Abstract
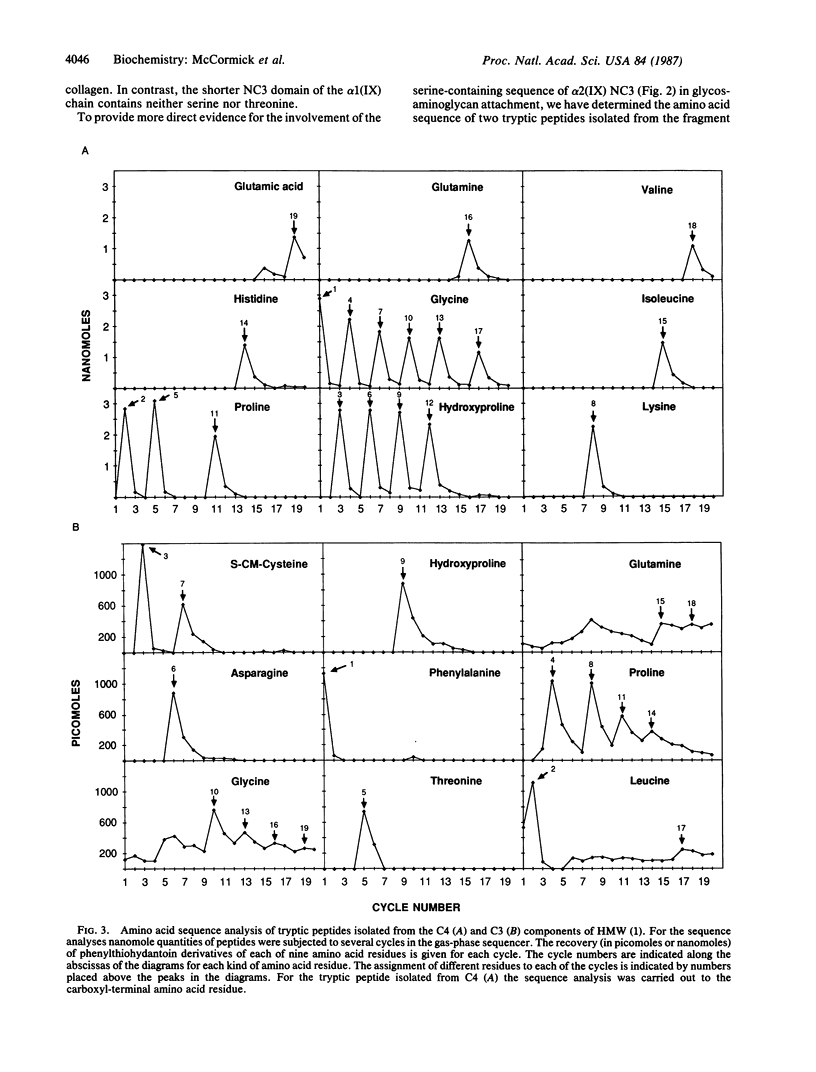
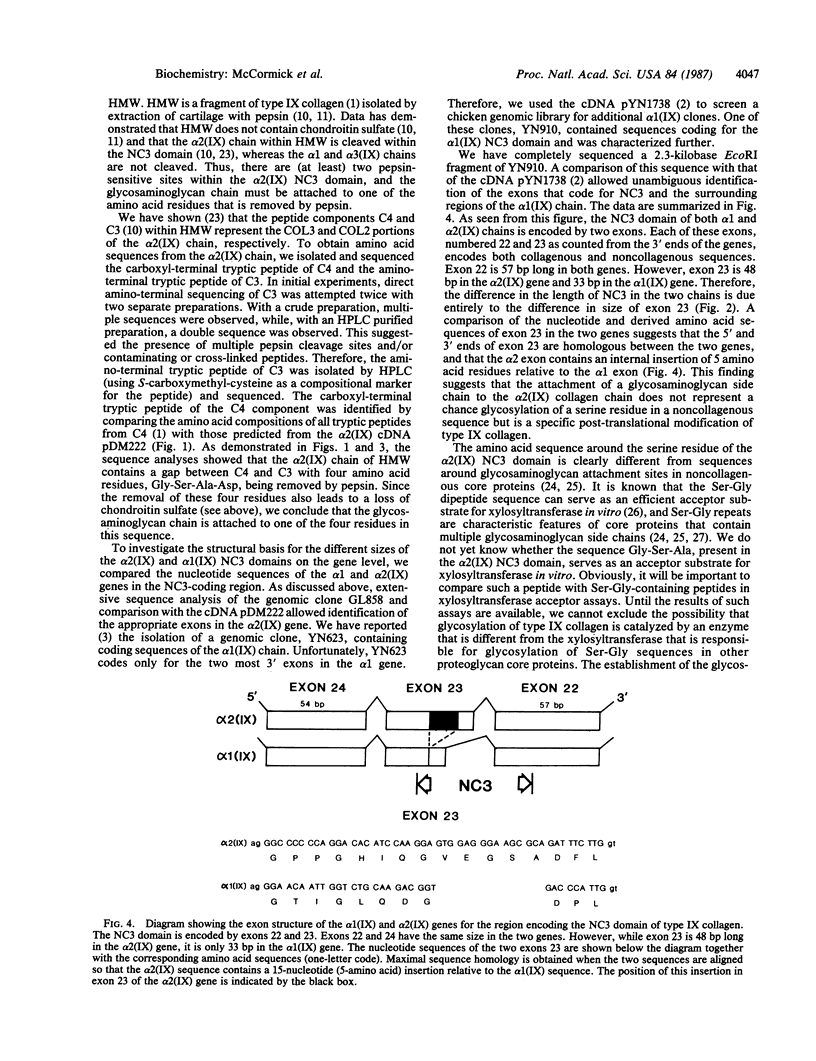
Type IX collagen represents 5-20% of the total collagen in hyaline cartilage. The molecules of this collagen are composed of three genetically distinct polypeptide subunits. One of these subunits, alpha 2(IX), contains covalently bound glycosaminoglycan (chondroitin sulfate or dermatan sulfate). We report here on the structure of the glycosaminoglycan attachment site of type IX collagen-proteoglycan. We show, by a combination of cDNA and peptide sequencing, that the attachment region contains the sequence Gly-Ser-Ala-Asp, located within the noncollagenous domain NC3 of the alpha 2(IX) chain. By comparing the exons encoding the NC3 domain in the alpha 2(IX) and alpha 1(IX) genes, we find that the exon coding for the glycosaminoglycan attachment site in the alpha 2(IX) gene is 48 base pairs long, whereas the homologous alpha 1(IX) exon is 33 base pairs. The NC3 domain is, therefore, five amino acid residues longer in alpha 2(IX) than in alpha 1(IX). The extra sequence in alpha 2(IX), Val-Glu-Gly-Ser-Ala, provides a simple explanation for the kink observed at the NC3 domain of type IX molecules when examined by electron microscopy. The inserted block of amino acid residues also provides the NC3 domain of alpha 2(IX) chains with a serine residue, not present in alpha 1(IX) that serves as attachment site for a glycosaminoglycan side chain. Our data show that the amino acid sequence that surrounds the glycosylated serine residue in type IX collagen-proteoglycan differs from glycosylated sequences in noncollagenous core proteins. The data also provide strong evidence that glycosylation of type IX collagen is not a chance glycosylation of a serine residue in a noncollagenous domain, but is a specific post-translational modification of this unusual collagen molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon M. A., Krusius T., Campbell S., Schwartz N. B., Ruoslahti E. Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. Proc Natl Acad Sci U S A. 1987 May;84(10):3194–3198. doi: 10.1073/pnas.84.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon M. A., Oldberg A., Pierschbacher M., Ruoslahti E. Molecular cloning and sequence analysis of a chondroitin sulfate proteoglycan cDNA. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1321–1325. doi: 10.1073/pnas.82.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner P., Vaughan L., Winterhalter K. H. Type IX collagen from sternal cartilage of chicken embryo contains covalently bound glycosaminoglycans. Proc Natl Acad Sci U S A. 1985 May;82(9):2608–2612. doi: 10.1073/pnas.82.9.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Huber S., van der Rest M., Bruckner P., Rodriguez E., Winterhalter K. H., Vaughan L. Identification of the type IX collagen polypeptide chains. The alpha 2(IX) polypeptide carries the chondroitin sulfate chain(s). J Biol Chem. 1986 May 5;261(13):5965–5968. [PubMed] [Google Scholar]
- Irwin M. H., Mayne R. Use of monoclonal antibodies to locate the chondroitin sulfate chain(s) in type IX collagen. J Biol Chem. 1986 Dec 15;261(35):16281–16283. [PubMed] [Google Scholar]
- Konomi H., Seyer J. M., Ninomiya Y., Olsen B. R. Peptide-specific antibodies identify the alpha 2 chain as the proteoglycan subunit of type IX collagen. J Biol Chem. 1986 May 25;261(15):6742–6746. [PubMed] [Google Scholar]
- Kroeker W. D., Kowalski D. Gene-sized pieces produced by digestion of linear duplex DNA with mung bean nuclease. Biochemistry. 1978 Aug 8;17(16):3236–3243. doi: 10.1021/bi00609a010. [DOI] [PubMed] [Google Scholar]
- Lazure C., Seidah N. G., Chrétien M., Lallier R., St-Pierre S. Primary structure determination of Escherichia coli heat-stable enterotoxin of porcine origin. Can J Biochem Cell Biol. 1983 May;61(5):287–292. doi: 10.1139/o83-039. [DOI] [PubMed] [Google Scholar]
- Lozano G., Ninomiya Y., Thompson H., Olsen B. R. A distinct class of vertebrate collagen genes encodes chicken type IX collagen polypeptides. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4050–4054. doi: 10.1073/pnas.82.12.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y., Olsen B. R. Synthesis and characterization of cDNA encoding a cartilage-specific short collagen. Proc Natl Acad Sci U S A. 1984 May;81(10):3014–3018. doi: 10.1073/pnas.81.10.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y., van der Rest M., Mayne R., Lozano G., Olsen B. R. Construction and characterization of cDNA encoding the alpha 2 chain of chicken type IX collagen. Biochemistry. 1985 Jul 16;24(15):4223–4229. doi: 10.1021/bi00336a061. [DOI] [PubMed] [Google Scholar]
- Noro A., Kimata K., Oike Y., Shinomura T., Maeda N., Yano S., Takahashi N., Suzuki S. Isolation and characterization of a third proteoglycan (PG-Lt) from chick embryo cartilage which contains disulfide-bonded collagenous polypeptide. J Biol Chem. 1983 Aug 10;258(15):9323–9331. [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek P. A., Merlino G. T., Peters N. K., Cohn V. H., Moore G. P., Kleinsmith L. J. Characterization of five members of the actin gene family in the sea urchin. Biochim Biophys Acta. 1981 Dec 28;656(2):195–205. doi: 10.1016/0005-2787(81)90087-3. [DOI] [PubMed] [Google Scholar]
- Reese C. A., Mayne R. Minor collagens of chicken hyaline cartilage. Biochemistry. 1981 Sep 15;20(19):5443–5448. doi: 10.1021/bi00522a014. [DOI] [PubMed] [Google Scholar]
- Reese C. A., Wiedemann H., Kühn K., Mayne R. Characterization of a highly soluble collagenous molecule isolated from chicken hyaline cartilage. Biochemistry. 1982 Mar 2;21(5):826–830. doi: 10.1021/bi00534a002. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Bourdon M., Krusius T. Molecular cloning of proteoglycan core proteins. Ciba Found Symp. 1986;124:260–271. doi: 10.1002/9780470513385.ch14. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan L., Winterhalter K. H., Bruckner P. Proteoglycan Lt from chicken embryo sternum identified as type IX collagen. J Biol Chem. 1985 Apr 25;260(8):4758–4763. [PubMed] [Google Scholar]
- van der Rest M., Fietzek P. P. A comprehensive approach to the study of collagen primary structure based on high-performance liquid chromatography. Eur J Biochem. 1982 Jul;125(3):491–496. doi: 10.1111/j.1432-1033.1982.tb06709.x. [DOI] [PubMed] [Google Scholar]
- van der Rest M., Mayne R., Ninomiya Y., Seidah N. G., Chretien M., Olsen B. R. The structure of type IX collagen. J Biol Chem. 1985 Jan 10;260(1):220–225. [PubMed] [Google Scholar]


