in schizophrenia, mood disorders and substance abuse. Comorbidity, shared traits, or molecular phenocopies? Int J Neuropsychopharmacol. 2007;10:547–555. doi: 10.1017/S1461145706007322. [DOI] [PubMed] [Google Scholar]
- Son GH, Geum D, Chung S, Kim EJ, Jo JH, Kim CM, Lee KH, Kim H, Choi S, Kim HT, Lee CJ, Kim K. Maternal stress produces learning deficits associated with impairment of NMDA receptor-mediated synaptic plasticity. J Neurosci. 2006;26:3309–3318. doi: 10.1523/JNEUROSCI.3850-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spauwen J, Krabbendam L, Lieb R, Wittchen HU, van Os J. Early maternal stress and health behaviours and offspring expression of psychosis in adolescence. Acta Psychiatr Scand. 2004;110:356–364. doi: 10.1111/j.1600-0447.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, Zheng X, Gu N, Feng G, Sham P, He L. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. Jama. 2005;294:557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Stein AD, Pierik FH, Verrips GH, Susser ES, Lumey LH. Maternal exposure to the Dutch famine before conception and during pregnancy: quality of life and depressive symptoms in adult offspring. Epidemiology. 2009;20:909–915. doi: 10.1097/EDE.0b013e3181b5f227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KE, Adams CE, Mellott TJ, Robbins E, Kisley MA. Perinatal choline deficiency produces abnormal sensory inhibition in Sprague-Dawley rats. Brain Res. 2008;1237:84–90. doi: 10.1016/j.brainres.2008.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF. The genetics of schizophrenia. PLoS Med. 2005;2:e212. doi: 10.1371/journal.pmed.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Szymanska M, Budziszewska B, Jaworska-Feil L, Basta-Kaim A, Kubera M, Leskiewicz M, Regulska M, Lason W. The effect of antidepressant drugs on the HPA axis activity, glucocorticoid receptor level and FKBP51 concentration in prenatally stressed rats. Psychoneuroendocrinology. 2009;34:822–832. doi: 10.1016/j.psyneuen.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Rawlings RR, Ennis JM, Merrill DD, Flores DS. Birth seasonality in bipolar disorder, schizophrenia, schizoaffective disorder and stillbirths. Schizophr Res. 1996;21:141–149. doi: 10.1016/0920-9964(96)00022-9. [DOI] [PubMed] [Google Scholar]
- Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- Van den Hove DL, Blanco CE, Aendekerk B, Desbonnet L, Bruschettini M, Steinbusch HP, Prickaerts J, Steinbusch HW. Prenatal restraint stress and long-term affective consequences. Dev Neurosci. 2005;27:313–320. doi: 10.1159/000086711. [DOI] [PubMed] [Google Scholar]
- Van Den Hove DL, Steinbusch HW, Scheepens A, Van De Berg WD, Kooiman LA, Boosten BJ, Prickaerts J, Blanco CE. Prenatal stress and neonatal rat brain development. Neuroscience. 2006;137:145–155. doi: 10.1016/j.neuroscience.2005.08.060. [DOI] [PubMed] [Google Scholar]
- van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34:1066–1082. doi: 10.1093/schbul/sbn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Totoki K, Schoch H, Whitaker KW, Hill-Smith T, Lucki I, Reyes TM. Early life protein restriction alters dopamine circuitry. Neuroscience. 2010;168:359–370. doi: 10.1016/j.neuroscience.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillermot S, Weber L, Feldon J, Meyer U. A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J Neurosci. 2010;30:1270–1287. doi: 10.1523/JNEUROSCI.5408-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Estradiol decreases anxiety behavior and enhances inhibitory avoidance and gestational stress produces opposite effects. Stress. 2007;10:251–260. doi: 10.1080/00958970701220416. [DOI] [PubMed] [Google Scholar]
- Watson JB, Mednick SA, Huttunen M, Wang X. Prenatal teratogens and the development of adult mental illness. Dev Psychopathol. 1999;11:457–466. doi: 10.1017/s0954579499002151. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11beta-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 2000;12:1047–1054. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Thrivikraman KV, Plotsky PM. Chronic maternal stress inhibits the capacity to up-regulate placental 11beta-hydroxysteroid dehydrogenase type 2 activity. J Endocrinol. 2005;186:R7–R12. doi: 10.1677/joe.1.06374. [DOI] [PubMed] [Google Scholar]
- Winter C, Reutiman TJ, Folsom TD, Sohr R, Wolf RJ, Juckel G, Fatemi SH. Dopamine and serotonin levels following prenatal viral infection in mouse—implications for psychiatric disorders such as schizophrenia and autism. Eur Neuropsychopharmacol. 2008;18:712–716. doi: 10.1016/j.euroneuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C, Djodari-Irani A, Sohr R, Morgenstern R, Feldon J, Juckel G, Meyer U. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int J Neuropsychopharmacol. 2009;12:513–524. doi: 10.1017/S1461145708009206. [DOI] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring. Behav Brain Res. 2008;190:156–159. doi: 10.1016/j.bbr.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Wong-Goodrich SJ, Glenn MJ, Mellott TJ, Blusztajn JK, Meck WH, Williams CL. Spatial memory and hippocampal plasticity are differentially sensitive to the availability of choline in adulthood as a function of choline supply in utero. Brain Res. 2008;1237:153–166. doi: 10.1016/j.brainres.2008.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Xu MQ, Sun WS, Liu BX, Feng GY, Yu L, Yang L, He G, Sham P, Susser E, St Clair D, He L. Prenatal malnutrition and adult schizophrenia: further evidence from the 1959–1961 Chinese famine. Schizophr Bull. 2009;35:568–576. doi: 10.1093/schbul/sbn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Salomon S, Matzner H, Weinstock M. Effect of varied gestational stress on acquisition of spatial memory, hippocampal LTP and synaptic proteins in juvenile male rats. Behav Brain Res. 2007;179:126–132. doi: 10.1016/j.bbr.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Yang L, Lindholm K, Konishi Y, Li R, Shen Y. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J Neurosci. 2002;22:3025–3032. doi: 10.1523/JNEUROSCI.22-08-03025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Han H, Cao J, Li L, Xu L. Prenatal stress modifies hippocampal synaptic plasticity and spatial learning in young rat offspring. Hippocampus. 2006;16:431–436. doi: 10.1002/hipo.20181. [DOI] [PubMed] [Google Scholar]
- Zagron G, Weinstock M. Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behav Brain Res. 2006;175:323–328. doi: 10.1016/j.bbr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- Zuena AR, Mairesse J, Casolini P, Cinque C, Alema GS, Morley-Fletcher S, Chiodi V, Spagnoli LG, Gradini R, Catalani A, Nicoletti F, Maccari S. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS ONE. 2008;3:e2170. doi: 10.1371/journal.pone.0002170. [DOI] [PMC free article] [PubMed] [Google Scholar]